In this issue of Blood, Sorvillo and colleagues demonstrate that a macrophage mannose receptor (MR) on antigen-presenting cells facilitates uptake of ADAMTS13 antigen.1 The findings provide the first hint on how ADAMTS13 antigen may be endocytosed, processed, and presented to immune T cells.
Most cases of idiopathic thrombotic thrombocytopenic purpura (TTP) in adults are caused by acquired immunoglobulin G (IgG) autoantibodies against the metalloprotease ADAMTS13. The mechanism underlying the formation of autoantibodies is poorly understood. TTP, a potentially fatal syndrome, is a characterized by profound thrombocytopenia and microangiopathic hemolytic anemia with various symptoms and signs of organ dysfunction. Nearly all adult idiopathic TTP patients harbor IgG autoantibodies that inhibit plasma ADAMTS13 activity, thus resulting in compromised proteolytic processing of ultra large von Willebrand factor (VWF) multimers anchored on endothelial cells and in circulating blood. The ultra large VWF multimers are hyperactive, interacting with platelets in flowing blood and leading to unwanted thromboses in small arteries and capillaries. Epitope mapping demonstrates that almost all patients' IgG autoantibodies recognize the ADAMTS13 spacer domain, particularly exosite 3 and its surrounding residues.2,3 These residues have been shown to play an essential role in recognition and cleavage of VWF. Whether the spacer domain of ADAMTS13 is particularly immunogenic or resembles epitopes found in microbial pathogens is not known.
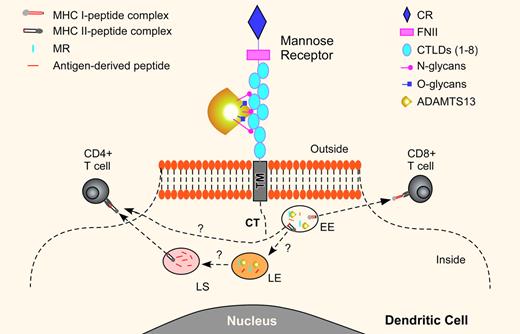
The association between HLA-DRB1*11 and acquired idiopathic TTP indicates that antigen-specific CD4+ T cells may contribute to formation of autoantibodies against ADAMTS13 in these patients.4 Sorvillo et al convincingly demonstrate that a fluorescein-labeled ADAMTS13 protein is readily taken up by immature monocyte-derived dendritic cells (iDCs) in culture.1 This process can be blocked by EGTA and mannan, a polymer of mannose. The results suggest an involvement of a calcium- and mannan-sensitive C-type lectin receptor in the endocytosis of ADAMTS13. Further experiments with a monoclonal antibody against an MR or small interfering RNA silencing the MR gene confirm that the MR mediates the endocytosis of ADAMTS13 by iDCs. Other C-type lectin receptors on iDCs, such as the dendritic cell specific ICAM3 grabbing nonintegrin receptor (DC-SIGN), do not appear to be involved in ADAMTS13 endocytosis under these conditions. Binding experiments show that ADAMTS13 interacts with 4 to 7 C-type lectin-like carbohydrate recognition domains (CTLDs) of the MR (see figure). A removal of N-linked glycans from ADAMTS13 protein dramatically reduces ADAMTS13 endocytosis, but the removal of O-linked oligosaccharides has no effect. Together, these results suggest that the MR plays an important role in facilitating endocytosis of N-glycosylated ADAMTS13 by dendritic cells.
A proposed of model for mannose receptor–mediated endocytosis of ADAMTS13 in dendritic cells. The mannose receptor (MR) consists of an N-terminal cysteine-rich domain (CR) and 8 C-type lectin-like carbohydrate recognition domains (CTLDs 1-8) that bind glycoproteins terminated by D-mannose, L-fucose, or N-acetylglucosamine. The 4 to 7 CTLDs are shown to bind ADAMTS13. Once the MR-ADAMTS13 complex is internalized, it is transported to the endosomal pathway including early endosome (EE), late endosome (LE), and lysosome (LS). In the early endosome, the endocytosed ADAMTS13 may be dissociated from the MR and loaded on the MHC class I molecules (MHC I) for activation of the CD8+ T cells, termed cross-presentation. Other studies have demonstrated that certain soluble protein antigens taken up through the MR pathway can be targeted to the LE and LS for proteolytic degradation. The antigen-derived peptides can then be loaded on the MHC II molecules for presentation to the CD4+ T cells. The endocytosed ADAMTS13 is primarily detected in the early endosome of iDCs, suggesting that other pathways for endocytosis of ADAMTS13 may also be necessary for presenting the antigenic peptides to the CD4+ cells. It may be possible that on induction of iDC maturation, the antigen can be transported to the MHC II molecules for presentation to the CD4+ cells.
A proposed of model for mannose receptor–mediated endocytosis of ADAMTS13 in dendritic cells. The mannose receptor (MR) consists of an N-terminal cysteine-rich domain (CR) and 8 C-type lectin-like carbohydrate recognition domains (CTLDs 1-8) that bind glycoproteins terminated by D-mannose, L-fucose, or N-acetylglucosamine. The 4 to 7 CTLDs are shown to bind ADAMTS13. Once the MR-ADAMTS13 complex is internalized, it is transported to the endosomal pathway including early endosome (EE), late endosome (LE), and lysosome (LS). In the early endosome, the endocytosed ADAMTS13 may be dissociated from the MR and loaded on the MHC class I molecules (MHC I) for activation of the CD8+ T cells, termed cross-presentation. Other studies have demonstrated that certain soluble protein antigens taken up through the MR pathway can be targeted to the LE and LS for proteolytic degradation. The antigen-derived peptides can then be loaded on the MHC II molecules for presentation to the CD4+ T cells. The endocytosed ADAMTS13 is primarily detected in the early endosome of iDCs, suggesting that other pathways for endocytosis of ADAMTS13 may also be necessary for presenting the antigenic peptides to the CD4+ cells. It may be possible that on induction of iDC maturation, the antigen can be transported to the MHC II molecules for presentation to the CD4+ cells.
What is the fate of endocytosed ADAMTS13 in dendritic cells? Sorvillo and colleagues show that a significant amount of the endocytosed ADAMTS13 is detected in the early endosome as indicated by early endosomal antigen-1 (EEA-1).1 This observation is consistent with the localization of endocytosed ovalabumin through the MR pathway. The endocytosed ovalabumin is excluded from late endosome or lysosome marked by Rab7 or lysosomal-associated membrane protein-1 (LAMP-1).5 It has been well documented in the literature that the endocytosed antigens through the MR pathway into early endosomes associate with the MHC class I molecules and are presented to CD8+ T cells for cross presentation, a pathway to activate cytolytic CD8+ T cells (see figure). Whether the MR pathway plays a major role in the antigen presentation and processing by the MHC class II molecules that activate CD4+ T cells remains controversial. The MR involvement in the antigen presentation through the MHC class II molecules is supported by the delivery of lipoglycan antigens to the late endosome and lysosome for presentation to the CD4+ T cells by CD1b molecules6 and by the generation of an isotype-switching antibody in response to immunization with anti-MR monoclonal antibody in vivo.7 Furthermore, the MR expression on the inflammatory macrophages is increased in response to inflammatory cytokines, such as IL-4, IL-13, and IL-10.8 In cytokine-treated cells, the MR is detected in the late endosome, suggesting that antigen-derived peptides can be loaded on the MHC class II molecules for presentation. The MHC class I and/or MHC class II presentation may depend on the activation status and maturation stage of the dendritic cells.9 These findings may help us understand how bacterial or viral infections may trigger the formation of autoantibody against ADMTS13, thereby resulting in an acute burst of TTP episodes.
In conclusion, the discovery of the role of mannose receptor in facilitating ADAMTS13 endocytosis by the antigen-presenting cells may promote further research on immune biology of ADAMTS13. The results of these investigations may shed new light on the pathogenesis of acquired autoimmune TTP.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal