In this issue of Blood, Ting et al identify the cell polarity–associated gene Ap2a2 as a positive regulator of hematopoietic stem cell (HSC) function and a candidate determinant of asymmetric HSC divisions.1
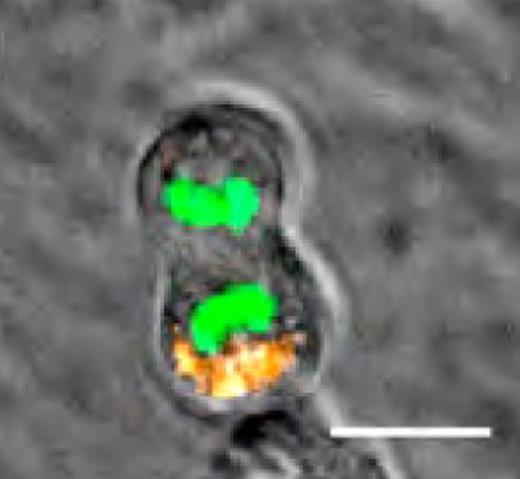
Asymmetrical segregation of AP2A2 during mitosis. CD150+48− LSK cells were transduced with an Ap2a2-Cherry fusion gene. Still frame from live cell videomicroscopy of dividing cells: DNA (Fitc-green); AP2A2 (Cherry-orange). Image from Ting et al.1
Asymmetrical segregation of AP2A2 during mitosis. CD150+48− LSK cells were transduced with an Ap2a2-Cherry fusion gene. Still frame from live cell videomicroscopy of dividing cells: DNA (Fitc-green); AP2A2 (Cherry-orange). Image from Ting et al.1
Hematopoiesis is maintained by stem cells with the ability to both self-renew and to give rise to differentiated progeny. A proper balance between these fates is essential to prevent hematopoietic failure or uncontrolled stem cell expansion. So how do HSCs undergo self-renewal? In theory, this can occur in 2 ways; either through symmetrical cell division (SCD) where 2 new stem cells are formed, or through asymmetrical cell division (ACD) giving rise to 1 stem cell and 1 cell committed for differentiation.2 Symmetrical division must occur because this is the only way by which the stem cell pool can be expanded. However, whether asymmetrical division really occurs in HSCs has remained an unresolved and elusive question.
Evidence supporting ACD in HSCs has been provided by detailed functional studies of the progeny of highly purified HSC populations, so-called paired daughter cell analysis.3,4 While these studies have clearly demonstrated that the 2 progeny of an HSC division can have different functional properties, it cannot be ruled out that these differences have been inferred by extrinsic factors immediately after cell division and they may therefore not be the result of an ACD per se. On the other hand, in lower organisms like Drosophila melanogaster and Caenorhabditis elegans, elegant studies have formally proven the existence of asymmetric stem cell divisions.2 Cells have been shown to be polarized during division and to unequally localize cell fate–determining molecules, which are asymmetrically inherited in the daughter cells. To ultimately prove that ACD occurs in HSCs it will be key to define molecules that segregate asymmetrically during mitosis and to show that the unequal partitioning of these molecules has functional consequences.5
It is in this context that Ting et al now report on Ap2a2 as a novel regulator of HSC function.1 Informed mainly by studies in lower organisms,6 Ting and colleagues decided to screen genes involved in cell polarity, for their ability to regulate HSC function in mice with the aim of identifying potential determinants of ACD in HSCs. They tested 43 candidate genes using retroviral overexpression in a combined in vitro/in vivo screening assay,7 and found 6 genes that had a clear, positive impact on HSC function. They decided to first focus on the endocytic gene, Ap2a2, as it was the top scorer with the most potent effect in the screening assays. From a series of in vitro expansion assays and in vivo transplantation experiments they could confirm that Ap2a2 dramatically enhances HSC activity. However, unlike many other positive HSC regulators identified in previous screens by the Sauvageau laboratory,7 Ap2a2 appeared to enhance HSC function without any substantial expansion of HSC numbers. As suggested by Ting et al, this could indicate a preference for asymmetric self-renewal divisions induced by Ap2a2.
To test whether Ap2a2 could act as a cell-fate determinant with an impact on ACD, Ting and colleagues studied its segregation during HSC mitosis using videomicroscopy and retroviral expression of Ap2a2 fused to a fluorescent protein (cherry). Remarkably, they found that cell divisions in some cases were associated with an asymmetrical partitioning of the fluorescently labeled AP2A2 protein (see figure).
The findings that Ap2a2 positively regulates HSC activity and also shows unequal segregation during mitosis are highly intriguing and indicate that Ap2a2 may in fact act as a cell-fate determinant influencing ACD. However, more work is required to support this. For example, it would be of great interest to isolate the immediate progeny of HSC divisions based on unequal segregation of AP2A2 and then determine the functional consequences of presence or absence of AP2A2 in the daughter cells. This would not only give more insights about Ap2a2 as a potential cell-fate determinant but could ultimately help resolve the long-standing question of whether or not asymmetric self-renewal occurs in HSCs.
One important aspect of ACD is malignant development. Studies in Drosophila have shown that normal stem cells can transition to tumor stem cells when genes involved in asymmetric cell division become mutated.8 Proving the concept of ACD in HSCs and gaining a better understanding of the process will therefore have implications for cancer-related questions. Cell-fate determinants active in HSCs may also be involved in leukemogenesis. The impressive strategy used by Ting and colleagues, combining functional genetic screens in HSCs with the tracking of protein segregation, promises to reveal additional candidate ACD-associated genes in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal