In this issue of Blood, Till et al present 3 patients with relapsed B-cell lymphomas treated with autologous T cells genetically modified to express a CD20-targeted chimeric antigen receptor (CAR) demonstrating both safety and clinical efficacy.1
A patient's own T cells may be genetically modified in the laboratory to target antigens expressed on tumor cells through the introduction and expression of CAR genes encoding tumor-targeted monoclonal antibody–derived single fragment length antibodies (scFvs) fused to T cell–derived cytoplasmic signaling domains. When expressed by T cells, these CARs mediate redirected T-cell specificity to antigens expressed on the tumor as well as normal cells that express the targeted antigen. Significantly, this approach has recently been translated into the clinical setting with an emphasis on trials treating patients with B-cell malignancies given the attractive target antigens expressed by these tumors including CD19 and CD20. While to date most published clinical reports demonstrating promising clinical responses use CAR-modified T cells targeted to CD19,2-5 in their report Till et al report similarly promising clinical responses targeting CD20,1 indicating that efficacy using this approach is not necessarily dependent on the antigen targeted by the CAR-modified T cells.
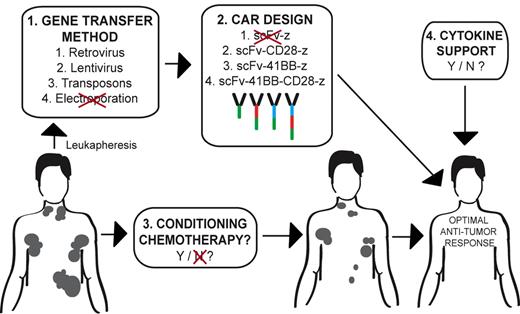
Many variables exist in the design of an optimized clinical protocol using autologous CAR-modified tumor-targeted T cells in the treatment of cancer. Patients undergo an initial leukapheresis to isolate autologous T cells. 1. CAR gene transfer of T cells may variably be achieved through retroviral-, lentiviral-, or transposon-mediated technology, with electroporation deemed unsuitable in this current report. 2. Optimal CAR design currently remains unclear although previously published data support a second- or third-generation CAR design over a first-generation CAR. 3. Conditioning therapy appears to favor T-cell persistence and an improved antitumor response. 4. However, the role of postinfusion exogenous cytokine support in obtaining an optimal antitumor response requires additional clinical study. Illustration by Hollie Pegram.
Many variables exist in the design of an optimized clinical protocol using autologous CAR-modified tumor-targeted T cells in the treatment of cancer. Patients undergo an initial leukapheresis to isolate autologous T cells. 1. CAR gene transfer of T cells may variably be achieved through retroviral-, lentiviral-, or transposon-mediated technology, with electroporation deemed unsuitable in this current report. 2. Optimal CAR design currently remains unclear although previously published data support a second- or third-generation CAR design over a first-generation CAR. 3. Conditioning therapy appears to favor T-cell persistence and an improved antitumor response. 4. However, the role of postinfusion exogenous cytokine support in obtaining an optimal antitumor response requires additional clinical study. Illustration by Hollie Pegram.
While this study serves as an additional report highlighting the therapeutic potential of CAR-modified T-cell adoptive therapy, it raises significant questions regarding protocol design to achieve optimal antitumor efficacy using this technology (see figure). In other words, among the multiple currently ongoing phase 1 clinical trials targeting B-cell malignancies with CAR-modified T cells there remain a significant number of confounding variables in protocol design that impair both the ability of investigators to compare clinical trial results between studies as well as assess the conditions that would result in an optimal antitumor response.6
There exists a high degree of variability in gene transfer approaches with groups using retroviral vectors, lentiviral vectors, transposons, and, in this report, electroporation. Given the reported difficulties in generating suitable numbers of CAR-modified T cells using electroporation, as well as the lengthy requisite periods of in vitro expansion in this current report,1 the authors in future trials plan to switch to a lentiviral gene transfer system that allows for a more reliable and rapid generation of targeted T cells. However, the question of whether optimal safety, gene transfer, and subsequent CAR expression may be achieved with retroviral- versus lentiviral- versus transposon-mediated gene transfer remains an unanswered question.
In addition, based on published preclinical and clinical trial data, optimal CAR design continues to be debated. Most current clinical trials use second-generation CARs containing a co-stimulatory signaling domain, either CD28 or 4-1BB, with both constructs demonstrating promising clinical responses and long-term T-cell persistence.2-5 A recent study reported enhanced modified in vivo persistence of T cells bearing a CD28-containing second-generation CAR versus a first-generation CAR targeting the CD19 antigen.7 Collectively, these studies support the use of a second-generation CAR over a first-generation CAR. In the current report the authors used a third-generation CAR containing 2 co-stimulatory signaling domains, CD28 and 4-1BB, demonstrating safety as well as long-term persistence of modified T cells.1 This finding is significant given a previously reported death on study of a colon cancer patient treated with a similar third-generation CAR targeted to the ERBB2 antigen,8 suggesting that the adverse outcome seen in this case is more likely related to the targeted antigen rather than the CAR design. However, what remains unanswered is whether these third-generation CARs will offer improved antitumor efficacy compared with the better-studied second-generation CARs. In addition, with respect to second-generation CARs, it remains to be seen whether optimal clinical outcomes are achieved with the inclusion of CD28 or 4-1BB signaling domains given the promising results in B-cell malignancies reported with both CAR designs targeted to the CD19 antigen.2-5
The current report further raises the question of conditioning or lymphodepleting chemotherapy before infusion with CAR-modified T cells. The beneficial role of conditioning therapy is well established in the setting of tumor infiltrating lymphocyte (TIL) therapy of patients with melanoma.9 In contrast to initial findings by Till and colleagues demonstrating at best modest antitumor efficacy using CD20 CAR-targeted T cells in the absence of prior conditioning chemotherapy,10 in the current report, conditioning with cyclophosphamide before CAR-modified T-cell infusion resulted in more clinically relevant disease responses although the number of treated patients is small and the prior study used a first-generation CAR. In a recently published report of CLL patients, an initial cohort of patients with CD19-targeted T cells in the absence of prior conditioning therapy demonstrated no objective clinical disease responses. In contrast, a second cohort of patients treated with cyclophosphamide before CAR-modified T-cell infusion demonstrated either stable disease or marked tumor regression although patients treated in this second cohort were infused with a lower T-cell dose compared with the first cohort.2 Similarly, other reported promising clinical outcomes using CD19-targeted CAR-modified T cells each used a conditioning chemotherapy regimen just before adoptive T-cell infusion.3-5 However, the relevance of tumor sensitivity to the conditioning regimen in overall patient outcomes warrants additional investigation.
Finally, what is the role of cytokine support after modified T-cell infusion in protocol design? This question remains unanswered given the promising published clinical responses after infusion of targeted T cells both in the absence and presence of subsequent exogenous cytokine support with IL-2.1-5,10
Given the fact that there are multiple currently ongoing phase 1 clinical trials with highly variable protocol designs evaluating B cell-targeted CAR-modified T cells,6 there is a growing need in the field to address these variables head-to-head in multicenter trials to establish an optimal protocol design. Significantly, to this end our center, in collaboration with the University of Pennsylvania and Children's Hospital of Philadelphia through a National Institutes of Health–funded Special Transplantational Research Acceleration Projects (STRAP) award, has harmonized our existing protocols to compare lentiviral versus retroviral CAR gene transfer, as well as second-generation CD28 versus 4-1BB containing CD19-targeted CAR design, treating patients with equal numbers of T cells expressing either CAR construct. Outcomes from this unique multicenter trial will assess disease response as well as compare T-cell persistence of each CAR-modified T-cell population, and additionally will assess the role of retroviral versus lentiviral vector gene transfer on gene transfer and subsequent levels of T-cell CAR expression. While this study will directly address several of the persistent questions in the field, it is clear that additional future comparative multicenter clinical studies addressing additional variables cited above will be required to ultimately optimize the antitumor efficacy of this promising novel approach.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal