Abstract
Stroke is a devastating complication of sickle cell anemia (SCA) with high recurrence if untreated. Chronic transfusions reduce recurrent strokes but have associated morbidities including iron overload. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) was a multicenter phase 3 randomized trial comparing standard treatment (transfusions/chelation) to alternative treatment (hydroxyurea/phlebotomy) for children with SCA, stroke, and iron overload. SWiTCH was a noninferiority trial with a composite primary end point, allowing an increased stroke risk but requiring superiority for removing iron. Subjects on standard treatment received monthly transfusions plus daily deferasirox iron chelation. Subjects on alternative treatment received hydroxyurea plus overlap transfusions during dose escalation to maximum tolerated dose (MTD), followed by monthly phlebotomy. Subjects on standard treatment (N = 66) maintained 30% sickle hemoglobin (HbS) and tolerated deferasirox at 28.2 ± 6.0 mg/kg/d. Subjects on alternative treatment (N = 67) initiated hydroxyurea and 60 (90%) reached MTD at 26.2 ± 4.9 mg/kg/d with 29.1% ± 6.7% fetal hemoglobin (HbF). Adjudication documented no strokes on transfusions/chelation but 7 (10%) on hydroxyurea/phlebotomy, still within the noninferiority stroke margin. The National Heart, Lung, and Blood Institute closed SWiTCH after interim analysis revealed equivalent liver iron content, indicating futility for the composite primary end point. Transfusions and chelation remain a better way to manage children with SCA, stroke, and iron overload. This clinical trial was registered at ClinicalTrials.gov NCT00122980.
Introduction
Decades of observational data have documented that cerebrovascular disease is common in sickle cell disease and causes substantial morbidity.1 Stroke is the most devastating neurologic manifestation, occurring most frequently in children and adults with homozygous sickle cell anemia (SCA). The incidence of primary stroke in children with SCA is 0.6-0.8 events per 100 patient-years,2,3 with a cumulative incidence of 7.8% by age 14 years in the Jamaican cohort4 and 11% by age 20 years in the US Cooperative Study of Sickle Cell Disease.3 Once stroke has occurred, the incidence of recurrent (secondary) stroke ranges from 47% to 93% in untreated patients.2,4,5
Chronic erythrocyte transfusions help prevent secondary stroke. Blood transfused every 3 to 4 weeks raises the hemoglobin concentration, reduces sickle hemoglobin (HbS), improves blood flow with nonsickled erythrocytes, and suppresses endogenous sickle erythropoiesis.6 An effective therapeutic target for transfusions is 30% HbS, associated with a 14% to 23% incidence of secondary stroke and event rate of 2.2-6.4 recurrent strokes per 100 patient-years.3,7,8 Unfortunately, chronic transfusions are administered indefinitely, because of the high stroke recurrence rate after discontinuation of short-term9 or long-term10 transfusions. Consequently, young patients with SCA and stroke remain on lifelong chronic transfusions with almost no available alternatives, and develop transfusion-associated problems including infections, erythrocyte auto/alloimmunization, iron overload requiring chelation therapy, and reduced expected number of quality-adjusted life years.11-15
Hydroxyurea has proven laboratory benefits and clinical efficacy for acute complications,16,17 and could potentially provide protection against recurrent stroke. After discontinuing transfusions, therapeutic phlebotomy can reduce the iron burden. After an anecdotal report of 2 patients18 and then prospective results demonstrating the feasibility of this approach,19 extended single-institution pilot data supported the use of hydroxyurea and phlebotomy as an alternative to transfusions and chelation for reduction of secondary stroke and management of iron overload, respectively.20 Thirty-five children received hydroxyurea/phlebotomy, with stroke recurrence of 5.7 events per 100 patient-years; serial phlebotomy significantly lowered iron burden and normalized hepatic iron.20 Based on these results, Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) was designed as a phase 3 multicenter trial to determine the efficacy of hydroxyurea/phlebotomy, compared with transfusions/chelation for children with SCA, stroke, and iron overload.
Methods
Study design
SWiTCH was a noninferiority trial, comparing alternative treatment (hydroxyurea/phlebotomy) to standard treatment (transfusions/chelation) for reduction of secondary stroke and improved management of iron overload, respectively. As described,21 subjects with SCA, previous stroke, and ≥ 18 months of transfusions with documented iron overload were recruited from 26 pediatric sickle cell programs (supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After local institutional review board approval from all SWiTCH sites and written informed consent in accordance with the Declaration of Helsinki, eligibility screening included initial stroke verification, brain magnetic resonance imaging/magnetic resonance angiography, liver biopsy with quantitative liver iron content (LIC; Mayo Laboratories) to document iron overload (defined as LIC ≥ 5 mg/g dry weight liver), transcranial Doppler (TCD), abdominal ultrasonography, neurocognitive testing, and quality of life assessment; subjects were then randomized to study treatment (Figure 1).
Enrollment, randomization, and follow-up of the SWiTCH study patients.
Study end points
Investigator interest in hydroxyurea for secondary stroke prevention was tied closely to the use of serial phlebotomy for removing iron burden. Accordingly, a composite primary end point was conceived, including both secondary stroke recurrence rate and quantitative LIC.21 Based on pilot data,19,20 the efficacy of hydroxyurea to reduce secondary stroke rate was not predicted to be equivalent to transfusions. As described,21 an increased stroke rate (inferiority margin = 0.20) was allowed by study design, with the recurrent stroke rate predicted to be 0.06 (6%) for subjects in the standard treatment arm and 0.12 (12%) for the alternative treatment arm. This “acceptable” stroke margin was offset by the likelihood of improved management of iron overload through serial phlebotomy, compared with chelation. Secondary study end points included nonstroke neurologic events, nonneurologic sickle cell clinical events, quality of life, and measures of organ function.21
Study treatments
Subjects randomized to standard treatment continued monthly blood transfusions designed to maintain ≤ 30% HbS, with local discretion regarding transfusion type (eg, simple or erythrocytapheresis). These subjects also received daily iron chelation typically with deferasirox (Exjade). Children already on chelation maintained their current dose, while those starting deferasirox received 20 mg/kg/d, with dose escalation in both groups as indicated and tolerated. Subjects randomized to alternative treatment commenced hydroxyurea at 20 mg/kg/d with stepwise escalation to maximum tolerated dose (MTD) defined by mild myelosuppression (absolute neutrophil count [ANC] 2-4 × 109/L) as described.22,23 Transfusions continued for 4 to 9 months during an overlap phase using a modified schedule20 designed to protect against recurrent stroke during hydroxyurea dose escalation. Once MTD was reached and transfusions were discontinued, phlebotomy commenced with a target of 10 mL/kg (maximum volume, 500 mL) blood removed monthly to reduce iron burden.20,21 Lower phlebotomy volumes (5 mL/kg) were recommended when subjects were excessively anemic (hemoglobin concentration, 7.0-7.9 g/dL); phlebotomy was not performed below 7.0 g/dL. The total duration of study treatment was 30 months after randomization, with a final study visit scheduled 6 months after discontinuation of study treatments.
Statistical design
SWiTCH was designed as a randomized, single-masked, noninferiority trial with a 2-component (LIC, stroke rate) composite primary end point.21 Concluding that alternative treatment is better than the standard treatment required rejecting the iron null hypothesis in favor of the alternative: alternative treatment baseline-adjusted mean LIC is less than for standard treatment and rejecting the stroke null hypothesis in favor of the alternative: alternative treatment recurrent stroke rate is less than standard treatment rate plus 0.20, the noninferiority margin, in the intention-to-treat population, as described in the design article.21 Interim analyses were scheduled after 33% and 67% of subjects completed exit studies.
Stroke adjudications
The unique SWiTCH stroke adjudication process provided inclusive and systematic evaluation of all new acute neurologic events by treatment-masked neurologists and neuroradiologists. As described,21 independent opinions were first rendered with limited data (ie, 3 neurologists forming opinions without imaging results and conversely 3 neuroradiologists without clinical data), then group consensus opinions were formed, followed by a combined consensus opinion. Subjects with “likely” stroke, based on new neurologic clinical findings and corresponding radiologic changes, were given the diagnosis of recurrent stroke, part of the primary end point. Subjects with “likely” stroke and new neurologic findings but no corresponding radiologic changes were diagnosed with transient ischemic attack (TIA).
Study monitoring
The National Heart, Lung, and Blood Institute (NHLBI)–appointed Data and Safety Monitoring Board (DSMB) periodically reviewed all enrollment, safety, toxicity, and efficacy data, including all new stroke events and interim analyses. The SWiTCH principal investigator was masked to all treatment-specific results, including laboratory tests and clinical events.
Results
Screening and enrollment
A total of 202 children with SCA and stroke were screened, with 161 consented and enrolled in SWiTCH (Figure 1). During screening, 27 became ineligible for randomization (13 for low LIC) and 134 were randomized, 2 more than the protocol specified. One subject moved before initiating treatment and was lost to follow-up, leaving 133 subjects in the intention-to-treat population (66 standard arm, 67 alternative arm). There were no statistically significant imbalances in selected baseline demographic, clinical, and laboratory characteristics, including previous recurrent stroke and baseline LIC (Table 1).
Selected baseline characteristics of the intention-to-treat population
| Characteristic . | Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P . |
|---|---|---|---|
| HbSS | 66 (100%) | 66 (99%) | > .999 |
| Sex, male | 31 (47%) | 41 (61%) | .100 |
| Age at study enrollment, y | 13.3 ± 3.8 | 13.0 ± 4.0 | .733 |
| Stroke history | |||
| Age at index stroke, y | 6.2 ± 2.8 | 5.6 ± 3.0 | .257 |
| Previous recurrent stroke | 4 (6%) | 10 (15%) | .096 |
| History of TIA | 11 (17%) | 10 (15%) | .783 |
| Baseline brain MRI/MRA | |||
| Infarction | 65 (98%) | 67 (100%) | .312 |
| Vasculopathy | 54 (82%) | 53 (79%) | .693 |
| Moya-moya | 5 (8%) | 11 (16%) | .117 |
| Transfusion history | |||
| Duration, y | 7.0 ± 3.6 | 7.4 ± 3.8 | .592 |
| Simple transfusions | 41 (62%) | 43 (64%) | .806 |
| RBC alloantibodies | 17 (26%) | 26 (39%) | .108 |
| RBC autoantibodies | 9 (14%) | 16 (24%) | .131 |
| Iron overload status | |||
| LIC, mg Fe/g dw liver | 14.5 (9.5-23.3) | 13.9 (8.7-22.9) | .721 |
| Serum ferritin, ng/mL | 3282.0 (2321.0-4306.0) | 3346.0 (2202.0-4682.0) | .984 |
| Previous desferrioxamine | 44 (70%) | 47 (71%) | .864 |
| Previous deferasirox | 55 (87%) | 57 (86%) | .875 |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 9.2 (8.6-9.7) | 9.2 (8.5-9.6) | .998 |
| MCV, fL | 86.2 (83.6-87.7) | 85.8 (83.6-88.8) | .932 |
| HbA, % | 67.5 (56.3-74.5) | 66.1 (56.0-71.8) | .478 |
| HbS, % | 27.0 (21.2-38.6) | 30.3 (23.8-39.6) | .356 |
| HbF, % | 1.7 (1.0-2.5) | 1.4 (0.8-2.2) | .303 |
| ARC, ×109/L | 335.4 (246.2-391.0) | 367.0 (213.2-464.0) | .560 |
| WBC, ×109/L | 13.2 (11.0-16.6) | 14.0 (10.3-16.9) | .864 |
| ANC, ×109/L | 7.4 (6.2-9.2) | 7.1 (5.5-10.2) | .519 |
| ALT, U/L | 41.0 (36.0-58.0) | 45.0 (34.0-77.0) | .371 |
| Creatinine, mg/dL | 0.4 (0.3-0.5) | 0.4 (0.4-0.5) | .140 |
| Total bilirubin, mg/dL | 2.8 (1.8-3.9) | 2.6 (2.2-4.1) | .554 |
| Characteristic . | Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P . |
|---|---|---|---|
| HbSS | 66 (100%) | 66 (99%) | > .999 |
| Sex, male | 31 (47%) | 41 (61%) | .100 |
| Age at study enrollment, y | 13.3 ± 3.8 | 13.0 ± 4.0 | .733 |
| Stroke history | |||
| Age at index stroke, y | 6.2 ± 2.8 | 5.6 ± 3.0 | .257 |
| Previous recurrent stroke | 4 (6%) | 10 (15%) | .096 |
| History of TIA | 11 (17%) | 10 (15%) | .783 |
| Baseline brain MRI/MRA | |||
| Infarction | 65 (98%) | 67 (100%) | .312 |
| Vasculopathy | 54 (82%) | 53 (79%) | .693 |
| Moya-moya | 5 (8%) | 11 (16%) | .117 |
| Transfusion history | |||
| Duration, y | 7.0 ± 3.6 | 7.4 ± 3.8 | .592 |
| Simple transfusions | 41 (62%) | 43 (64%) | .806 |
| RBC alloantibodies | 17 (26%) | 26 (39%) | .108 |
| RBC autoantibodies | 9 (14%) | 16 (24%) | .131 |
| Iron overload status | |||
| LIC, mg Fe/g dw liver | 14.5 (9.5-23.3) | 13.9 (8.7-22.9) | .721 |
| Serum ferritin, ng/mL | 3282.0 (2321.0-4306.0) | 3346.0 (2202.0-4682.0) | .984 |
| Previous desferrioxamine | 44 (70%) | 47 (71%) | .864 |
| Previous deferasirox | 55 (87%) | 57 (86%) | .875 |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 9.2 (8.6-9.7) | 9.2 (8.5-9.6) | .998 |
| MCV, fL | 86.2 (83.6-87.7) | 85.8 (83.6-88.8) | .932 |
| HbA, % | 67.5 (56.3-74.5) | 66.1 (56.0-71.8) | .478 |
| HbS, % | 27.0 (21.2-38.6) | 30.3 (23.8-39.6) | .356 |
| HbF, % | 1.7 (1.0-2.5) | 1.4 (0.8-2.2) | .303 |
| ARC, ×109/L | 335.4 (246.2-391.0) | 367.0 (213.2-464.0) | .560 |
| WBC, ×109/L | 13.2 (11.0-16.6) | 14.0 (10.3-16.9) | .864 |
| ANC, ×109/L | 7.4 (6.2-9.2) | 7.1 (5.5-10.2) | .519 |
| ALT, U/L | 41.0 (36.0-58.0) | 45.0 (34.0-77.0) | .371 |
| Creatinine, mg/dL | 0.4 (0.3-0.5) | 0.4 (0.4-0.5) | .140 |
| Total bilirubin, mg/dL | 2.8 (1.8-3.9) | 2.6 (2.2-4.1) | .554 |
Selected baseline demographic, clinical, and laboratory characteristics of the intention-to-treat population at the time of study enrollment. Measures of central tendency for laboratory values, LIC, and ferritin are listed as median (interquartile range); treatment group differences were assessed via Wilcoxon rank sum tests. Other continuous variables are summarized as mean ± SD; treatment group differences were assessed via ANOVA. Categorical values are summarized as count (%); treatment group differences were assessed via χ2 tests, with the exception that the Fisher exact test was used for sickle cell genotype. There were no significant differences in characteristics between the two study groups.
TIA indicates transient ischemic attack; MRI/MRA, magnetic resonance imaging/magnetic resonance angiography; LIC, liver iron concentration; MCV, mean corpuscular volume; ARC, absolute reticulocyte count; ANC, absolute neutrophil count; WBC, white blood cell; HbSS, homozygous sickle cell anemia; HbA, adult hemoglobin; HbS, sickle hemoglobin; HbF, fetal hemoglobin; and ALT, alanine transaminase.
Study treatments
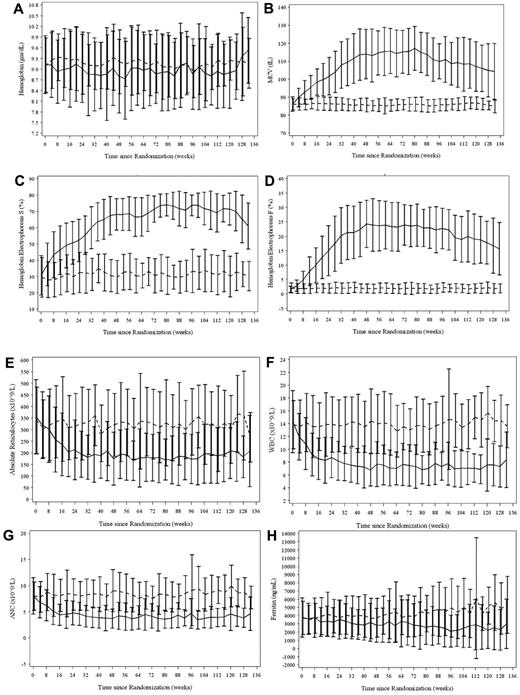
Selected laboratory effects are illustrated in Figure 2. Most subjects in the standard treatment arm received simple transfusions, with some partial exchange procedures or automated exchange (erythrocytapheresis) procedures. Their percentage of HbS averaged ∼ 30% throughout study treatment (Figure 2B). Deferasirox was provided initially at 25.1 ± 5.6 mg/kg/d with a final dose of 28.2 ± 6.0 mg/kg/d. Three subjects on standard treatment received desferrioxamine (Desferal) chelation.
Laboratory parameters based on intention-to-treat population. (A) Hemoglobin concentration; (B) MCV; (C) percentage of HbS; (D) percentage of HbF; (E) ARC; (F) WBC; (G) ANC; (H) ferritin. Complete blood counts and reticulocytes were obtained locally, while hemoglobin electrophoresis and serum ferritin were measured centrally. The standard treatment arm is indicated by dashes, while the alternative treatment arm is indicated by the solid line. Values are illustrated as medians with 25%-75% whisker plots. All parameters are significantly different (P < .001) between treatment groups except for panel A, which had no difference.
Laboratory parameters based on intention-to-treat population. (A) Hemoglobin concentration; (B) MCV; (C) percentage of HbS; (D) percentage of HbF; (E) ARC; (F) WBC; (G) ANC; (H) ferritin. Complete blood counts and reticulocytes were obtained locally, while hemoglobin electrophoresis and serum ferritin were measured centrally. The standard treatment arm is indicated by dashes, while the alternative treatment arm is indicated by the solid line. Values are illustrated as medians with 25%-75% whisker plots. All parameters are significantly different (P < .001) between treatment groups except for panel A, which had no difference.
Subjects in the alternative treatment arm initiated hydroxyurea at 20 mg/kg/d and then had dose escalation to MTD. Sixty of 67 subjects (90%) eventually reached MTD and had transfusions discontinued per protocol; the remaining 7 did not reach MTD either from nonadherence (n = 4), study withdrawal (n = 2), or recurrent stroke (n = 1). Mean time to MTD was 32 weeks (median 28, range 16-80 weeks); mean hydroxyurea dose at MTD was 26.2 ± 4.9 mg/kg/d (median 27.6, range 9.9-32.8 mg/kg/d). On hydroxyurea, the average hemoglobin concentration remained stable at ∼ 9 g/dL, with expected significant increases in mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and percentage of fetal hemoglobin (%HbF) plus expected significant decreases in white blood cell count (WBC), ANC, absolute reticulocyte count (ARC), total bilirubin, and lactate dehydrogenase (LDH; Table 2). Most subjects reached target myelosuppression with mean ANC at MTD of 3.5 ± 1.5 × 109/L (median 3.4, range 1.5-7.2 × 109/L) and final average ANC of 3.8 × 109/L (Table 2). The %HbF responses included a mean Hb(F/F+S) at MTD of 29.1% ± 6.7% (median 29.2, range 13.6-43.7%) and final average HbF of 19.5%. Serum ferritin was significantly lower on the alternative treatment arm (Figure 2H).
Laboratory evaluations of the intention-to-treat population at final assessment
| Laboratory parameter . | Final assessment . | Change from baseline . | ||||
|---|---|---|---|---|---|---|
| Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P* . | Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P† . | |
| Hemoglobin, g/dL | 9.0 (8.7-9.6) | 9.0 (8.4-9.6) | .934 | 0.0 (−0.5-0.6) | 0.0 (−0.7-0.7) | .898 |
| MCV, fL | 86.1 (83.3-88.5) | 103.3 (93.5-114.9) | < .001 | 0.1 (−2.0-2.5) | 19.5 (7.5-28.5) | < .001 |
| HbA, % | 63.6 (56.4-69.1) | 1.7 (0.0-31.4) | < .001 | 0.0 (−12.7-6.7) | −50.9 (-66.8-−33.7) | < .001 |
| HbF, % | 1.3 (0.8-2.7) | 19.5 (10.0-24.3) | < .001 | −0.2 (−0.8-0.4) | 17.9 (9.2-22.9) | < .001 |
| HbS, % | 32.3 (25.0-38.3) | 64.1 (52.6-76.4) | < .001 | 0.3 (−7.5-12.3) | 35.0 (21.7-46.2) | < .001 |
| ARC, ×109/L | 312.4 (226.3-419.6) | 176.7 (115.1-303.4) | < .001 | −11.8 (−88.2-93.2) | −149.1 (−231.0-−19.0) | < .001 |
| WBC, ×109/L | 13.2 (10.4-17.0) | 7.2 (5.6-11.6) | < .001 | 0.2 (−2.0-2.3) | −5.4 (−8.1-−2.2) | < .001 |
| ANC, ×109/L | 7.8 (6.5-10.0) | 3.8 (2.6-5.5) | < .001 | 0.8 (−1.3-2.4) | −3.3 (−5.1-−1.4) | < .001 |
| Platelets, ×109/L | 368.0 (315.0-437.0) | 315.0 (242.0-435.0) | .0183 | −28.0 (−70.0-18.0) | −83.0 (−171.0-−8.0) | .0022 |
| Total bilirubin, mg/dL | 3.3 (2.2-4.2) | 1.6 (1.1-2.5) | < .001 | 0.4 (−0.3-1.2) | −1.1 (−1.9-−0.6) | < .001 |
| LIC, mg Fe/g dw liver‡ | 17.3 (8.8-30.7) | 17.2 (10.0-30.6) | .7920 | −2.2 (−5.5-4.9) | −1.2 (−2.8-7.2) | .4888 |
| Serum ferritin, ng/mL | 4064.0 (2330.0-7126.0) | 1994.0 (998.0-3475.0) | < .001 | 1159.5 (−662.0-2724.0) | −966.0 (−1629.0-49.0) | < .001 |
| LDH, U/L | 434.0 (350.0-533.0) | 311.0 (274.0-434.0) | < .001 | −8.5 (−74.0-74.0) | −67.0 (−143.0-7.0) | .0015 |
| Laboratory parameter . | Final assessment . | Change from baseline . | ||||
|---|---|---|---|---|---|---|
| Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P* . | Transfusions and chelation group, N = 66 . | Hydroxyurea and phlebotomy group, N = 67 . | P† . | |
| Hemoglobin, g/dL | 9.0 (8.7-9.6) | 9.0 (8.4-9.6) | .934 | 0.0 (−0.5-0.6) | 0.0 (−0.7-0.7) | .898 |
| MCV, fL | 86.1 (83.3-88.5) | 103.3 (93.5-114.9) | < .001 | 0.1 (−2.0-2.5) | 19.5 (7.5-28.5) | < .001 |
| HbA, % | 63.6 (56.4-69.1) | 1.7 (0.0-31.4) | < .001 | 0.0 (−12.7-6.7) | −50.9 (-66.8-−33.7) | < .001 |
| HbF, % | 1.3 (0.8-2.7) | 19.5 (10.0-24.3) | < .001 | −0.2 (−0.8-0.4) | 17.9 (9.2-22.9) | < .001 |
| HbS, % | 32.3 (25.0-38.3) | 64.1 (52.6-76.4) | < .001 | 0.3 (−7.5-12.3) | 35.0 (21.7-46.2) | < .001 |
| ARC, ×109/L | 312.4 (226.3-419.6) | 176.7 (115.1-303.4) | < .001 | −11.8 (−88.2-93.2) | −149.1 (−231.0-−19.0) | < .001 |
| WBC, ×109/L | 13.2 (10.4-17.0) | 7.2 (5.6-11.6) | < .001 | 0.2 (−2.0-2.3) | −5.4 (−8.1-−2.2) | < .001 |
| ANC, ×109/L | 7.8 (6.5-10.0) | 3.8 (2.6-5.5) | < .001 | 0.8 (−1.3-2.4) | −3.3 (−5.1-−1.4) | < .001 |
| Platelets, ×109/L | 368.0 (315.0-437.0) | 315.0 (242.0-435.0) | .0183 | −28.0 (−70.0-18.0) | −83.0 (−171.0-−8.0) | .0022 |
| Total bilirubin, mg/dL | 3.3 (2.2-4.2) | 1.6 (1.1-2.5) | < .001 | 0.4 (−0.3-1.2) | −1.1 (−1.9-−0.6) | < .001 |
| LIC, mg Fe/g dw liver‡ | 17.3 (8.8-30.7) | 17.2 (10.0-30.6) | .7920 | −2.2 (−5.5-4.9) | −1.2 (−2.8-7.2) | .4888 |
| Serum ferritin, ng/mL | 4064.0 (2330.0-7126.0) | 1994.0 (998.0-3475.0) | < .001 | 1159.5 (−662.0-2724.0) | −966.0 (−1629.0-49.0) | < .001 |
| LDH, U/L | 434.0 (350.0-533.0) | 311.0 (274.0-434.0) | < .001 | −8.5 (−74.0-74.0) | −67.0 (−143.0-7.0) | .0015 |
Laboratory evaluations of the intention-to-treat population upon final assessment—either at study exit (N = 50), at the time of study closure (N = 62), or upon early termination for other reasons (N = 21). Median results are provided (interquartile range) unless indicated. Treatment group differences for laboratory parameters were assessed via Wilcoxon rank sum.
See Table 1 for abbreviation definitions.
P value based on final assessment.
P value based on change from baseline to final assessment.
Values for LIC represent 25 subjects in the standard treatment arm and 31 on the alternative treatment arm.
During hydroxyurea dose escalation, overlap transfusions continued for stroke prevention until MTD was reached. A mean of 8 ± 3 monthly transfusions (median 7) was given during this overlap period, before discontinuing transfusions. Phlebotomy commenced initially with 5 mL/kg removed followed by 10 mL/kg every 4 weeks. All 60 subjects reaching MTD received phlebotomy; 935 procedures (mean 16/subject) were performed with a mean of 127 ± 74 mL/kg blood removed per subject. Partial phlebotomy volumes were required at least once for 36 subjects, most often because of low hemoglobin concentration but also for miscellaneous problems including inadequate venous access.
Stroke adjudications
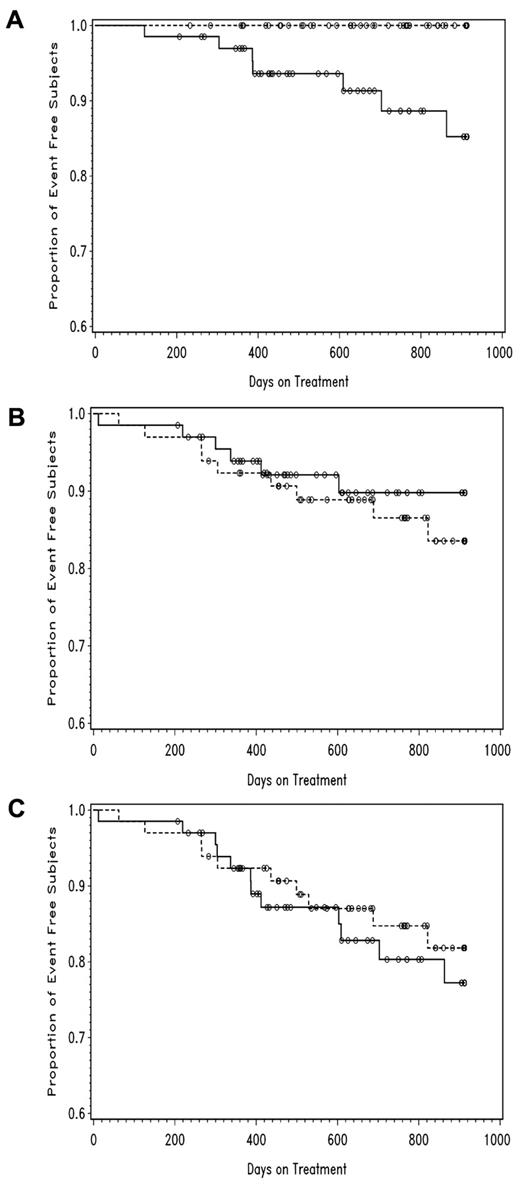
Formal stroke adjudication occurred for 91 new neurologic events (41 on transfusions/chelation, 50 on hydroxyurea/phlebotomy). Seven subjects had positive stroke adjudication, all within the alternative treatment arm (Figure 3A); 6 were infarctive strokes and clinically mild with no postevent worsening by Barthel index, plus 1 fatal hemorrhagic stroke, the only death in that treatment group. There were 20 TIA adjudications among 15 subjects, 9 on standard treatment and 6 on alternative treatment (Figure 3B). Considering all severe events (stroke, TIA, and death) together, the 2 treatment groups were similar with 10 subjects affected in the standard arm and 12 in the alternative treatment arm (Figure 3C). The single death in the standard treatment arm occurred at week 72, a sudden event at home from pulmonary embolism with right heart failure.
Event-free (Kaplan-Meier) plots of adjudicated neurologic events for the SWiTCH trial, by treatment group. The standard treatment arm is indicated by dashes, while the alternative treatment arm is indicated by the solid line. (A) Stroke with P < .05; (B) TIA with P = NS; (C) stroke, TIA, or death with P = NS.
Event-free (Kaplan-Meier) plots of adjudicated neurologic events for the SWiTCH trial, by treatment group. The standard treatment arm is indicated by dashes, while the alternative treatment arm is indicated by the solid line. (A) Stroke with P < .05; (B) TIA with P = NS; (C) stroke, TIA, or death with P = NS.
Study closure
The study remained open despite the imbalance of 7 strokes in 67 subjects on hydroxyurea/phlebotomy compared with 0 strokes in 66 subjects on transfusions/chelation because this rate difference (0.10) was still within the study's noninferiority margin and 10% stroke recurrence on hydroxyurea/phlebotomy was below the predicted 12% rate. However, the first scheduled interim analysis indicated that LIC values were not significantly different between treatment groups (16.6 mg/g dry weight liver in the standard arm compared with 15.7 mg/g in the alternative arm). Because reduction in LIC was not superior on hydroxyurea/phlebotomy, the DSMB concluded that the composite primary study end point would not be met and recommended study closure. NHLBI closed SWiTCH, and subjects exited the study with final laboratory values provided in Table 2.
Subsequent analyses
Posthoc analyses examined characteristic features of the 7 subjects who developed stroke recurrence on study treatment. Six were male with previous transfusion difficulties (2 with alloantibodies, 3 with autoantibodies). Only 1 had previous recurrent stroke before enrolling in SWiTCH, but all had severe vasculopathy at entry with MRA vessel stenosis/occlusion and 2 had moya-moya. Compared with 60 subjects on alternative treatment without stroke recurrence, these 7 were significantly younger at index stroke (median 2.1 vs 5.9 years) with higher prevalence of previous TIA at enrollment (57% vs 10%). They also had lower average hemoglobin concentrations and lower %HbF on hydroxyurea treatment, although the differences were not statistically significant. Six were in the phlebotomy phase at stroke recurrence, but without direct temporal association to individual phlebotomy procedures. Several received multiple partial phlebotomy procedures for low hemoglobin concentration, reflecting chronic severe anemia while on hydroxyurea.
Discussion
With full enrollment achieved in SWiTCH and ∼ 78% of the study treatment patient-years completed, the first interim analysis (performed after one-third of the subjects had completed all exit studies) documented no difference in LIC between the 2 treatment arms. Without superiority in LIC reduction, the imbalance of recurrent secondary strokes (10% of subjects on hydroxyurea/phlebotomy vs 0% on transfusions/chelation) was no longer justifiable, and SWiTCH was closed because of statistical futility for reaching the composite primary study end point.
The potential efficacy of hydroxyurea for secondary stroke prophylaxis, which led to the SWiTCH trial design, was based both on theoretical grounds and pilot data. Mechanistically, hydroxyurea has several therapeutic effects that might prevent stroke recurrence including higher %HbF, decreased sickling, lower WBC because of myelosuppression, fewer circulating adhesive reticulocytes, increased erythrocyte size and deformability, and improved rheology.24 In our single-institution experience using hydroxyurea for secondary stroke prophylaxis, patients and families were eager for an alternative to lifelong transfusions, with 35 of 36 eligible patients enrolling in the prospective pilot study.19,20 These preliminary data documented a stroke recurrence rate of 3 to 7 events per 100 patient-years, with lower values observed with transfusion overlap during escalation to hydroxyurea MTD.
Subjects in the 2 SWiTCH treatment arms were similar based on baseline clinical, laboratory, and radiographic parameters (Table 1). In the alternative treatment arm with hydroxyurea/phlebotomy, there were more subjects with previous recurrent stroke and baseline moyamoya vasculopathy, as well as a higher prevalence of auto- and alloantibody formation, but these differences did not reach statistical significance. Laboratory goals were achieved in both study treatment arms: subjects randomized to standard treatment with monthly transfusions maintained an average HbS of ∼ 30% throughout the study (Figure 2C) and had an average final HbS of 32% (Table 2), and also tolerated deferasirox at an average dose of 28 mg/kg/d. Subjects randomized to daily hydroxyurea demonstrated mild myelosuppression with an ANC of ∼ 4.0 × 109/L throughout the study (Figure 2D), plus a final average ANC of 3.8 × 109/L and final average %HbF of 19.5% (Table 2). Subjects on hydroxyurea had the expected statistically significant increases in MCV and MCH, plus the predicted decreases in WBC, ARC, platelets, bilirubin, and LDH (Figure 2, Table 2), and in general tolerated monthly phlebotomy well. Most subjects continued their assigned study treatments without difficulty, and remained in the trial until completing study treatment or until the study closure. A total of 4 subjects randomized to transfusions/chelation had early dropout (3 withdrew consent and 1 was removed for nonadherence to transfusions), compared with 9 subjects on hydroxyurea/phlebotomy (2 withdrew consent, 5 were deemed nonadherent with hydroxyurea treatment, and 2 were removed from the study by their local investigator).
The stroke recurrence rate observed in the SWiTCH alternative treatment arm was within the expected range, but the imbalance of strokes between treatment arms was not predicted. The observed stroke recurrence rate on hydroxyurea/phlebotomy was 10% (5.6 events per 100 patient-years), recognizing that ∼ 22% of patient-years of treatment still remained at study closure. In contrast, the stroke recurrence rate in the standard treatment arm (0%, 0.0 events per 100 patient-years) was unexpected, especially compared with data from other retrospective and prospective studies.3,7,8,25 It should be emphasized, however, that SWiTCH was not closed solely because of excessive or imbalanced strokes, but rather to futility in achieving the composite primary end point. Without reaching superior iron unloading with alternative treatment, the observed unbalanced recurrence rates did not warrant study continuation.
At the first interim analysis, both treatment groups had essentially unchanged LIC values at study exit, compared with baseline values. The lack of superior iron reduction observed on the alternative treatment has several potential explanations. First, for subjects randomized to the standard treatment arm, adherence with oral deferasirox chelation was better than historical use of subcutaneous desferrioxamine, and these subjects were able to maintain near-neutral iron balance over the 30-month treatment period (Table 2). Second, for subjects randomized to the alternative treatment arm, the overlap transfusions added to the iron burden before phlebotomy could commence. With an average of 8 extra simple transfusions given during the initial overlap phase, followed by an average of 127 mL/kg blood removed by phlebotomy, the observed neutral iron balance on alternative treatment seems reasonable. Third, the duration of phlebotomy may have been too short to observe a net iron reduction; continued phlebotomy would likely have unloaded excess iron and eventually normalized LIC, as previously documented in this population19,20 and other settings with iron overload.26,27 As a supplement to liver biopsy, newer MRI techniques of liver iron assessment28-30 could have provided additional data on hepatic iron distribution and unloading, and should be considered for future clinical trials involving transfusional iron overload. In retrospect, the SWiTCH study trial design itself may have contributed to the early study closure because superiority in iron reduction was part of the composite primary end point. However, this end point reflected the important clinical linkage between the goals of reducing secondary stroke and removing excess iron. Therefore, SWiTCH was not designed as an equivalence trial between treatments, but as a noninferiority trial allowing an increased number of recurrent stroke events, given the opportunity to manage iron overload better.
The stroke adjudication process documented a new stroke in 7 subjects, all randomized to the alternative treatment arm (Figure 3A). Posthoc analysis of these subjects indicated that a younger age at initial stroke, previous TIA, severe vasculopathy, and history of auto/alloimmunization appear to be risk factors for stroke recurrence; a lower hemoglobin concentration on hydroxyurea may also increase the risk. TIA was more common than stroke with 20 positive adjudications among 15 subjects (Figure 3B), and was diagnosed when the clinical presentation and features supported stroke but without radiologic evidence of new infarction. When 3 measures of severity (stroke, TIA, and death) were analyzed together, there were similar numbers of subjects in each treatment arm (10 in the standard treatment arm, 12 in the alternative treatment arm). At the protocol-designated 6-month follow-up visit, 31% of SWiTCH subjects were still taking hydroxyurea (compared with 50% during study treatment) including some treatment crossovers. Whether they will continue hydroxyurea treatment is unknown, but recent long-term data from the original pre-SWiTCH pilot study has documented that ∼ 70% of patients with SCA and stroke treated with hydroxyurea/phlebotomy remain stroke-free with low iron burdens.31
Based on the SWiTCH trial results, transfusion and chelation remain the better way to manage children with SCA, stroke, and iron overload. However, management of existing cerebrovascular disease in patients with SCA is still an extremely difficult clinical problem, and prevention of vasculopathy and stroke is the preferred goal. TCD screening lowers primary stroke risk when performed comprehensively,32,33 but then requires indefinite transfusions.34 Hydroxyurea will be compared with transfusions for children with abnormally elevated TCD velocities but no primary stroke, in the TCD With Transfusions Changing to Hydroxyurea (TWiTCH) trial (ClinicalTrials.gov NCT01425307) that has just begun subject enrollment. Prevention of cerebrovascular disease in SCA, however, is the ideal goal. Hydroxyurea for very young patients with SCA now has proven safety and efficacy in the 2-year phase 3 BABY HUG randomized clinical trial (ClinicalTrials.gov NCT00006400),35 with similar results observed as for older children and adults. The potential neurologic benefits of hydroxyurea therapy early in life are currently under investigation in the BABY HUG follow-up studies. If salutary, early initiation of hydroxyurea could help prevent or retard the development of cerebrovascular disease in this vulnerable patient population, while simultaneously avoiding serious complications of transfusions including iron overload and auto/alloimmunization.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Medical Coordinating Center and the Statistics and Data Management Center for their support throughout the study. They also appreciate the efforts of the study consultants, central laboratory personnel, site investigators, and study coordinators as listed in the supplemental Appendix, plus nursing staff at each participating institution. They provide special recognition for the numerous sacrifices made by the children and families who participated in this study. They also acknowledge Novartis Inc for donating deferasirox chelation used in the standard treatment arm.
This clinical trial was supported by National Heart, Lung, and Blood Institute grants U01-HL078787 (R.E.W.) and U01-HL078987 (R.W.H.).
National Institutes of Health
Authorship
Contribution: R.E.W. and R.W.H. designed the study, supervised the trial, analyzed the results, and wrote the manuscript, on behalf of the entire team of SWiTCH investigators.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of SWiTCH investigators, see the online supplemental Appendix.
Correspondence: Russell E. Ware, MD, PhD, Director, Center for Global Health, Baylor College of Medicine and Texas Children's Hospital, 1102 Bates St, Ste FC-1145, Houston, TX 77030; e-mail: reware@bcm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal