Abstract
ADAMTS13 is a plasma metalloproteinase that regulates platelet adhesion and aggregation by cleaving ultra-large VWF multimers on the surfaces of endothelial cells. Autoantibodies directed against ADAMTS13 prohibit the processing of VWF multimers, initiating a rare and life-threatening disorder called acquired thrombotic thrombocytopenic purpura. The formation of autoantibodies depends on the activation of CD4+ T cells. This process requires immune recognition, endocytosis, and subsequent processing of ADAMTS13 into peptides that are presented on MHC class II molecules to CD4+ T cells by dendritic cells (DCs). In the present study, we investigated endocytosis of recombinant ADAMTS13 by immature monocyte-derived DCs using flow cytometry and confocal microscopy. After incubation of fluorescently labeled ADAMTS13 with DCs, significant uptake of ADAMTS13 was observed. Endocytosis of ADAMTS13 was completely blocked by the addition of EGTA and mannan. ADAMTS13 endocytosis was decreased in the presence of a blocking mAb directed toward the macrophage mannose receptor (MR). Furthermore, siRNA silencing of MR reduced the uptake of ADAMTS13 by DCs. In addition, in vitro binding studies confirmed the interaction of ADAMTS13 with the carbohydrate recognition domains of MR. The results of the present study indicate that sugar moieties on ADAMTS13 interact with MR, thereby promoting its endocytosis by APCs.
Introduction
APCs continuously sample antigens from their environment and, after their processing, load antigen-derived peptides on MHC class I or II molecules for presentation to CD8+ or CD4+ T cells, respectively. To appropriately respond to incoming pathogens, APCs are equipped with a large diversity of cell-surface receptors, so-called pattern recognition receptors that recognize specific pathogen-associated molecular patterns.1 This provides APCs such as dendritic cells (DCs) with the capacity to recognize a wide diversity of foreign pathogens. After encountering a pathogen, DCs undergo maturation and migrate to the draining lymph nodes, where they encounter and prime naive T cells. This mechanism ultimately generates a humoral response that is capable of rapidly neutralizing re-entering pathogens. It is well established that, in addition to recognizing pathogen-associated molecular patterns, pattern recognition receptors can also interact with molecular patterns present on endogenous proteins.2 This results in the continuous presentation of “self-derived” peptides to CD4+ T cells.3 The efficient removal of potentially self-reactive T cells in the thymus prevents the stimulation of CD4+ T cells by self-antigens presented on MHC class II molecules. Nevertheless, autoimmunity can develop as a result of activation of low-affinity CD4+ T cells that have not been efficiently eliminated from the repertoire in the thymus.4 In addition, shared T-cell epitopes between pathogen-encoded and self-antigens are a potential source for cross-reactive T cells.5
The autoimmune disorder thrombotic thrombocytopenic purpura (TTP) results from the development of autoantibodies directed toward the metalloprotease ADAMTS13.6 Several studies have shown that these Abs target an exposed surface in the spacer domain,which mediates binding of ADAMTS13 to its substrate, VWF.6-13 Lack of cleavage of ultra-large VWF multimers by ADAMTS13 results in the accumulation of blood platelets at sites of vascular perturbation.14 This promotes microvascular obstruction, resulting in low platelet counts (thrombocytopenia) and fragmentation of RBCs (hemolytic anemia).15,16
Our current knowledge on the etiology of acquired TTP is limited. A large number of case reports suggest that microbial infections are linked to the onset or recurrence of this autoimmune disease,6 leading us to hypothesize that triggering of the innate immune system is associated with the initiation of acquired TTP.6 It was established recently that HLA-DRB1*11 is more frequent, whereas HLA-DRB1*04 is underrepresented, in patients with acquired TTP compared with controls.17,18 This observation implies that ADAMTS13-derived peptides presented on HLA-DRB1*11 on the surface of APCs promote the activation of ADAMTS13-specific CD4+ T cells. Clonal and subclass analysis revealed that anti-ADAMTS13 Abs are composed of subclasses IgG1 and IgG4 and that the variable domains are highly modified by somatic hypermutation.11,19,20 Both isotype switching and somatic hypermutation of Abs depends on the presence of antigen-specific CD4+ T cells. Immune recognition, endocytosis, and subsequent processing of ADAMTS13 into peptides that are presented on MHC class II molecules by DCs are needed for the generation of ADAMTS13-specific CD4+ T cells. In the present study, we explored the requirements for immune recognition of ADAMTS13 by human monocyte-derived DCs. Our results indicate that ADAMTS13 is efficiently internalized by immature DCs (iDCs) in a mannose receptor (MR)–dependent manner.
Methods
Materials
The following Abs were used in this study: PE-conjugated CD14 (Sanquin Reagents); APC-conjugated CD83, FITC-conjugated CD80, and APC-conjugated mouse anti-CD206 mAb (MR; BD Biosciences); APC-conjugated mouse IgG isotype control, mouse monoclonal antibody directed against early endosome antigen 1 (EEA1; BD Biosciences), and PE-conjugated mouse anti–human CD209 (DC-specific ICAM3-grabbing non-integrin receptor [DC-SIGN]; AbD Serotec); mouse IgG isotype control conjugated with FITC and PE (Dako); anti–human CD209 (R&D Systems); anti–human CD206 (Santa Cruz Biotechnology); and monoclonal blocking Ab anti-MR clone 15.2 (BioLegend). MAb AZN-D1 directed toward CD209 has been described previously.21,22 Mannan, D-mannose, N-acetyl-D-glucosamine (GlcNac), and D-galactose were purchased from Sigma-Aldrich.
Fluorescent labeling of recombinant ADAMTS13
Wild-type full-length ADAMTS13 was produced in stable HEK293 cells and purified as described previously.7 Purified recombinant ADAMTS13 was labeled using the Microscale Alexa Fluor 488 protein-labeling kit (Invitrogen). Protein concentration and efficiency of labeling was spectrophotometrically determined at 280 and 495 nm. The integrity of Alexa Fluor 488–labeled ADAMTS13 (ADAMTS13-488) was confirmed by SDS-PAGE (data not shown).
Generation of iDCs and uptake of ADAMTS13
Human monocytes were isolated from PBMCs using anti-CD14+ magnetic beads (Miltenyi Biotec). Blood of healthy subjects was drawn in accordance with Dutch regulations and after approval from Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. Differentiation of monocytes into iDCs was obtained by culturing them in CellGro DC Medium for 5 days in the presence of 800 U/mL of IL-4 and 1000 U/mL of GM-CSF (CellGenix). In uptake experiments, 0.2 × 106 iDCs were incubated with 50nM ADAMTS13-488 at 37°C for 45 minutes in serum-free IMDM (Lonza). Uptake was analyzed by flow cytometry (LSRII flow cytometer; BD Biosciences). Histograms were processed using FlowJo Version 7.6.5 software (TreeStar). Data are expressed as percentage of mean fluorescence intensity (MFI) at 37°C, where 100% of uptake corresponds to the maximal fluorescent signal obtained. In all uptake experiments, we used fluorescently labeled isotype controls. In our data analysis, we have subtracted from each sample the background staining (MFI obtained with a control IgG-FITC).
To monitor uptake by confocal microscopy, 0.5 × 106 iDCs were allowed to adhere to fibronectin-coated glass slides for 4-5 hours. Next, ADAMTS13-488 (100nM) was added to the cells in serum-free IMDM and uptake was performed for 45 minutes at 37°C. Cells were fixed in 3% paraformaldehyde in PBS for 15 minutes. Cells were stained with Abs against DC-SIGN (CD209), EEA1, or MR (CD206) and subsequently with Alexa Fluor 568–conjugated secondary Abs (Molecular Probes) in PBS supplemented with 0.5% human serum albumin with or without 0.1% saponin. Stained coverslips were mounted with Mowiol (Polysciences) and viewed by confocal microscopy using the appropriate filter settings and using a Plan-Neofluar 63×/1.3 oil-immersion lens (LSM 510; Carl Zeiss).
Blocking experiments and siRNA gene silencing
To analyze the involvement of C-type lectin receptors (CLRs) in the uptake and binding of ADAMTS13-488, iDCs were first pre-incubated for 20 minutes at 37°C with mannan (1 mg/mL), D-mannose (10mM), GlcNac (10mM), or D-galactose (10mM). Subsequently, 50nM of ADAMTS13-488 was added to the cells for 45 minutes at 37°C and uptake was analyzed by flow cytometry. Blockage of antigen uptake was also monitored after preincubation of the cells with sucrose (0.75M) and EGTA (5mM) or with mAb clone AZN-D1 directed against DC-SIGN and mAb clone 15.2 directed against MR.
For gene-silencing experiments, 6 μL of 100μM MR, DC-SIGN–specific, or nontargeting control siRNA pools (Dharmacon, Thermo Fisher Scientific) were added to 4 × 106 iDCs on day 2 in antibiotic- and serum-free medium (CellGro; Cellgenix). Cells were then pulsed at 250 V, 150 μF, and ∞ Ω in a Gene Pulser (Bio-Rad). After 78 hours, cells were analyzed for MR and DC-SIGN expression using APC-conjugated mouse monoclonal anti-MR Ab (BD Biosciences) and PE-conjugated mouse anti–DC-SIGN Ab (AbD Serotec). Subsequently, uptake experiments were performed as described previously with ADAMTS13-488.
In vitro binding of ADAMTS13 to MR and DC-SIGN-Fc chimeras
Binding of ADAMTS13 to soluble DC-SIGN-Fc chimeras (R&D Systems) and to MR-Fc chimera containing the major ligand–binding C-type lectin domains CTLD 4-7, prepared as described previously,23 was performed as follows. ADAMTS13 (5 μg/mL) was immobilized onto a MaxiSorp plate (Nunc) in 50mM NaHCO3 (pH 9.5) overnight at 4°C. Wells were blocked for 1 hour in TSM buffer (20mM Tris-HCl pH 7.4, 150mM NaCl, 2mM CaCl2, and 2mM MgCl2) containing 1% BSA. Subsequently, 10 μg/mL of the soluble receptor was added for 1 hour at 37°C. Unbound fragments were washed away and Fc-chimera binding to ADAMTS13 was determined by incubation with a peroxidase-labeled murine anti–human IgG1 Ab (Sanquin Reagents). Binding specificity was verified by the preincubation of the recombinant receptors with 1 mg/mL of mannan.
Results
ADAMTS13 is endocytosed by monocyte-derived iDCs
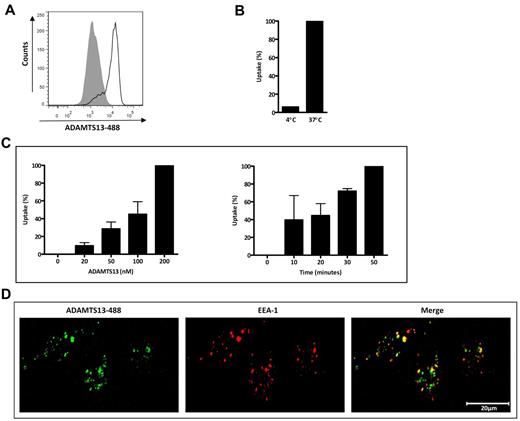
We used monocyte-derived iDCs to study ADAMTS13 endocytosis by APCs. To determine the ratio of internalized and cell-surface–bound ADAMTS13-488, incubation with 50nM ADAMTS13-488 was performed at both 37°C (uptake) and 4°C (binding). A strong increase in MFI was observed after incubation with ADAMTS13-488 at 37°C (Figure 1A-B); however, only 6% of ADAMTS13-488 was detected when the incubation was performed at 4°C (Figure 1B). This observation suggests that ADAMTS13-488 is mainly endocytosed by iDCs. Increased concentrations of ADAMTS13 and prolonged incubation time resulted in an increase of the MFI, demonstrating that ADAMTS13 endocytosis is dose and time dependent (Figure 1C). We subsequently investigated the intracellular targeting of ADAMTS13 after its uptake by iDCs. Cells were first incubated with ADAMTS13-488 for 45 minutes at 37°C, fixed, and then stained for the early endosome marker EEA-1. Confocal microscopy revealed that a significant part of endocytosed ADAMTS13-488 was present within early endosomes (Figure 1D). Inspection of these cells with Image-Pro Plus Version 6.1 software (Media Cybernetics) revealed that approximately 55% ± 6% of the endocytosed ADAMTS13 is present within the endosomal compartment (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Our data show effective endocytosis of ADAMTS13 by iDCs.
ADAMTS13 uptake by iDCs. (A) ADAMTS13-488 (50nM) was incubated with iDCs for 45 minutes at 37°C and cells were analyzed by FACS. The gray histogram represents control cells without ADAMTS13-488. (B) Cell-surface–bound versus internalized ADAMTS13 was determined by incubating iDCs with ADAMTS13-488 at 4°C or 37°C for 45 minutes. (C) Uptake was performed with increasing concentrations (0-200nM) of ADAMTS13-488 (45 minutes) or at prolonged time intervals (0-50 minutes with 50nM ADAMTS13-488). Graphs represent data from 2-3 independent experiments ± SD. Data are expressed as the percentage of mean fluorescence intensity at 37°C, where 100% corresponds to the highest mean fluorescence signal observed for individual experiments such as 50nM (B) or 200nM ADAMTS13-488 and 50 minutes of ADAMTS13-488 uptake (C). (D) ADAMTS13-488 was added to iDCs for 45 minutes at 37°C. DCs were fixed, permeabilized, and stained with anti-EEA1 Ab, followed by Alexa Fluor 568–labeled secondary Ab (EEA1 staining is shown in red).
ADAMTS13 uptake by iDCs. (A) ADAMTS13-488 (50nM) was incubated with iDCs for 45 minutes at 37°C and cells were analyzed by FACS. The gray histogram represents control cells without ADAMTS13-488. (B) Cell-surface–bound versus internalized ADAMTS13 was determined by incubating iDCs with ADAMTS13-488 at 4°C or 37°C for 45 minutes. (C) Uptake was performed with increasing concentrations (0-200nM) of ADAMTS13-488 (45 minutes) or at prolonged time intervals (0-50 minutes with 50nM ADAMTS13-488). Graphs represent data from 2-3 independent experiments ± SD. Data are expressed as the percentage of mean fluorescence intensity at 37°C, where 100% corresponds to the highest mean fluorescence signal observed for individual experiments such as 50nM (B) or 200nM ADAMTS13-488 and 50 minutes of ADAMTS13-488 uptake (C). (D) ADAMTS13-488 was added to iDCs for 45 minutes at 37°C. DCs were fixed, permeabilized, and stained with anti-EEA1 Ab, followed by Alexa Fluor 568–labeled secondary Ab (EEA1 staining is shown in red).
Receptor-mediated endocytosis of ADAMTS13
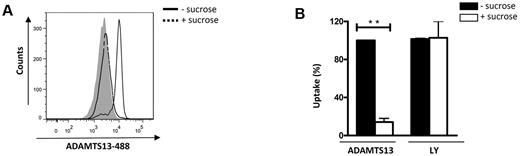
Uptake of antigens by iDCs can occur through 2 main pathways: receptor-mediated endocytosis and macropinocytosis.24 To identify the mechanism that mediates ADAMTS13 endocytosis, we evaluated the binding and uptake of ADAMTS13-488 after preincubation of iDCs with sucrose. Sucrose blocks receptor recycling through the formation of clathrin microcages on the inner surface of the plasma membrane and therefore prevents clathrin-mediated endocytosis.25,26 Uptake of ADAMTS13 was completely abolished by preincubation of iDCs with 0.75M sucrose (Figure 2A-B). In contrast, uptake of lucifer yellow by iDCs, which proceeds via macropinocytosis, was not inhibited by sucrose24 (Figure 2B). These findings indicate that ADAMTS13 is internalized by a cell-surface receptor on iDCs.
Uptake of ADAMTS13 by iDCs is mediated by a clathrin-dependent cell surface receptor. Immature DCs were pre-incubated with 0.75M sucrose before the addition of 50nM ADAMTS13-488. (A) Cells were analyzed by FACS. Gray histogram represents control cells without ADAMTS13-488. (B) Data from 3 independent experiments ± SD are presented and are expressed as a percentage of mean fluorescence intensity, where 100% corresponds to the mean fluorescence signal obtained for ADAMTS13-488 in the absence of sucrose. Lucifer yellow (LY), which is internalized by macropinocytosis, was used as a control.
Uptake of ADAMTS13 by iDCs is mediated by a clathrin-dependent cell surface receptor. Immature DCs were pre-incubated with 0.75M sucrose before the addition of 50nM ADAMTS13-488. (A) Cells were analyzed by FACS. Gray histogram represents control cells without ADAMTS13-488. (B) Data from 3 independent experiments ± SD are presented and are expressed as a percentage of mean fluorescence intensity, where 100% corresponds to the mean fluorescence signal obtained for ADAMTS13-488 in the absence of sucrose. Lucifer yellow (LY), which is internalized by macropinocytosis, was used as a control.
C-type lectins are involved in uptake of ADAMTS13
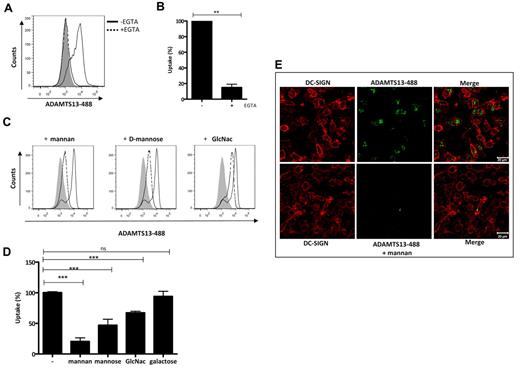
iDCs express several endocytic receptors, such as C-type lectin receptors (CLRs), which are abundantly expressed on iDCs. These receptors share at least one Ca2+/carbohydrate recognition domain and bind sugars in a calcium-dependent manner.2 To determine whether CLRs contribute to the uptake of ADAMTS13, we pre-incubated iDCs with either 5mM EGTA or 1 mg/mL of mannan, 10mM D-mannose, GlcNAc, or D-galactose. These blocking reagents did not affect the viability of the cells (supplemental Figure 3). We found that endocytosis of ADAMTS13-488 by iDCs was significantly inhibited by EGTA and mannan (Figure 3A-D). Only 18% of residual uptake of ADAMTS13-488 was observed when cells were incubated with EGTA or mannan (Figure 3B,D). Preincubation of iDCs with D-mannose or GlcNAc resulted in a reduction of uptake of approximately 33%-50%, whereas, as expected, preincubation of the cells with D-galactose did not affect the uptake of ADAMTS13 (Figure 3D). Mannan, D-mannose, and GlcNAc bind with different affinity to CLRs.27 We observed a more pronounced inhibition of ADAMTS13 uptake when cells were pre-incubated with mannan (Figure 3D). Mannan is a polysaccharide consisting of multiple mannose residues and therefore has a more than 100-fold higher affinity for mannan-sensitive CLRs compared to monomeric D-mannose. The inhibitory effect of mannan on the endocytosis of ADAMTS13-488 was further confirmed by confocal microscopy (Figure 3E). Preincubation of iDCs with 1 mg/mL of mannan resulted in a significantly reduced amount of intracellular ADAMT13-488. To demonstrate the specificity of ADAMTS13 uptake by iDCs, we analyzed the uptake of α2-macroglobulin, which is mediated by the low-density lipoprotein-related protein LRP (CD91).28 Endocytosis of α2-macroglobulin was not reduced by mannan, D-mannose, GlcNAc, D-galactose, EGTA, and mAb 15.2 directed toward the mannose-receptor (supplemental Figure 2). These results show specific involvement of a mannan-sensitive CLR in the uptake of ADAMTS13 by iDCs. We also analyzed whether ADAMTS13 N- or O-linked glycans moieties were important in mediating uptake by iDCs. ADAMTS13 was untreated or treated overnight at 37°C with peptide-N-glycosidase F or O-glycosidase together with neuraminidase to remove, respectively, N-linked and O-linked sugars (supplemental Figure 4). We found that treatment of ADAMTS13 with peptide-N-glycosidase F resulted in a 70% of reduction of uptake compared with untreated ADAMTS13. O-glycosidase treatment did not have any effect on ADAMTS13 endocytosis by iDCs (supplemental Figure 4B). These data indicate that N-linked sugar moieties present on ADAMTS13 are involved in its uptake by iDCs.
CLRs mediate ADAMTS13 uptake by iDCs. FACS analysis of ADAMTS13-488 uptake by iDCs is shown. (A-B) Uptake of ADAMTS13 (expressed as a percentage) was prevented by preincubation of the cells with 5mM EGTA. (C) Inhibition of ADAMTS13-488 endocytosis by different monosaccharides. The gray histograms represent controls incubated in the absence of ADAMTS13. Cells incubated with ADAMTS13 are indicated by a solid line; cells incubated with ADAMTS13 and different sugar components are indicated by a dotted line. (D) Blocking of ADAMTS13 uptake by different sugars is expressed as a percentage. (E) Confocal analysis of the effect of mannan on ADAMTS13 endocytosis. iDCs were incubated with or without mannan before the addition of ADAMTS13-488 and stained with anti–DC-SIGN Ab (red).
CLRs mediate ADAMTS13 uptake by iDCs. FACS analysis of ADAMTS13-488 uptake by iDCs is shown. (A-B) Uptake of ADAMTS13 (expressed as a percentage) was prevented by preincubation of the cells with 5mM EGTA. (C) Inhibition of ADAMTS13-488 endocytosis by different monosaccharides. The gray histograms represent controls incubated in the absence of ADAMTS13. Cells incubated with ADAMTS13 are indicated by a solid line; cells incubated with ADAMTS13 and different sugar components are indicated by a dotted line. (D) Blocking of ADAMTS13 uptake by different sugars is expressed as a percentage. (E) Confocal analysis of the effect of mannan on ADAMTS13 endocytosis. iDCs were incubated with or without mannan before the addition of ADAMTS13-488 and stained with anti–DC-SIGN Ab (red).
In vitro binding of ADAMTS13 to MR and DC-SIGN
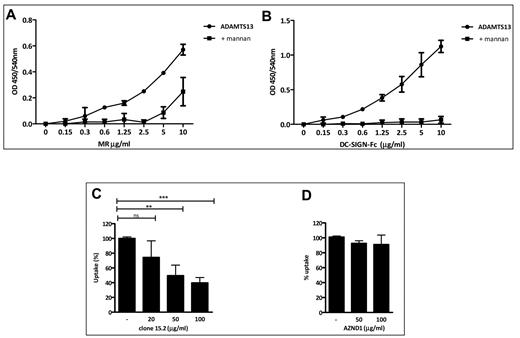
It has been reported previously that the 2 major CLRs expressed on iDCs displaying mannan binding specificity are DC-SIGN and MR.2,22 Therefore, we investigated the in vitro binding of ADAMTS13 to MR and DC-SIGN. Increasing concentrations of recombinant DC-SIGN or MR CTLD 4-7 Fc chimeras were added to immobilized ADAMTS13 for 2 hours. A dose-dependent binding to both receptors was observed (Figure 4A-B). After preincubation of the receptor chimeras with 1 mg/mL of mannan, binding to ADAMTS13 was completely abolished (Figure 4A-B). These data demonstrate that ADAMTS13 can bind to both MR and DC-SIGN in vitro in a mannan-sensitive manner.
Binding of ADAMTS13 to MR and DC-SIGN. ADAMTS13 was immobilized on microtiter plates. Increasing concentrations (0-10 μg/mL) of recombinant MR CTLD 4-7-Fc (A) or DC-SIGN-Fc (B) chimeras were added to the wells. To determine whether ADAMTS13 binding to these receptors is specific, the Fc-chimera receptors were pre-incubated with mannan prior to being added to the wells. Data are from 2 independent experiments. (C-D) iDCs were pre-incubated at 37°C in normal medium or in medium containing different concentrations of anti-MR Ab (C) or anti–DC-SIGN blocking Ab AZND1 (D) for 20 minutes, and then 50nM ADAMTS13-488 was added to the cells. Uptake was measured after 45 minutes by FACS and is expressed as a percentage of uptake. Graphs represent data from 3 independent experiments and are expressed as means ± SD.
Binding of ADAMTS13 to MR and DC-SIGN. ADAMTS13 was immobilized on microtiter plates. Increasing concentrations (0-10 μg/mL) of recombinant MR CTLD 4-7-Fc (A) or DC-SIGN-Fc (B) chimeras were added to the wells. To determine whether ADAMTS13 binding to these receptors is specific, the Fc-chimera receptors were pre-incubated with mannan prior to being added to the wells. Data are from 2 independent experiments. (C-D) iDCs were pre-incubated at 37°C in normal medium or in medium containing different concentrations of anti-MR Ab (C) or anti–DC-SIGN blocking Ab AZND1 (D) for 20 minutes, and then 50nM ADAMTS13-488 was added to the cells. Uptake was measured after 45 minutes by FACS and is expressed as a percentage of uptake. Graphs represent data from 3 independent experiments and are expressed as means ± SD.
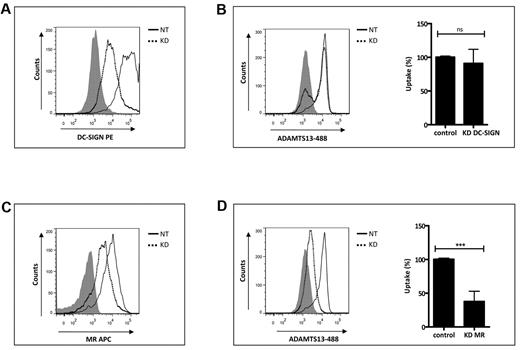
MR contributes to the uptake of ADAMTS13 by human iDCs
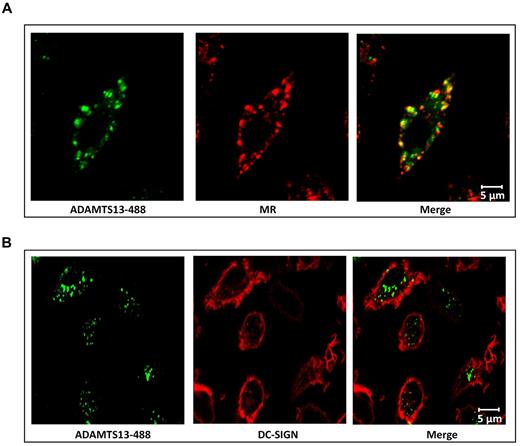
To further explore the involvement of MR and DC-SIGN in the uptake of ADAMTS13 by iDCs, we first investigated whether we could interfere with the uptake of ADAMTS13-488 by pre-incubating iDCs with increasing concentrations of blocking Abs directed toward DC-SIGN and MR.21,22,29,30 Endocytosis of 50nM ADAMTS13-488 was reduced by the addition of anti-MR Ab clone 15.2 (Figure 4C). In contrast, AZN-D1 directed toward DC-SIGN did not affect the uptake of ADAMTS13 (Figure 4D). To confirm the involvement of the MR in the uptake of ADAMTS13, we used small interfering RNA (siRNA) pools to knock down the expression of MR or DC-SIGN on iDCs. Strong reduction of MR and DC-SIGN expression was observed (Figure 5A,C). ADAMTS13-488 uptake in iDCs treated with siRNAs targeting MR was reduced compared with cells receiving nontargeting siRNAs (Figure 5D). No reduction in ADAMTS13-488 uptake was observed in cells transfected with DC-SIGN siRNAs (Figure 5B), indicating that this receptor is not involved in the uptake of ADAMTS13 by iDCs. Confocal analysis was performed to verify the involvement of MR in ADAMTS13 uptake. iDCs were incubated with ADAMTS13-488, fixed, permeabilized, and stained with specific Abs against DC-SIGN and MR. Endocytosed ADAMTS13 co-localized with MR but not with DC-SIGN (Figure 6). These data suggest the involvement of MR in ADAMTS13 endocytosis. Once endocytosed, both ADAMTS13 and MR co-localize within early endosomes (Figure 1 and supplemental Figure 1). Additional in vitro binding studies between CTLD 4-7-Fc and ADAMTS13 were performed using surface plasmon resonance analysis and revealed an apparent equilibrium dissociation constant (KD) value of 474nM (supplemental Figure 5). Although our in vitro data suggest a role for DC-SIGN in binding to ADAMTS13, the lack of inhibition of the prototypic blocking Ab AZN-D1, together with the absence of a reduction in ADAMTS13 uptake after knock-down of the receptor, suggests that DC-SIGN is not involved in the binding and uptake of ADAMTS13 by iDCs.
Gene silencing of MR reduces uptake of ADAMTS13 by iDCs. Silencing of MR in iDCs reduces uptake of ADAMTS13. iDCs were transfected with nontargeting siRNA or targeting MR and DC-SIGN siRNAs. After 72 hours, the expression of MR and DC-SIGN was measured by FACS (A,C). After siRNA transfection, cells were incubated with 50nM ADAMTS13-488 and its uptake was monitored by FACS (B,D). Dotted line histograms represent cells transfected with siRNAs targeting MR and DC-SIGN (KD). Solid line histograms represent cells transfected with nontargeting siRNAs (NT). Gray histograms represent cells stained with isotype control Abs. Graphs represent data from 3 independent experiments and are expressed as means ± SD. Results are expressed as a percentage of the mean fluorescence intensity, where 100% corresponds to the highest mean fluorescence intensity obtained with ADAMTS13-488.
Gene silencing of MR reduces uptake of ADAMTS13 by iDCs. Silencing of MR in iDCs reduces uptake of ADAMTS13. iDCs were transfected with nontargeting siRNA or targeting MR and DC-SIGN siRNAs. After 72 hours, the expression of MR and DC-SIGN was measured by FACS (A,C). After siRNA transfection, cells were incubated with 50nM ADAMTS13-488 and its uptake was monitored by FACS (B,D). Dotted line histograms represent cells transfected with siRNAs targeting MR and DC-SIGN (KD). Solid line histograms represent cells transfected with nontargeting siRNAs (NT). Gray histograms represent cells stained with isotype control Abs. Graphs represent data from 3 independent experiments and are expressed as means ± SD. Results are expressed as a percentage of the mean fluorescence intensity, where 100% corresponds to the highest mean fluorescence intensity obtained with ADAMTS13-488.
Colocalization of intracellular ADAMTS13 and MR in iDCs. MR mediates endocytosis of ADAMTS13. iDCs were incubated with ADAMTS13-488 for 45 minutes at 37°C. Subsequently, samples were fixed and labeled for MR (A) or DC-SIGN (B) and analyzed by confocal microscopy. (A) Colocalization of ADAMTS13 (green) with MR (red) was observed (merge). (B) Labeling of DC-SIGN (red) showed no colocalization with ADAMTS13 (green).
Colocalization of intracellular ADAMTS13 and MR in iDCs. MR mediates endocytosis of ADAMTS13. iDCs were incubated with ADAMTS13-488 for 45 minutes at 37°C. Subsequently, samples were fixed and labeled for MR (A) or DC-SIGN (B) and analyzed by confocal microscopy. (A) Colocalization of ADAMTS13 (green) with MR (red) was observed (merge). (B) Labeling of DC-SIGN (red) showed no colocalization with ADAMTS13 (green).
Discussion
In the present study, we have demonstrated that ADAMTS13 is effectively internalized by human monocyte–derived iDCs. Internalization of ADAMTS13 can be blocked by mannan and EGTA, suggesting a prominent role of CLRs. In agreement with these findings, we show in vitro interaction of ADAMTS13 with 2 mannan-sensitive CLRs, DC-SIGN and MR. Despite the capacity of ADAMTS13 to interact in vitro with DC-SIGN, ADAMTS13 endocytosis appears to be independent from this C-type lectin, because its uptake by iDCs is not influenced by the presence of the blocking anti–DC-SIGN Ab AZN-D1 nor by siRNA-mediated knockdown of DC-SIGN. In contrast, our experiments suggest MR as a candidate receptor for ADAMTS13 uptake by iDCs. Both MR knockdown and preincubation of anti-MR Ab clone 15.2 with iDCs resulted in significantly reduced amounts of internalized ADAMTS13. MR is a 175-kDa transmembrane glycoprotein belonging to the type I C-type lectin family. The extracellular portion of this receptor consists of a N-terminal cysteine-rich domain that binds sulfated sugars in a calcium-independent manner and 8 carbohydrate recognition domains (CTLD 1-8) that bind in a calcium-dependent manner to glycoconjugates or glycoproteins terminating in D-mannose, L-fucose, or N-acetylglucosamine.31 Our surface plasmon resonance studies revealed that ADAMTS13 binds to the 4-7 CTLD repeats of the MR. Eight complex N-linked glycans associated with ADAMTS13 have been described previously,32 together with O-linked disaccharide glucose-β1,3 fucose residues in the thrombospondin repeats.33 Lectin-binding assays have provided evidence for the presence of high mannose residues also on plasma-derived ADAMTS13.34 We demonstrate that removal of N-linked sugar moieties from ADAMTS13 reduces its binding and uptake by iDCs. These findings support a model in which exposed mannosylated or fucosylated sugar moieties mediate the binding of ADAMTS13 to the MR, thereby promoting its internalization by iDCs. MR internalization is dependent on the interaction of tyrosine-based and dihydrophobic motifs in its cytoplasmic tail with the clathrin adaptor complex AP-2, thereby recruiting MR to clathrin-coated pits.35 After its recruitment into clathrin-coated pits, MR is rapidly internalized. MR is predominantly internalized into early endosomes (supplemental Figure 1). The low pH in early endosomes disrupt one of the Ca2+-binding sites in CRD4 of MR, which destabilizes the sugar-binding properties of this domain,36 resulting in the release of ligands from MR, which subsequently recycles to the plasma membrane.31 Several recent studies in murine DCs have shown that antigens internalized via the MR are targeted to a “stable” population of early endosomes that have been implicated in cross-presentation of internalized antigen to CD8+ T cells.37,38 However, in cytokine-treated cells, MR can also be detected in late endosomes.31,39,40 Under these conditions, MR, together with its ligand, is transported to late endocytic compartments, where antigen-derived peptides can be loaded on MHC class II molecules.31,39,40 This suggests that, depending on the nature of the ligand, the amount of antigen internalized, the maturation level of DCs, or the cytokine environment, MR internalization can lead to cross-presentation to CD8+ T cells or presentation of antigen-derived peptides to CD4+ T cells. The described association between HLA-DRB1*11 strongly suggests that ADAMTS13-specific CD4+ T cells contribute to the pathogenesis of acquired TTP.17,18 Based on these findings, we suggest a model in which MR-mediated internalization of ADAMTS13 by DCs results in priming of naive CD4+ T cells, thereby initiating autoimmune responses to ADAMTS13, which results in acquired TTP.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Zappelli and Dr M. van den Biggelaar for help with the surface plasmon resonance analysis, and Dr A. ten Brinke for helpful discussions.
This study was supported by PPOC-08-021 (to N.S.) and by the Dutch Burns Foundation (08.109 to L.M.v.d.B.).
Authorship
Contribution: N.S., W.P., and E.H. performed the experiments; N.S., R.F., and J.V. conceived the study; L.M.v.d.B., L.M.-P., and T.B.G. provided expertise and crucial reagents; N.S. and J.V. wrote the manuscript; and all authors provided input on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for W.P. is Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Boston, MA.
Correspondence: Jan Voorberg PhD, Department of Plasma Proteins, Sanquin Research, Plesmanlaan 125, 1066 CX, Amsterdam, The Netherlands; e-mail: j.voorberg@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal