Abstract
IgE and IgE receptors (FcϵRI) are well-known inducers of allergy. We recently found in mice that active systemic anaphylaxis depends on IgG and IgG receptors (FcγRIIIA and FcγRIV) expressed by neutrophils, rather than on IgE and FcϵRI expressed by mast cells and basophils. In humans, neutrophils, mast cells, basophils, and eosinophils do not express FcγRIIIA or FcγRIV, but FcγRIIA. We therefore investigated the possible role of FcγRIIA in allergy by generating novel FcγRIIA-transgenic mice, in which various models of allergic reactions induced by IgG could be studied. In mice, FcγRIIA was sufficient to trigger active and passive anaphylaxis, and airway inflammation in vivo. Blocking FcγRIIA in vivo abolished these reactions. We identified mast cells to be responsible for FcγRIIA-dependent passive cutaneous anaphylaxis, and monocytes/macrophages and neutrophils to be responsible for FcγRIIA-dependent passive systemic anaphylaxis. Supporting these findings, human mast cells, monocytes and neutrophils produced anaphylactogenic mediators after FcγRIIA engagement. IgG and FcγRIIA may therefore contribute to allergic and anaphylactic reactions in humans.
Introduction
We recently reported that neutrophils are sufficient to induce active systemic anaphylaxis (ASA) in mice.1 Not only mouse neutrophils, but also human neutrophils, could indeed restore ASA when transferred into mice that are resistant to ASA because they lack activating IgG receptors (FcγR). Mouse neutrophils express 2 FcγRs, FcγRIIIA and FcγRIV, which accounted for ASA induction.1 However, human neutrophils express neither FcγRIIIA nor FcγRIV. They express 2 other FcγRs, FcγRIIA and FcγRIIIB, which do not exist in mice.2 Noticeably, FcγRIIA, but not FcγRIIIB, can bind mouse IgG.1 FcγRIIA may therefore be responsible for inducing human neutrophil activation when transferred into ASA-resistant mice.
Anaphylaxis is a systemic hyperacute allergic reaction that develops within minutes after antigen/allergen exposure in humans. It can be reproduced experimentally by injecting antigen in animals immunized with the same antigen (active anaphylaxis), or in mice preinjected with antigen-specific IgE or IgG antibodies (passive anaphylaxis). Not only systemic anaphylaxis leading to hypothermia, hypotension, and respiratory distress, but also local anaphylaxis leading to extravasation and inflammation, can be induced in mice depending on the route used for antigen challenge. Different models were found to depend on different mechanisms. IgE-induced and IgG1-induced passive cutaneous anaphylaxis (PCA) required mast cells.3,4 IgE-induced passive systemic anaphylaxis (PSA) also required mast cells.5,6 However, IgG1-induced PSA was reported to require basophils,7 whereas IgG2-induced PSA required neutrophils.1 Mast cells5 and basophils7,8 were not required for ASA that depended on monocytes/macrophages,9 or on neutrophils1 depending on the experimental model. Therefore, each of these 4 cell types contribute to a specific model of anaphylaxis, but their respective contribution in humans remains to be determined.
In mice, mast cells, basophils, neutrophils, and monocytes/macrophages express activating FcRs that require the association of the ITAM-containing FcRγ-subunit to be expressed and functional at the cell membrane. Importantly, FcRγ−/− mice developed neither PCA, nor PSA or ASA, indicating that activating FcRs are mandatory for the induction of these reactions. Mast cells and basophils express specifically the murine high-affinity IgE receptor FcϵRI, and neutrophils and monocytes/macrophages express specifically the murine high-affinity IgG receptor FcγRIV.10 However, all of these cells express the low-affinity IgG receptor FcγRIIIA. Passive anaphylaxis models have demonstrated that FcϵRI is mandatory for IgE-induced PCA and PSA,11 FcγRIIIA for IgG1-induced PCA12 and PSA,6 and FcγRIV for IgG2-induced PSA.1 FcγRIIIA and FcγRIV,1 but not FcϵRI,6 contributed detectably to ASA models.
Human neutrophils do not express FcγRIIIA, and FcγRIV does not exist in humans.10 Instead human neutrophils express the low-affinity activating IgG receptor FcγRIIA. FcγRIIA possesses its own ITAM in its intracytoplasmic domain, and is not associated with the FcRγ-subunit.2 The FcγRIIA ITAM, however, is noncanonical and has been described to be less potent in inducing cell activation in vitro than the FcRγ ITAM.13,14 FcγRIIA binds all 4 human IgG subclasses,15 as well as mouse IgG1, IgG2a, and IgG2b subclasses.1 Polymorphisms in the gene encoding FcγRIIA have been reported to be linked to bronchial asthma and allergic rhinitis,16 suggesting a role for FcγRIIA in allergic reactions. Mice transgenic for the Fcgr2a gene have been generated that recapitulate the expression of FcγRIIA in humans.17 These FcγRIIAtg mice spontaneously developed autoimmune diseases on a wild-type (WT) background (ie, pneumonitis, glomerulonephritis, and rheumatoid arthritis).18 FcγRIIA, expressed on the FcRγ−/− background, was sufficient to induce experimental models of thrombocytopenia19 and rheumatoid arthritis.20 The ability of FcγRIIA to induce allergic reactions has not been investigated.
FcγRIIA is the most widely expressed FcR in humans,18 and remarkably the only activating IgG receptor constitutively expressed by mast cells, basophils, neutrophils, and eosinophils. Mast cells, basophils, and eosinophils are well-known effectors of allergic reactions, and our recent work suggests that neutrophils might be effectors of anaphylaxis.1 We therefore studied the ability of human FcγRIIA to induce passive and active anaphylaxis, and models of allergic inflammation in skin and airways. To this aim, we used FcγRIIA-transgenic mice on backgrounds deficient for endogenous FcRs. We found that FcγRIIA was sufficient to induce mast cell and macrophage activation in vitro, and mast cell–dependent PCA and lung inflammation in vivo. FcγRIIA-induced PSA was dependent on monocytes/macrophages and neutrophils, but not on mast cells and basophils. Noticeably, FcγRIIA was sufficient to induce fatal ASA. Finally, human mast cells, monocytes, and neutrophils produced anaphylactogenic mediators after FcγRIIA engagement. FcγRIIA may therefore be the major activating IgG receptor contributing to allergic reactions and anaphylaxis in humans.
Methods
Mice
C57BL/6J FcγRIIAtg mice were provided by M. P. Reilly (Jefferson Medical College, Philadelphia, PA), FcγRI/IIB/IIIA triple-deficient (3KO) mice (N6 C57BL/6J) by S. Verbeek (Leiden University Medical Center, Leiden, The Netherlands), KRNtg mice by D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and IGBMC (Strasbourg, France). WT C57BL/6J mice were purchased from Charles River, Wsh/Wsh and FcRγ−/− C57BL/6J mice from The Jackson Laboratory. FcγRI/IIB/IIIA−/− FcϵRI−/− FcϵRII−/− (5KO; N6 C57BL/6J) mice were previously described.21 FcγRIIAtg mice were intercrossed with 3KO, 5KO, FcRγ−/−, and/or Wsh/Wsh mice to obtain 3KOIIA, 5KOIIA, FcRγ−/−IIA, and Wsh3KOIIA-transgenic mice, respectively. All mice carrying the hFcγRIIA transgene were used as heterozygous animals. Nontransgenic littermates served as controls. Mice in all experiments were 6-10 weeks old. All mouse protocols were approved by the Animal Care and Use Committees of Paris, Ile de France, France.
Active systemic anaphylaxis
Mice were injected intraperitoneally on day 0 with 200 μg BSA, either in CFA or in alum, and boosted intraperitoneally on day 14 with 200 μg BSA in IFA or in alum, respectively. BSA-specific IgG1 and IgG2a/b/c antibodies in serum were titered by ELISA on day 17 as described.1 Mice with comparable antibody titers were challenged intravenously with 500 μg BSA, 8 days after the last immunization. Central temperature was monitored using a digital thermometer (YSI).
Passive systemic anaphylaxis
Immune complexes made of 1 mg OVA and 1 mg anti-OVA mAb (clone OVA-14), or 20 μL K/BxN serum and 50 μg GPI in 200 μL physiologic solution were preformed at 37°C and injected intravenously. Alternatively, 50 or 150 μg of mAb IV.3 were injected intravenously. Central body temperature was recorded.
Passive cutaneous anaphylaxis
ICs were preformed by incubating OVA and OVA-14 in a 1:1 ratio for 15 minutes at 37°C. Indicated amounts of these ICs, or heat-aggregated (1 hour at 63°C) purified human IgG or anti-FcγRIIA mAb IV.3 were injected intradermally in 20 μL total volume, immediately followed by an intravenous (IV) injection of 100 μL PBS containing 2% Evans Blue. Thirty minutes later, Evans blue was extracted from 1-cm-diameter skin pieces using formamide at 63°C and quantified by absorbance (620 nm).
Airway inflammation
Mice were injected intranasally with 50 μL of rabbit anti-OVA antiserum and 500 μg of OVA intravenously. After indicated periods of time, mice were lethally anesthetized, and 4 broncho-alveolar lavages of, respectively, 0.5, 1, 1, and 1 mL PBS were performed. The supernatant of the first lavage was used to quantify MPO and keratinocyte-derived chemokine (KC) content. The cells from all lavages were pooled for cell count analysis. Hemorrhage score was determined on pooled lavages and ranged from 0 (no blood present), 1 (detectable hemorrhage), to 3 (strong hemorrhage).
Alveolar macrophages, recovered by 3 consecutive 1 mL broncho-alveolar lavages from individual mice, were exposed for 3 hours to 3 different conditions: (1) no stimulant, (2) plate-bound ICs made of 100 μg/mL OVA and 30 μg/mL rabbit anti-OVA, and (3) plate-bound anti-FcγRIIA mAbs (100 μg/mL). Supernatants were assayed for KC and MIP-1α by ELISA (R&D Systems).
In vivo blocking and depletion
Anti-FcγRIIA mAb (50 μg/mouse) was injected intravenously either once (24 hours), or twice (24 hours and 12 hours) before the experiment. 300 μL/mouse PBS- or clodronate-liposomes (at 7 mg/mL), 300 μg/mouse anti-Gr1, 300 μg/mouse anti–Ly-6G, 10 μg/mouse anti-CD200R3 mAbs, or 1 mg/mouse GdCl3 were injected 24 hours before the experiment. Depletion of specific populations was ascertained using flow cytometry on blood samples taken during or after the experiment (examples are shown in supplemental Figure 3B-E, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Please refer to supplemental Methods for information on antibodies, reagents, and cells; flow cytometry analysis; lung histology; statistical analyses; and in vitro cell activation.
Results
FcγRIIA can trigger active systemic anaphylaxis
Active systemic anaphylaxis was induced by an IV antigen challenge in mice immunized with the same antigen. This protocol induced a body temperature decrease and mortality in WT mice, but not in FcRγ−/− mice, immunized with antigen in Alum (supplemental Figure 1A) or in Freund adjuvant (supplemental Figure 1B). Immunizations in either adjuvant lead to the production of IgG1 and IgE antibodies, but only immunizations in Freunds adjuvant lead to the production of IgG2 antibodies in both mouse strains (data not shown). Human neutrophils express both FcγRIIA and FcγRIIIB (Figure 1A), but only FcγRIIA and not FcγRIIIB can bind mouse IgG (Figure 1B). To analyze the capacity of FcγRIIA to induce ASA, we developed transgenic mouse models expressing human FcγRIIA under the control of its own promoter, and deficient for endogenous FcRs. FcγRIIAtg mice express FcγRIIA not only on neutrophils, but also on eosinophils, monocytes, macrophages, and weakly on basophils (Figure 1C). FcγRIIAtg mice therefore reproduce the expression pattern found in humans (Figure 1D). Noticeably, whereas human neutrophils and basophils express FcγRIIA (supplemental Figure 1C), mouse neutrophils express FcγRIIIA and FcγRIV, and mouse basophils only FcγRIIIA,1 as ITAM-bearing activating FcγR.
FcγRIIA can induce active systemic anaphylaxis. (A) Representative histogram plots of human FcR expression on human blood neutrophils. (B) Representative histogram plots of anti-FLAG mAb (top panel) or preformed mouse polyclonal IgG-immune complexes (bottom panel) binding to CHO transfectants expressing the indicated FLAG-tagged human polymorphic variants of FcγRIIA (H131 or R131) and FcγRIIIB (NA1, NA2, or SH). (C) Representative expression of FcγRIIA on blood and peritoneal cells from 3KOIIA mice (open histograms) or nontransgenic 3KO littermate controls (filled histograms): T cells (CD3+), B cells (CD19+), NK cells (DX5+/NK1.1+), neutrophils (Gr1hi/CD11b+), eosinophils (Gr1int/SiglecF+), basophils (IgE+/DX5+), and monocytes/macrophages (CD11b+/Gr1−). (D) Representative expression of FcγRIIA (open histograms) on human blood cells: T cells (CD3+), B cells (CD19+), NK cells (CD56+), neutrophils (CD24+), eosinophils (CCR3+/CDw125+), basophils (FcϵRI+/CD203c+), and monocytes (CD14+); or isotype control (closed histograms). (E-G) Indicated mice were immunized with BSA, (E) in Freunds adjuvant, or (F-G) in alum, challenged with BSA and central temperatures and survival rates were monitored. (E) ASA in FcRγ−/− (n = 7) and FcRγ−/−IIA mice (n = 5). (F) ASA in FcRγ−/− (n = 4) and FcRγ−/−IIA (n = 5) mice. (G) ASA in FcRγ−/−IIA mice injected twice with anti-FcγRIIA mAb IV.3 (n = 5) or not (n = 6), before BSA-challenge. FcRγ−/− mice were used as controls (n = 7). (E-G) Data are represented as mean ± SEM. (A-G) Data are representative of at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
FcγRIIA can induce active systemic anaphylaxis. (A) Representative histogram plots of human FcR expression on human blood neutrophils. (B) Representative histogram plots of anti-FLAG mAb (top panel) or preformed mouse polyclonal IgG-immune complexes (bottom panel) binding to CHO transfectants expressing the indicated FLAG-tagged human polymorphic variants of FcγRIIA (H131 or R131) and FcγRIIIB (NA1, NA2, or SH). (C) Representative expression of FcγRIIA on blood and peritoneal cells from 3KOIIA mice (open histograms) or nontransgenic 3KO littermate controls (filled histograms): T cells (CD3+), B cells (CD19+), NK cells (DX5+/NK1.1+), neutrophils (Gr1hi/CD11b+), eosinophils (Gr1int/SiglecF+), basophils (IgE+/DX5+), and monocytes/macrophages (CD11b+/Gr1−). (D) Representative expression of FcγRIIA (open histograms) on human blood cells: T cells (CD3+), B cells (CD19+), NK cells (CD56+), neutrophils (CD24+), eosinophils (CCR3+/CDw125+), basophils (FcϵRI+/CD203c+), and monocytes (CD14+); or isotype control (closed histograms). (E-G) Indicated mice were immunized with BSA, (E) in Freunds adjuvant, or (F-G) in alum, challenged with BSA and central temperatures and survival rates were monitored. (E) ASA in FcRγ−/− (n = 7) and FcRγ−/−IIA mice (n = 5). (F) ASA in FcRγ−/− (n = 4) and FcRγ−/−IIA (n = 5) mice. (G) ASA in FcRγ−/−IIA mice injected twice with anti-FcγRIIA mAb IV.3 (n = 5) or not (n = 6), before BSA-challenge. FcRγ−/− mice were used as controls (n = 7). (E-G) Data are represented as mean ± SEM. (A-G) Data are representative of at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
After antigen challenge, ASA developed in FcRγ−/−IIA mice, but not in nontransgenic FcRγ−/− littermates, immunized with antigen in Freunds adjuvant (Figure 1E) or in Alum (Figure 1F) leading to a severe temperature drop and 100% mortality. IV injections of anti-FcγRIIA blocking mAbs abolished ASA-induced temperature drop and mortality in FcRγ−/−IIA mice immunized in Freunds adjuvant (data not shown) or in Alum (Figure 1G). FcγRIIA is therefore sufficient to trigger fatal active systemic anaphylaxis.
FcγRIIA can trigger passive systemic anaphylaxis
To investigate the potential of FcγRIIA to induce PSA, we used divalent (anti-FcγRIIA mAbs) or multivalent (IgG-immune complexes) ligands. An IV injection of 150 μg, but not of 50 μg, anti-FcγRIIA mAb IV.3 induced a modest temperature drop in FcRγ−/−IIA mice, but not in FcRγ−/− mice (Figure 2A). Noticeably, FcRγ−/− mice lack all activating FcγRs but express inhibitory FcγRIIB, which has been reported to negatively regulate PSA induced by mouse-activating FcγRs.22 FcγRIIB binds, like human FcγRIIA, mouse IgG1 and IgG2 subclasses. IgG-immune complexes may therefore coaggregate human FcγRIIA with mouse FcγRIIB, leading to the inhibition of FcγRIIA-dependent activation,23 and consequently of FcγRIIA-induced PSA. We therefore crossed FcγRIIAtg mice to FcγRI/FcγRIIB/FcγRIIIA−/− (3KO) mice or to FcγRI/FcγRIIB/FcγRIIIA−/− FcϵRI/FcϵRII−/− (5KO) mice (Figure 2B). 3KO and 5KO mice lack all IgG receptors except the activating IgG2 receptor FcγRIV,21 whereas FcRγ−/− mice lack all IgG receptors except the inhibitory IgG1/IgG2 receptor FcγRIIB. An injection of 150 μg mAb IV.3 induced a significant temperature drop in 3KOIIA mice (Figure 2C) and in 5KOIIA mice (Figure 2D). Because 50 μg mAb IV.3 induced no or a very weak temperature drop, we used this dose to block FcγRIIA in 3KOIIA and 5KOIIA mice.
In vivo aggregation of FcγRIIA induces passive systemic anaphylaxis. (A,C-D) Indicated FcγRIIA-transgenic mice were injected with 50 μg (gray symbols) or 150 μg (black symbols) of mAb IV.3, and central temperatures were monitored (n = 3). Nontransgenic littermates injected with 150 μg mAb IV.3 were used as controls (open symbols, n = 3). (A) FcRγ−/−, (C) 3KO, (D) 5KO backgrounds. (B) Schematic representation of Fc receptors expressed in the different mouse models used in this study. (E-F) Mice were injected with indicated preformed mouse IC and central temperatures were monitored. Gray symbols indicate mice injected with 50 μg of mAb IV.3 24 hours before challenge (n = 4). Top panel (E) n = 5, (F) n = 4. Bottom panel (E) 3KO or 3KOIIA n = 3, (F) 3KO n = 3, 3KOIIA+iso n = 4. (A,C-F) Data are represented as mean ± SEM and are representative of at least 2 independent experiments (**P < .01; ***P < .001).
In vivo aggregation of FcγRIIA induces passive systemic anaphylaxis. (A,C-D) Indicated FcγRIIA-transgenic mice were injected with 50 μg (gray symbols) or 150 μg (black symbols) of mAb IV.3, and central temperatures were monitored (n = 3). Nontransgenic littermates injected with 150 μg mAb IV.3 were used as controls (open symbols, n = 3). (A) FcRγ−/−, (C) 3KO, (D) 5KO backgrounds. (B) Schematic representation of Fc receptors expressed in the different mouse models used in this study. (E-F) Mice were injected with indicated preformed mouse IC and central temperatures were monitored. Gray symbols indicate mice injected with 50 μg of mAb IV.3 24 hours before challenge (n = 4). Top panel (E) n = 5, (F) n = 4. Bottom panel (E) 3KO or 3KOIIA n = 3, (F) 3KO n = 3, 3KOIIA+iso n = 4. (A,C-F) Data are represented as mean ± SEM and are representative of at least 2 independent experiments (**P < .01; ***P < .001).
Likewise, an IV injection of monoclonal IgG1- (Figure 2E) or polyclonal IgG-immune complexes (Figure 2F) induced a significant temperature drop in 3KOIIA mice, but not in 3KO mice. Pretreatment with anti-FcγRIIA mAb IV.3 abolished these temperature drops in 3KOIIA mice (Figure 2E-F bottom panels). FcγRIIA is therefore sufficient to trigger monoclonal IgG1-induced PSA and polyclonal IgG-induced PSA.
Neutrophils and monocytes/macrophages mediate FcγRIIA-dependent PSA
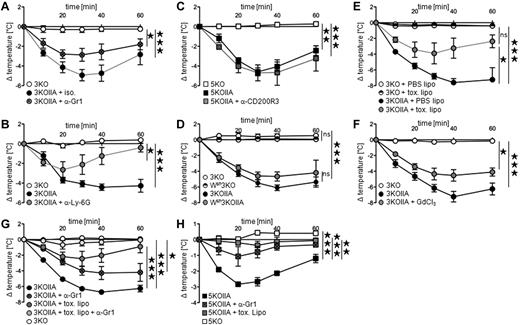
All cell types that express FcγRIIA, ie, neutrophils, monocytes/macrophages, basophils, and eosinophils (Figure 1C) can potentially contribute to PSA. We found recently that neutrophils are responsible for polyclonal IgG-IC–induced PSA,1 whereas basophils were reported to be responsible for monoclonal IgG1-IC–induced PSA.7 Polyclonal IgG-IC–induced PSA was reduced by neutrophil depletion after injection of anti-Gr1 (Figure 3A) or anti–Ly-6G (Figure 3B) mAbs in 3KOIIA mice. Neutrophils therefore contribute to FcγRIIA-dependent PSA. Surprisingly, basophil depletion did not affect PSA (Figure 3C). To investigate whether FcγRIIA-triggered PSA depends on mast cells, 3KOIIA mice, and as negative controls 3KO mice were crossed with Wsh/Wsh mice. Wsh 3KOIIA mice developed unaltered FcγRIIA-triggered PSA, that is, in the absence of mast cells (Figure 3D). However, depletion of monocytes/macrophages induced by toxic liposomes, but not by control liposomes, reduced PSA (Figure 3E). Supporting this result, PSA was reduced in 3KOIIA mice injected with gadolinium, which inhibits monocytes/macrophage function (Figure 3F). Monocytes/macrophages therefore also contribute to FcγRIIA-dependent PSA. Depletion of a single cell population reduced PSA, but depletion of both neutrophils and monocytes/macrophages abrogated PSA in 3KOIIA mice (Figure 3G). In line with these results, monocytes/macrophage depletion and neutrophil depletion also inhibited anti-FcγRIIA mAb-induced PSA (Figure 3H). Collectively, these data demonstrate that neutrophils and monocytes/macrophages, together, account for FcγRIIA-dependent passive systemic anaphylaxis.
Neutrophils and monocytes/macrophages are necessary for FcγRIIA-dependent PSA. (A-G) Indicated mice were injected with preformed polyclonal IgG-IC (mouse anti-GPI antiserum plus GPI), and central temperatures were monitored. PSA in FcγRIIA transgenic mice injected with (A) anti-Gr1 mAbs (n = 8) or isotype (ISO) control (n = 3), (B) anti–Ly-6G mAbs (n = 4) or untreated control (n = 4), (C) anti-CD200R3 mAbs (n = 3) or untreated control (n = 3). Nontransgenic littermates were used as controls (A, n = 2; B, n = 4; C, n = 3). (D) PSA in Wsh3KOIIA mice and 3KOIIA mice (n = 3). Nontransgenic littermate controls 3KO (n = 3) and Wsh3KO mice (n = 4) were used as controls. (E-F) PSA in 3KOIIA mice injected with (E) PBS liposomes (PBS lipo) or clodronate liposomes (toxic lipo; n = 3), (F) Gadolinium chloride (GdCl3) or not (n = 4). Nontransgenic littermates were used as controls (E, n = 2; F: n = 4). (G) PSA in 3KOIIA mice left untreated (n = 7), or injected with anti-Gr1 mAbs (n = 5), toxic liposomes (n = 6) or anti-Gr1 mAbs plus toxic liposomes (n = 6). Data are a compilation of 2 experiments. 3KO served as negative control (n = 2). Statistical significances are indicated among 3KOIIA groups. (H) mAb IV.3-induced PSA in indicated mice injected with anti-Gr1 mAbs, toxic liposomes, or left untreated (n = 3). (A-H) Data are represented as mean ± SEM, and (A-F,H) are representative of at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
Neutrophils and monocytes/macrophages are necessary for FcγRIIA-dependent PSA. (A-G) Indicated mice were injected with preformed polyclonal IgG-IC (mouse anti-GPI antiserum plus GPI), and central temperatures were monitored. PSA in FcγRIIA transgenic mice injected with (A) anti-Gr1 mAbs (n = 8) or isotype (ISO) control (n = 3), (B) anti–Ly-6G mAbs (n = 4) or untreated control (n = 4), (C) anti-CD200R3 mAbs (n = 3) or untreated control (n = 3). Nontransgenic littermates were used as controls (A, n = 2; B, n = 4; C, n = 3). (D) PSA in Wsh3KOIIA mice and 3KOIIA mice (n = 3). Nontransgenic littermate controls 3KO (n = 3) and Wsh3KO mice (n = 4) were used as controls. (E-F) PSA in 3KOIIA mice injected with (E) PBS liposomes (PBS lipo) or clodronate liposomes (toxic lipo; n = 3), (F) Gadolinium chloride (GdCl3) or not (n = 4). Nontransgenic littermates were used as controls (E, n = 2; F: n = 4). (G) PSA in 3KOIIA mice left untreated (n = 7), or injected with anti-Gr1 mAbs (n = 5), toxic liposomes (n = 6) or anti-Gr1 mAbs plus toxic liposomes (n = 6). Data are a compilation of 2 experiments. 3KO served as negative control (n = 2). Statistical significances are indicated among 3KOIIA groups. (H) mAb IV.3-induced PSA in indicated mice injected with anti-Gr1 mAbs, toxic liposomes, or left untreated (n = 3). (A-H) Data are represented as mean ± SEM, and (A-F,H) are representative of at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
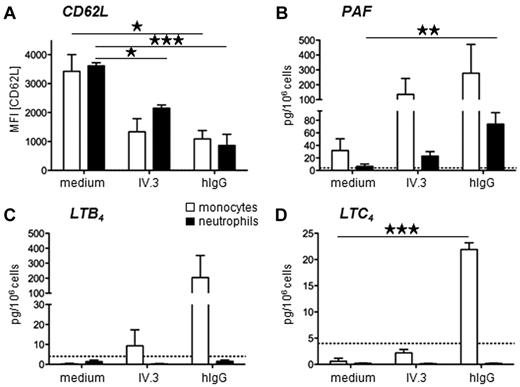
To investigate if neutrophils and monocytes may also contribute to anaphylactic reactions in humans, we investigated whether these FcγRIIA-expressing cell types produce anaphylactogenic mediators after FcγRIIA engagement. Monocytes and neutrophils purified from the blood of normal donors could be activated in vitro by anti-FcγRIIA mAb- or human IgG-heat aggregates, as revealed by their decreased CD62L expression (Figure 4A). In the same conditions, both human monocytes and human neutrophils produced platelet activating factor (PAF; Figure 4B),24 whereas monocytes, but not neutrophils, produced leukotriene B4 (LTB4; Figure 4C) and LTC4 (Figure 4D). Human monocytes and neutrophils therefore produce anaphylactogenic mediators (ie, PAF and leukotrienes) after FcγRIIA engagement, supporting a role for FcγRIIA in human allergic reactions.
Human neutrophils and monocytes produce anaphylactogenic mediators. (A-D) Purified human monocytes or neutrophils were incubated in vitro with heat-aggregated human IgG or anti-FcγRIIA mAb IV.3, and (A) CD62L expression, (B) PAF, (C) LTB4, and (D) LTC4 production are represented. Mean results from the analysis of 3 normal donors are represented (*P < .05; **P < .01; ***P < .001).
Human neutrophils and monocytes produce anaphylactogenic mediators. (A-D) Purified human monocytes or neutrophils were incubated in vitro with heat-aggregated human IgG or anti-FcγRIIA mAb IV.3, and (A) CD62L expression, (B) PAF, (C) LTB4, and (D) LTC4 production are represented. Mean results from the analysis of 3 normal donors are represented (*P < .05; **P < .01; ***P < .001).
FcγRIIA triggers mast cell–dependent passive cutaneous anaphylaxis
Mast cells are responsible for IgE-induced PSA6 and for PCA.25 IgG1-induced PCA, which depends on mouse FcγRIIIA, was also reported to depend on mast cells using mast cell–deficient W/Wv or Wsh/Wsh mice.26 Mast cells may also be responsible for cutaneous anaphylaxis in humans because degranulated mast cells are found in skin biopsies from allergic patients, and because mast cell–specific mediator levels correlate with the severity of allergic skin inflammation. As human mast cells express FcγRIIA,27 we investigated whether FcγRIIA could trigger IgG-induced PCA. Intradermal injections of increasing doses of IgG1-IC (supplemental Figure 2A) or of mAb IV.3 (supplemental Figure 2B) induced cutaneous anaphylaxis in 5KOIIA mice, but not in 5KO mice, as assessed by Evans blue extravasation. Intradermal injections of IgG1-IC, but not of antigen or Ab alone, induced PCA in 5KOIIA mice that was inhibited by an IV pretreatment with anti-FcγRIIA mAb (Figure 5A, supplemental Figure 2C). Similarly, intradermal injections of heat-aggregated (HA) human polyclonal IgG induced cutaneous reactions in 5KOIIA mice, but not in 5KO mice, that were abolished by anti-FcγRIIA mAb pretreatment (Figure 5B, supplemental Figure 2D). FcγRIIA can therefore trigger mouse and human IgG-induced PCA.
Mast cells are mandatory for FcγRIIA-dependent passive cutaneous anaphylaxis. (A-C) Mice were injected intradermally with indicated reagents and intravenously with Evans blue. Quantification of Evans blue extracted from skin tissue is represented. (A-B) PCA in 5KO (open symbols: A, n = 4; B, n = 3), 5KOIIA (black symbols: A, n = 3, B, n = 3), or 5KOIIA mice preinjected (A) once or (B) twice with 50 μg mAb IV.3 (gray symbols, n = 4). (C) PCA in 3KOIIA mice (black symbols, n = 4), in Wsh3KOIIA (gray symbols, n = 3), and as controls in Wsh3KO (open symbols, n = 2). NB: 2 points are represented per mouse, as each mouse was injected on 2 different sites with Ag and with IC. (D) Representative expression of FcγRIIA on peritoneal mast cells (c-kit+/IgE+) from 3KOIIA mice (open histograms) or 3KO littermate controls (filled histograms). (E-F) Peritoneal cells from 3KO (white bars) and 3KOIIA (black bars) mice were stimulated with indicated reagents. (E) The percentage of degranulated mast cells (a minimum of 200 cells were counted per experimental point), and (F) histamine content in the supernatant of each experimental point are represented. (A-C) Data are represented as single measured points, and mean ± SEM. (A-F) Data are representative of at least 2 independent experiments (*P < .05; ***P < .001).
Mast cells are mandatory for FcγRIIA-dependent passive cutaneous anaphylaxis. (A-C) Mice were injected intradermally with indicated reagents and intravenously with Evans blue. Quantification of Evans blue extracted from skin tissue is represented. (A-B) PCA in 5KO (open symbols: A, n = 4; B, n = 3), 5KOIIA (black symbols: A, n = 3, B, n = 3), or 5KOIIA mice preinjected (A) once or (B) twice with 50 μg mAb IV.3 (gray symbols, n = 4). (C) PCA in 3KOIIA mice (black symbols, n = 4), in Wsh3KOIIA (gray symbols, n = 3), and as controls in Wsh3KO (open symbols, n = 2). NB: 2 points are represented per mouse, as each mouse was injected on 2 different sites with Ag and with IC. (D) Representative expression of FcγRIIA on peritoneal mast cells (c-kit+/IgE+) from 3KOIIA mice (open histograms) or 3KO littermate controls (filled histograms). (E-F) Peritoneal cells from 3KO (white bars) and 3KOIIA (black bars) mice were stimulated with indicated reagents. (E) The percentage of degranulated mast cells (a minimum of 200 cells were counted per experimental point), and (F) histamine content in the supernatant of each experimental point are represented. (A-C) Data are represented as single measured points, and mean ± SEM. (A-F) Data are representative of at least 2 independent experiments (*P < .05; ***P < .001).
Mouse IgG1-induced FcγRIIIA-dependent PCA is abrogated in W/Wv4 and Wsh/Wsh mice (data not shown). We investigated whether FcγRIIA-triggered PCA also depends on mast cells by comparing 3KOIIA mice with Wsh3KOIIA mice. As expected, 3KOIIA mice developed mouse IgG1-induced PCA. Wsh3KOIIA mice, however, as well as Wsh3KO mice used as negative controls, were protected from mouse IgG1-induced PCA (Figure 5C, supplemental Figure 3E). Mast cells are therefore mandatory for FcγRIIA-triggered IgG-induced PCA.
FcγRIIA aggregation induces mast cell degranulation in vitro and ex vivo
Because IgG-induced mast cell–dependent PCA occurs in 3KOIIA mice, mast cells are expected to express FcγRIIA. Ex vivo peritoneal mast cells from 3KOIIA mice indeed express FcγRIIA (Figure 5D), but no other FcγR (not shown). IgG-IC induced ex vivo peritoneal mast cells from 3KOIIA mice, but not from 3KO mice to degranulate (Figure 5E) and release histamine (Figure 5F). Anti-IgE antibodies, however, induced IgE-bearing peritoneal mast cells from both naive strains to degranulate and release histamine. FcγRIIA aggregation can therefore activate mast cells.
Like peritoneal mast cells from 3KOIIA mice, in vitro cultured peritoneal cell–derived mast cells (PCMCs),28 fetal skin-derived mast cells (FSMCs), and bone marrow–derived mast cells (BMMCs) from the same mice all expressed FcγRIIA (Figure 6A). PCMCs from 3KOIIA mice, but not from 3KO mice, bound monoclonal IgG1-IC and polyclonal IgG-IC (Figure 6B). Incubation of 3KOIIA PCMCs with either of these immune complexes induced mast-cell activation as revealed by Ca2+ fluxes (Figure 6C) and intracellular protein phosphorylation (Figure 6D). FcγRIIA aggregation by IgG-IC induced consistent syk, PLCγ1, Akt, IκB, and ERK1/2 phosphorylation, but a barely detectable LAT phosphorylation compared with that induced by the aggregation of FcϵRI by IgE plus antigen on the same cells (Figure 6D). FcγRIIA aggregation, similar to FcϵRI aggregation, induced a sustained phosphorylation of SHIP1, as previously reported in monocytes.29 This SH2-containing inositol-5-phosphatase negatively regulates signaling by FcRγ-chain associated FcRs, and is mandatory for FcγRIIB-dependent negative regulation of mast cell activation.30,31
FcγRIIA activates mouse and human mast cells in vitro. (A) Representative expression of FcγRIIA on PCMCs, FSMCs, and BMMCs from 3KOIIA (open histogram) and 3KO mice (filled histogram). (B) PCMCs from indicated mice were incubated with indicated preformed mouse IgG-IC (αOVA: anti-OVA mAb; αGPI: polyclonal anti-GPI antiserum; open histograms) or not (filled histograms). Binding of ICs was detected by staining with F(ab′)2 GAM-PE. (C) Calcium fluxes in PCMCs from 3KO or 3KOIIA mice incubated with indicated IC (black curves) or Ag alone (gray curves). Ionomycin was used as control. (D) Western blot analysis of PCMC lysates after stimulation with indicated reagents for different periods of time. PCMCs sensitized overnight with IgE anti-DNP and challenged with DNP-HSA for 3 minutes served as positive controls. Actin was used as a loading control. FcγRIIA was used as a genotype control (reprobe after pERK1/2 staining). (E-F) Mediator release by PCMCs from 3KO (open bars) and 3KOIIA (black bars) mice challenged with indicated reagents. PCMCs sensitized overnight with IgE anti-DNP and challenged with DNP-HSA served as positive controls. NB: GPI+αGPI correspond to ICs made of GPI and polyclonal anti-GPI antiserum in panel E, and to ICs made of GPI and IgG purified from anti-GPI antiserum in panel F. (G) Representative histogram plots of human FcR expression on human SMCs. (H) Percentage of β-hexosaminidase release and quantification of histamine release by human SMCs incubated with anti-FcϵRI mAb or with preformed complexes of mAb IV.3 and GAM. (E-F,H) Data are represented as mean ± SEM. (A-H) Data are representative from at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
FcγRIIA activates mouse and human mast cells in vitro. (A) Representative expression of FcγRIIA on PCMCs, FSMCs, and BMMCs from 3KOIIA (open histogram) and 3KO mice (filled histogram). (B) PCMCs from indicated mice were incubated with indicated preformed mouse IgG-IC (αOVA: anti-OVA mAb; αGPI: polyclonal anti-GPI antiserum; open histograms) or not (filled histograms). Binding of ICs was detected by staining with F(ab′)2 GAM-PE. (C) Calcium fluxes in PCMCs from 3KO or 3KOIIA mice incubated with indicated IC (black curves) or Ag alone (gray curves). Ionomycin was used as control. (D) Western blot analysis of PCMC lysates after stimulation with indicated reagents for different periods of time. PCMCs sensitized overnight with IgE anti-DNP and challenged with DNP-HSA for 3 minutes served as positive controls. Actin was used as a loading control. FcγRIIA was used as a genotype control (reprobe after pERK1/2 staining). (E-F) Mediator release by PCMCs from 3KO (open bars) and 3KOIIA (black bars) mice challenged with indicated reagents. PCMCs sensitized overnight with IgE anti-DNP and challenged with DNP-HSA served as positive controls. NB: GPI+αGPI correspond to ICs made of GPI and polyclonal anti-GPI antiserum in panel E, and to ICs made of GPI and IgG purified from anti-GPI antiserum in panel F. (G) Representative histogram plots of human FcR expression on human SMCs. (H) Percentage of β-hexosaminidase release and quantification of histamine release by human SMCs incubated with anti-FcϵRI mAb or with preformed complexes of mAb IV.3 and GAM. (E-F,H) Data are represented as mean ± SEM. (A-H) Data are representative from at least 2 independent experiments (*P < .05; **P < .01; ***P < .001).
FcγRIIA aggregation also induced PCMC degranulation, as revealed by β-hexosaminidase and histamine release (Figure 6E), supporting our results obtained with ex vivo peritoneal mast cells (Figure 5E-F). In addition, FcγRIIA aggregation induced lipid mediator production, that is, LTC4 and prostaglandin D2 (PGD2) by PCMCs (Figure 6F). Thus, IgG-immune complexes induced FcγRIIA-triggered activation of mast cells leading to the release of mediators involved in vascular permeability (eg, histamine, LTC4, PGD2), which is consistent with FcγRIIA's ability to induce PCA.
We therefore analyzed the expression of Fc receptors on human skin–derived mast cells (SMC), and the ability of FcγRIIA to induce human skin mast cell activation. Human SMC constitutively express the high-affinity IgE receptor FcϵRI, as expected, and a single IgG receptor, FcγRIIA (Figure 6G), as described.27 FcγRIIA aggregation by anti-FcγRIIA mAb induced β-hexosaminidase and histamine release by human SMC (Figure 6H). FcϵRI aggregation was used as a positive control. FcγRIIA is therefore sufficient to activate human mast cells, and may be involved in the induction of mast cell–dependent inflammation and allergic reactions in humans.
FcγRIIA enables passive airway inflammation
Constriction of smooth muscles and subsequent granulocyte infiltration in the airways during asthmatic inflammation is thought to result from histamine and leukotriene release from mast cells after allergen inhalation. Thus, we wondered whether FcγRIIA may induce airway inflammation as it is expressed on mast cells and granulocytes. FcγRIIA is expressed in human lung tissue (Figure 7A), but also in lung sections (Figure 7B), and on alveolar macrophages (Figure 7C) from 3KOIIA mice. Unlike human asthma, most asthma models in mice are independent of antibody production by B cells,32,33 and consequently do not require FcRs. We therefore used a model of airway inflammation that depends on IgG and on FcγRs,34 and that consists of an IV injection of OVA and of an intranasal injection of anti-OVA rabbit serum, presumably forming ICs in vivo. Preformed OVA–anti-OVA rabbit serum ICs could bind to CHO cells expressing FcγRIIA, but not to untransfected CHO cells (Figure 7D). CD11c+/Gr1− alveolar macrophages represent more than 90% of the cells present in the alveolar space, as detected in broncho-alveolar lavages (BAL) of FcRγ−/−, FcRγ−/−IIA, and WT mice (Figure 7E). Concomitant intranasal instillation of anti-OVA rabbit serum and intravenous injection of OVA induced a massive infiltration of CD11c−/Gr1+ cells (> 80% of BAL content) in WT and in FcRγ−/−IIA mice, but not in FcRγ−/− mice (∼ 5% CD11c−/Gr1+ granulocytes; Figure 7F). FcγRIIA therefore induces granulocytes recruitment to the lung, and can replace endogenous FcRγ-associated activating FcRs. Total cell numbers in the BAL were unchanged at t = 3 hours after challenge, but increased starting t = 6 hours and reached 5 times the background value at t = 16 hours in FcRγ−/−IIA mice, but not in FcRγ−/− mice (Figure 7G). Granulocyte numbers in BAL represented most of this increase, whereas alveolar macrophage numbers did not vary statistically along the time course. Myeloperoxidase, which is mainly produced by neutrophils and by inflammatory macrophages in vivo,35,36 was detected at t = 16 hours postchallenge in FcRγ−/−IIA mice, but not in FcRγ−/− mice. (Figure 7H). Similar results were obtained when analyzing the hemorrhage score that reflects lung tissue damage (Figure 7I). KC, a chemokine produced by macrophages that can attract neutrophils to the site of inflammation, was found in BAL fluid of FcRγ−/−IIA and to a lesser extent in FcRγ−/− mice, as early as 3 hours after inoculation of antibody and antigen (Figure 7J). This result suggests that alveolar macrophages are activated after FcγRIIA aggregation by IgG-immune complexes, and release KC before neutrophil accumulation in the broncho-alveolar space, in agreement with the dependency on alveolar macrophages reported for this disease model.37 Supporting this hypothesis, purified alveolar macrophages from FcRγ−/−IIA mice, but not from FcRγ−/− mice, secreted KC ex vivo after IgG-IC or anti-FcγRIIA mAb stimulation (Figure 7K). Similar results were obtained when analyzing MIP-1α secretion (Figure 7L), suggesting that FcγRIIA-triggered alveolar macrophages contribute to chemokine-induced granulocyte recruitment to the lung. FcγRIIA can therefore induce airway inflammation characterized by granulocyte infiltration in a passive antibody-dependent mouse model.
FcγRIIA can induce acute airway inflammation. (A-B) Sections of (A) human lung or (B) lung from 3KO or 3KOIIA mice, stained with Hematoxilin (blue) and anti-FcγRIIA rabbit antiserum (red). (C) Representative expression of FcγRIIA on mouse alveolar macrophages (CD11c+/Gr1−) from 3KOIIA (open histogram) and 3KO (filled histogram) mice. (D) F(ab′)2 DAR-FITC staining of WT or FcγRIIA-expressing CHO transfectants incubated with preformed ICs made of OVA and rabbit anti-OVA antiserum (open histograms) or not (filled histograms). (E-F) Representative density plots of CD45+ BAL cells from indicated mice (E) left untreated or (F) injected with antigen intravenously and antiserum intranasally. Cell types were discriminated as alveolar macrophages (CD11c+/Gr1int, oval gate) and neutrophils (CD11c−/Gr1hi, rectangular gate). (G-J) Time course of (G) cell counts, (H) MPO level, (I) hemorrhage score, and (J) KC levels in BAL from indicated mice after injection with antigen intravenously and antiserum intranasally (n ≥ 3). (K) KC secretion or (L) MIP-1α secretion by purified alveolar macrophages from indicated mice incubated ex vivo on plate-bound rabbit IgG-ICs (OVA–anti-OVA) or IV.3 mAb. (G-L) Data are represented as mean ± SEM. (A-L) Data are representative from at least 2 independent experiments, and (J) data are a compilation of 2 experiments (*P < .05; **P < .01; ***P < .001).
FcγRIIA can induce acute airway inflammation. (A-B) Sections of (A) human lung or (B) lung from 3KO or 3KOIIA mice, stained with Hematoxilin (blue) and anti-FcγRIIA rabbit antiserum (red). (C) Representative expression of FcγRIIA on mouse alveolar macrophages (CD11c+/Gr1−) from 3KOIIA (open histogram) and 3KO (filled histogram) mice. (D) F(ab′)2 DAR-FITC staining of WT or FcγRIIA-expressing CHO transfectants incubated with preformed ICs made of OVA and rabbit anti-OVA antiserum (open histograms) or not (filled histograms). (E-F) Representative density plots of CD45+ BAL cells from indicated mice (E) left untreated or (F) injected with antigen intravenously and antiserum intranasally. Cell types were discriminated as alveolar macrophages (CD11c+/Gr1int, oval gate) and neutrophils (CD11c−/Gr1hi, rectangular gate). (G-J) Time course of (G) cell counts, (H) MPO level, (I) hemorrhage score, and (J) KC levels in BAL from indicated mice after injection with antigen intravenously and antiserum intranasally (n ≥ 3). (K) KC secretion or (L) MIP-1α secretion by purified alveolar macrophages from indicated mice incubated ex vivo on plate-bound rabbit IgG-ICs (OVA–anti-OVA) or IV.3 mAb. (G-L) Data are represented as mean ± SEM. (A-L) Data are representative from at least 2 independent experiments, and (J) data are a compilation of 2 experiments (*P < .05; **P < .01; ***P < .001).
Discussion
In this report, we provide evidence that human FcγRIIA contributes to IgG-mediated allergic reactions. Indeed, we demonstrate here for the first time that human FcγRIIA is sufficient to induce active and passive systemic anaphylaxis, cutaneous anaphylaxis, and lung inflammation in FcγRIIA-transgenic mice. Mast cells could be activated upon FcγRIIA engagement in vitro and were necessary for FcγRIIA-dependent PCA. Neither mast cells nor basophils, however, were mandatory for FcγRIIA-dependent PSA, which was induced by neutrophils and monocytes/macrophages. Finally, targeting FcγRIIA with specific blocking mAbs abolished passive and active anaphylaxis.
Human FcγRIIA has been described to contribute to several models of autoimmune diseases and inflammatory reactions in transgenic mice. When expressed on a WT background, ie, as an additional activating IgG receptor, FcγRIIA was reported to increase the severity of experimental thrombocytopenia,17 and to increase the incidence of autoimmune arthritis, pneumonitis, and glomerulonephritis at older age.18 These reports suggested that FcγRIIA may also induce inflammatory diseases in the absence of other FcRs. Indeed, when expressed on a mouse FcRγ−/− background, that is, in the absence of other activating IgG receptors, FcγRIIA was reported to induce experimental thrombocytopenia17 and hemolytic anaemia,38 rheumatoid arthritis,39 nephritis, and Arthus reaction.40 Along the same line, we show here that FcγRIIA induced IgG-dependent airway inflammation when expressed in FcRγ−/− mice. Inflammation was characterized by neutrophil infiltration of the broncho-alveolar space after KC (and MIP-1α) secretion, probably by FcγRIIA-triggered alveolar macrophages. In this passive model of airway inflammation, we also observed a trend toward increased metacholine-induced bronchial resistance as measured by plethysmography, in FcRγ−/−IIA but not FcRγ−/− mice (data not shown). In addition to its contribution to autoimmune disorders, FcγRIIA may therefore also contribute to allergic reactions. Polymorphisms in the gene encoding FcγRIIA have indeed been identified as risk factors for bronchial asthma and allergic rhinitis.16 Further supporting a role for FcγRIIA in allergic reactions, we show here that FcγRIIA restored immediate hypersensitivity reactions in resistant mice: (1) FcγRIIA was indeed sufficient to induce fatal ASA following 2 different immunization protocols; (2) FcγRIIA engagement by intravenously injected divalent or multivalent agonists induced PSA; and (3) FcγRIIA induced PCA when human IgG aggregates, mouse immune complexes or anti-FcγRIIA mAb IV.3 were injected intradermally. FcγRIIA can therefore reproduce by itself in a transgenic mouse model the allergic/anaphylactic pathologies reported to be triggered by the endogenous activating mouse FcγRs (FcγRI, FcγRIIIA, and FcγRIV), with similar severities and kinetics. Altogether these results, obtained in FcγRIIA-transgenic mice suggest that FcγRIIA might be a major player in allergic, autoimmune, and inflammatory pathologies mediated by IgG in humans.
Passive mouse models of inflammatory and allergic diseases have been reported to depend on specific cell types. Indeed, mast cells are required for PCA3 and IgE-PSA,5 macrophages for ITP,41 lung inflammation37 and passive rheumatoid arthritis,42 basophils for IgG1-PSA,7 and neutrophils for passive rheumatoid arthritis,43 ASA, and polyclonal IgG-PSA.1 All these myeloid cells express human FcγRIIA in transgenic mice (this paper and McKenzie et al17 ). Noticeably, FcγRIIA is the only activating (ITAM-bearing) IgG receptor expressed on these cells in humans, ie, on mast cells, neutrophils, eosinophils, and basophils. Of note, neutrophils, and to a much lower extent basophils,44 express FcγRIIIB, the activating capacities of which are debated.40,45 As a consequence, FcγRIIA should be able to activate in vivo all of the cell types reported to be necessary for the induction of models of inflammatory and allergic disease in mice. Mast cells were reported to be required for mouse FcγRIIIA-dependent and for mouse FcϵRI-dependent PCA.3,6 We demonstrate here that mast cells are also required for human FcγRIIA-dependent PCA using novel mast cell–deficient FcγRIIA-transgenic mice. Noticeably, mast cells were also reported to be required for IgE-induced PSA (mouse FcϵRI-dependent), but not for IgG1-induced PSA (mouse FcγRIIIA-dependent).5,6 Although basophils were proposed to be responsible for IgG1-induced PSA,7 basophil-deficient mice were, however, not protected.8 We reported recently that neutrophils are required for both IgG2-induced PSA and polyclonal IgG-induced PSA.1 Depending on the PSA model used, either mast cells, basophils, or neutrophils have therefore been reported to be mandatory for mouse FcR-dependent anaphylaxis.
We demonstrate here that neutrophils and monocytes/macrophages both contribute to FcγRIIA-dependent polyclonal IgG-PSA. Indeed, the depletion of both cell populations, but not of either one, was necessary to abolish the shock. Supporting these results, monocytes/macrophages and neutrophils contributed to another FcγRIIA-dependent PSA model, ie, when induced by IV injections of a high dose anti-FcγRIIA mAb. These results provide the first evidence that monocytes/macrophages contribute to a model of PSA. In agreement with IgG-induced PSA models in WT mice,1,6 mast cells were not mandatory for FcγRIIA-dependent PSA. Basophils did not detectably contribute to FcγRIIA-dependent PSA. This latter result could be explained by the lower expression level of FcγRIIA on basophils than on neutrophils or monocytes/macrophages in transgenic mice, as it is in humans.
Active models of allergic diseases in mice have been reported to depend on specific cell types. Indeed, monocytes/macrophages, but not neutrophils, have been reported to be mandatory for a model of ASA performed in mice immunized with goat IgG anti–mouse IgD, and challenged with goat IgG.9 Inversely, in a different model of ASA, performed in BSA-immunized mice challenged with BSA, we reported that neutrophils, but not monocytes/macrophages, were mandatory.1 In addition, we could show that the transfer of human neutrophils, which express FcγRIIA and FcγRIIIB, restored ASA in resistant mice. Because FcγRIIA, but not FcγRIIIB, binds mouse IgG-immune complexes, FcγRIIA is probably responsible for the activation of human neutrophils in this model. We could not address the contribution of neutrophils and basophils in FcγRIIA-dependent ASA because their depletion by specific mAbs is impaired on the FcRγ-deficient background. Indeed, whereas mouse FcγRs enabled efficient cell depletion using anti-Gr1, anti–Ly-6G, or anti-CD200R3 mAbs, human FcγRIIA did not. All of these mAbs are of the rat IgG2b isotype that is poorly bound by human FcγRIIA (data not shown). Nevertheless, one may speculate that monocytes/macrophages and neutrophils also contribute to FcγRIIA-dependent ASA, as they contribute to FcγRIIA-dependent PSA. Whatever the responsible cell population for FcγRIIA-dependent ASA, FcγRIIA is sufficient to promote the release of mediators leading to anaphylactic reactions. Depending on the immunization protocol, PAF was reported to be responsible,5,9 or to contribute partially1 to ASA. On one hand, PAF was found to be predominant in IgG1-induced PSA7 and we reported increased PAF levels in plasma during IgG2-induced PSA.1 On the other hand, histamine was found mandatory for IgG- and IgE-induced PCA using histidine decarboxylase-deficient mice46 as well as for IgE-induced PSA.7,47 It follows that FcγRIIA engagement should enable the release of PAF and/or of histamine by monocytes/macrophages, neutrophils, and mast cells. Supporting this line of reasoning, we report here that FcγRIIA-dependent cell activation in vitro induces human monocytes and neutrophils to produce PAF and human mast cells to release histamine. Whereas 3 to 5 times higher PAF amounts were produced by monocytes than by neutrophils, neutrophils are 10 times more numerous than monocytes in human blood, which may compensate their lower production of PAF. Intriguingly, activated monocytes but not neutrophils produced leukotrienes (ie, LTB4 and LTC4) which may also relate to anaphylaxis induction/severity in humans.
FcγRIIA is a nonconventional activating FcγR. Indeed, unlike all other activating FcγRs (FcγRI, FcγRIIIA, and FcγRIV in mice; FcγRI and FcγRIIIA in humans), FcγRIIA does not associate with the FcRγ-subunit, and contains an ITAM in its intracytoplasmic domain. The FcγRIIA ITAM is, however, noncanonical, as it is several amino acids longer and kinked because of the presence of proline residues.48 FcγRIIA has been considered less potent to activate cells than FcRs signaling through FcRγ-subunits as these subunits are expressed as dimers, thus providing 2 ITAMs per receptor. An elegant study using protein complementation49 and crystallographic data50 both suggest that FcγRIIA are expressed as noncovalent dimers. Thus, FcγRIIA and other activating FcγRs have the same number of ITAMs when expressed at the cell membrane. Whereas both types of receptors have the ability to induce intracellular calcium concentration increase,51 tyrosine phosphorylation events,52 and mediator release from granules,23,27 only the FcRγ ΙΤΑΜ was reported to enable antigen presentation and cytokine secretion13,14 upon receptor crosslinking. The inability of FcγRIIA to induce cytokine secretion after engagement has been contradicted by reports on human skin-derived mast cells27 or on human macrophages.53 Here, we detected chemokine (KC and MIP-1α) secretion by ex vivo alveolar macrophages, but failed to detect cytokine secretion by in vitro–derived murine mast cells (data not shown), after FcγRIIA engagement. Nevertheless, this latter stimulation led to the release of lipid mediators (LTC4, PGD2), granular mediators (histamine, β-hexosaminidase), calcium signaling, and to tyrosine phosphorylation of intracellular proteins. Among them, Syk, PLCγ1, Akt, Erk1/2, and SHIP1 were readily phosphorylated upon FcγRIIA engagement. These results correlate with previous reports showing that Syk and SHIP associate to FcγRIIA, are phosphorylated upon receptor aggregation,29,54 and that FcγRIIA requires PLCγ1 for calcium signaling.14 Phosphorylation of most intracellular signaling proteins was, however, less prominent after FcγRIIA engagement than after FcϵRI engagement. Similar results were reported when comparing FcγRIIA with FcγRI downstream signaling.14 Altogether, these data suggest that FcγRIIA enables cell activation through similar, although not identical, signaling pathways compared with those activated by FcRγ-associated FcRs. This may lead to differences in biologic outcome, in particular when considering cytokine production.
Finally, when considering its pattern of expression and its ability to activate myeloid cells, human FcγRIIA appears as a functional homolog of mouse FcγRIIIA. Noticeably, both receptors are the only activating FcγRs expressed on mast cells, basophils, and eosinophils in humans and mice, respectively. FcγRIIA is, however, coexpressed in humans with inhibitory FcγRIIB on basophils (L. Cassard and F.J., unpublished data, 2011), and on some monocytes and rare neutrophils.55 Because mouse and human FcγRIIB both inhibit cell activation by the same mechanism,56 and because human FcγRIIA is coexpressed with mouse FcγRIIB in FcRγ−/−IIA mice on basophils, monocytes, and neutrophils, we wondered if mouse FcγRIIB could negatively regulate human FcγRIIA-triggered PSA. We found that PSA was less profound in FcRγ−/−IIA mice (expressing mFcγRIIB) than in 3KOIIA (lacking mFcγRIIB; supplemental Figure 3A). Similar to mouse FcγRIIB, human FcγRIIB may therefore also negatively regulate FcγRIIA-triggered anaphylaxis in humans. In vivo, FcγRIIA demonstrated strong potential to activate myeloid cells and induce inflammatory and allergic pathologies in transgenic mice. Indeed, FcγRIIA engagement can activate neutrophils leading to nephritis40 or anaphylactic shock (this paper and Jönsson et al1 ). FcγRIIA engagement can also activate macrophages leading to rheumatoid arthritis,39 thrombocytopenia,17 lung inflammation, or anaphylactic shock and activate mast cells leading to PCA (this paper). Therefore, even though FcγRIIA may appear less efficient in vitro than other FcRγ-associated FcRs, its properties are sufficient for the induction of severe allergic, autoimmune and inflammatory pathologies in vivo. Targeting FcγRIIA with specific blocking molecules in inflammation and autoimmune/allergic reactions in humans might lead to similar inhibition as we reported recently for mouse FcγRIIIA in a murine model of rheumatoid arthritis,57 in PSA and in ASA.1 Supporting this assumption, we report here that blocking FcγRIIA protected transgenic mice from local and systemic anaphylaxis. Blocking FcγRIIA using divalent ligands (eg, mAb IV.3) to prevent allergic and autoimmune disease in humans, however, should not be envisioned, as we report here that high-doses of mAb IV.3 induced rather than prevented anaphylaxis. Small chemical entities, which prevent immune-complex binding to FcγRIIA, have proven efficient in a murine model of arthritis in FcγRIIA-transgenic mice,20 and may not induce these adverse effects. In conclusion, blocking FcγRIIA might be a potential approach for various allergic diseases, including non-IgE–mediated anaphylactic shocks that may be induced after FcγRIIA engagement on monocyte/macrophages and neutrophils.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank F. Hamano (Department of Lipidomics, Faculty of Medicine, The University of Tokyo, Tokyo, Japan) for help with lipid mediator measurements; O. Malbec, R. Peronet, and S. Mecheri for technical advice; and C. Detchepare (Institut Pasteur, Paris, France) for administrative help. They thank their colleagues for their generous gifts: M. P. Reilly, S. Verbeek, J.-P. Kinet (Harvard Institute of Medicine, Boston, MA), M. Lamers (MPII, Freiburg, Germany), D. Mathis, C. Benoist, and IGBMC (Illkirch, France) for mice; C. L. Anderson, R. Coffman, R. Good, H. Karasuyama, J. J. Lee, C. Leclerc and J. V. Ravetch, for antibodies. Cl2MDP was a gift of Roche Diagnostics GmbH.
This work was supported by the Institut Pasteur, Inserm, the Agence Nationale de la Recherche (ANR; grant GENOPAT-09-GENO-014-01), the Société Française d'Allergologie (SFA; Soutien de la recherche en allergologie 2010), and the Balsan company. F.J. is a recipient of a fellowship from the Fondation pour la Recherche Médicale (FRM). D.A.M. is a recipient of a fellowship from the Institut Pasteur (Bourse Roux). W.Z. and L.B.S. were funded in part by U19AI077435 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: F.J. performed experiments and designed part of the research; D.A.M. contributed to anaphylaxis experiments; W.Z. and L.B.S. performed analysis of human mast cells; Y.K. and T.S. performed analysis of lipid mediators; B.I. genotyped mice and produced essential reagents; H.K. performed histology; N.v.R. provided reagents; P.B., M.D., and F.J. analyzed results; P.B. designed and supervised the research; and P.B., with help from F.J. and M.D., wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Pierre Bruhns, PhD, Unité d'Allergologie Moléculaire et Cellulaire, Département d'Immunologie, Institut Pasteur, 25 rue du Docteur Roux, 75015 Paris, France; e-mail: bruhns@pasteur.fr.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal