In this issue of Blood, Jönsson et al show in an elegant transgenic study that human FcγRIIA, an activating IgG receptor, can trigger active and passive anaphylaxis and airway inflammation.1
The term “aphylaxis” was originally coined by Charles Richet in 1902 and later changed to “anaphylaxis.” Anaphylaxis is a hyperacute systemic allergic reaction that can occur within minutes or hours of allergen exposure in sensitized humans, leading to various symptoms including an itchy rash, throat swelling, low blood pressure, and death. Common causes include insect bites, foods, and medications. Anaphylaxis leads to 500-1000 deaths per year in the United States.
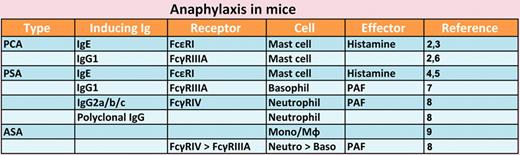
Presented are inducer immunoglobulins (Ig), their receptors, cell types, and effector molecules responsible for various types of experimental anaphylaxis.
Presented are inducer immunoglobulins (Ig), their receptors, cell types, and effector molecules responsible for various types of experimental anaphylaxis.
Because of the dramatic nature and serious outcomes of anaphylaxis, physicians and scientists have long been intrigued by this disease. However, only the past 3 decades after antibody Fc receptors were identified have the mechanism involved begun to be elucidated. Experimentally, 2 types of anaphylaxis have been studied: one can be induced by immunizing animals by antigen and followed by antigen challenge (active anaphylaxis) and the other by injecting antigen in animals previously sensitized with antigen-specific antibodies (passive anaphylaxis). Experimental anaphylaxis can be induced at the systemic level (systemic anaphylaxis) or locally, depending on the route of antigen challenge. IgE, the classic atopy-related antibody, plus allergen can cause passive systemic and cutaneous anaphylaxis (PSA and PCA). IgE was thought to be responsible for anaphylaxis, but surprisingly, active systemic anaphylaxis (ASA) can be induced in mice genetically made incapable of producing IgE. Later, IgG was also shown to have the ability to induce anaphylaxis. Use of knockout and transgenic mice allowed researchers to determine what types of antibodies, cells, Fc receptors, and effector molecules are involved in or responsible for different models of anaphylaxis (see figure). For example, IgE-induced PCA2,3 and PSA4,5 require FcϵRI (the high-affinity IgE receptor) expressed in mast cells and histamine released from activated mast cells. IgG1-induced PCA also depends on mast cells,2 but FcγRIIIA (not FcϵRI) is the receptor used.6 IgG1-induced PSA was reported to require FcγRIIIA on basophils,7 although this study was not replicated in basophil-deficient mice. Jönsson et al recently reported that IgG2b-induced PSA requires neutrophils expressing FcγRIV and platelet activating factor (PAF) and polyclonal IgG-induced PSA also requires neutrophils.8 Unlike a study showing that monocytes/macrophages are responsible for ASA induced by goat IgG in mice immunized with goat IgE anti–mouse IgD,9 Jönsson et al showed that neutrophils expressing FcγRIV and, to a lesser extent, basophils expressing FcγRIIIA contribute to ASA.
It is not easy to translate mouse results to human diseases, particularly anaphylaxis. The problems are many: there are multiple Fcγ receptors in both mice (FcγRI, FcγRIIB, FcγRIIIA, FcγRIV) and humans (FcγRI, FcγRIIA, FcγRIIB, FcγRIIC, FcγRIIIA, FcγRIIIB). Mouse FcγRI, FcγRIIIA, and FcγRIV and human FcγRI and FcγRIIIA are activating IgG receptors associated with a disulfide-bonded dimer of an ITAM-containing FcRγ subunit, while mouse and human FcγRIIB are inhibitory receptors with ITIM. FcγRIIA possesses an ITAM in its intracytoplasmice domain and is not associated with the FcRγ subunit. Human Fcγ receptors are not directly related to mouse Fcγ receptors. For example, mouse FcγRIV has no human ortholog. Mouse neutrophils express FcγRIIIA and FcγRIV, whereas human neutrophils express neither FcγRIIIA nor FcγRIV, but FcγRIIA and FcγRIIIB, which do not exist in mice. Of course, experiments on human patients for the study of disease pathogenesis may be prohibited. So, when Jönsson et al wanted to extend their previous study,8 they used transgenic mice that express human FcγRIIA in the WT background or in the absence of FcRγ, FcγRI/FcγRIIB/FcγRIIIA (3KO), or FcϵRI/FcϵRII/FcγRI/FcγRIIB/FcγRIIIA (5KO).1 Fortunately, FcγRIIA, but not FcγRIIIB, can bind mouse IgG.8 These situations allowed them to demonstrate that FcγRIIA can induce ASA, monoclonal IgG1- and polyclonal IgG-induced PSA (which is dependent on neutrophils and monocyte/macrophages), human and mouse IgG-induced PCA (which is dependent on mast cells), and airway inflammation triggered by immune complexes. Consistent with these in vivo results, stimulation of FcγRIIA in human monocytes and neutrophils produced anaphylactogenic mediators such as PAF and leukotrienes, and stimulation of FcγRIIA in mast cells induced degranulation.1
Importantly and in line with this study, polymorphisms in the gene encoding FcγRIIA has been linked to bronchial asthma and allergic rhinitis.10 FcγRIIA transgenic mice develop spontaneous autoimmune diseases such as pneumonitis, glomerulonephritis, rheumatoid arthritis, and thrombocytopenia.11 Another major reason that prompts the study of Fcγ receptors like this one is an extensive use of antibody-based immunotherapy. Further detailed information on the function of FcγRIIA and other Fcγ receptors will be important for safe and efficient use of antibody therapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal