In this issue of Blood, Borte et al describe a technique that expands the ability to detect primary immune deficiencies (PIDs) in newborns, particularly in the context of a screening program.1
Detection of PIDs is often easier for the laboratory technician than for the clinician. The disorders are rare, and most primary care physicians are likely to see at most 1 or 2 in their careers. Most infants have few signs or symptoms of disease before the development of infections. The infections that then occur are typically severe, often lethal, and always complicate treatment. The disease may go unrecognized even after the development of infections, as infants without PIDs may also suffer from severe infections. Without a family history to suggest a PID, which is rare, there is little chance the clinician will identify the disease early.2
Many PIDs are readily recognized and diagnosed using flow cytometry.3 Most SCID patients will have no T cells. Most X-linked agammaglobulinemia (XLA) patients will have no B cells. There is virtually no overlap with normal populations of infants, making these tests among the most sensitive and specific available in clinical practice. This is an ideal clinical situation for a newborn screening program.3 A treatable disorder that is difficult to detect clinically can be found by applying the screening test to all newborns at the time of birth. However, flow cytometry is far too expensive to use as a routine screening parameter for all infants, and the logistics of sample collection are difficult.
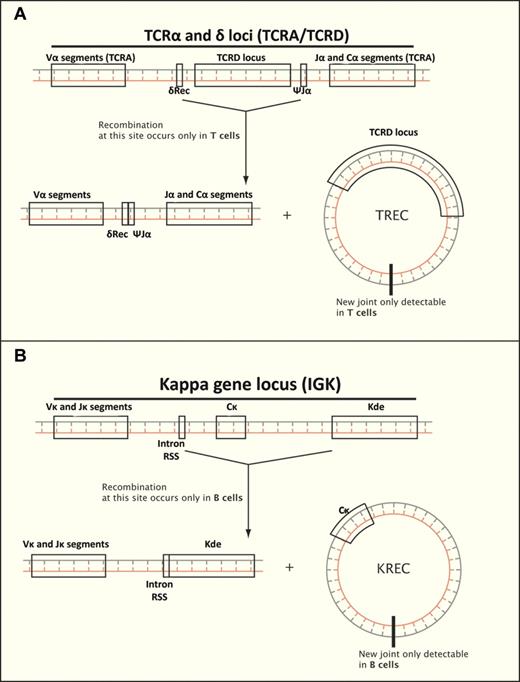
Screening of infants for SCID has already begun on a pilot basis in Michigan, Massachusetts, and California, with more states in the early process of implementation. These programs have used a screening method that detects T-cell receptor recombination excision circles (TRECs), an episomal byproduct of naive T-cell development, generated when the δ TCR genes are removed from the α TCR gene locus during the generation of a new αβ T cell (see figure).4 This genome-level rearrangement occurs nowhere else in the human body. Therefore, evidence that this rearrangement has taken place is prima facie evidence that naive T-cell development has occurred. The absence of TRECs indicates severe T-cell lymphopenia.5 Similar but distinct rearrangements occur in the B-cell receptor during the creation of the κ chain, the κ deleting recombination excision circle (KREC), creating the possibility to use these for detection of severe B-cell lymphopenia as well.6 The use of these rearrangements appears to be nearly as accurate as flow cytometry in detecting severe T- and B-cell lymphopenia, and additionally avoids the problems that might arise from maternal engraftment of T cells in the infant, because the maternal cells will have a much lower TREC number than an infant's own T cells.5
Rearrangements associated with antigen receptors occur only in T and B cells, and can be used to determine whether lymphocyte development has occurred. (A) Deletion of δ T-cell receptor genes results in a TREC, found only in T cells. (B) Deletion of κ constant region results in a KREC, found only in B cells.
Rearrangements associated with antigen receptors occur only in T and B cells, and can be used to determine whether lymphocyte development has occurred. (A) Deletion of δ T-cell receptor genes results in a TREC, found only in T cells. (B) Deletion of κ constant region results in a KREC, found only in B cells.
Borte et al extend these findings to a multiplexed test identifying low numbers of both KRECs and TRECs simultaneously. This allows detection of SCID and XLA simultaneously, but also appears to identify other important immune disorders as well, such as ataxia-telangiectasia (AT). These findings expand the potential reach of neonatal screening for PIDs beyond SCID, and would provide additional information about the nature of the immune deficiency at the time of newborn screening, permitting a more rapid response to the patient's problem.
This is a brave new world for primary immune deficiency, a field currently much more at home providing input on the nature of immune system function than considering the impact of their interventions on a public health and societal scale. Few immunologists have any particular expertise in public health interventions or the curious outcomes that can occur when an intervention is scaled up to apply to 300 million people.
For example, it is a common misconception that screening for such rare disorders as SCID and XLA could not possibly be cost-effective because they are uncommon and have expensive, although highly effective, therapies. In fact, it is relatively easy for screening for a rare disorder to be cost-effective, as long as the test is inexpensive and the benefit in healthy years of life saved is large.7,8 The cost of the test is the key driver of the expense of the intervention because it is applied broadly, while the cost of the therapy matters much less because it is applied infrequently.
The test reported here is inexpensive and the benefit large, but there is still some work to be done before expanding newborn screening in this direction can be recommended in an unqualified fashion. While it is easy to make the recommendation for SCID screening, the situation with XLA and AT is more complicated. While no one doubts that detecting XLA and AT is valuable, XLA is far less likely to be fatal or debilitating before being recognized than is SCID, so there is potentially more time for the clinical system to do its work at detecting disease before resorting to mass screening. And AT currently has no therapy beyond supportive care. Although very promising interventions are on the horizon, they are still on the horizon. There are other disorders detected on newborn screening without effective therapy, but they are found incidental to other disorders, and detecting them is not necessarily considered desirable.
The results of Borte et al also hint that there may be a large group of B-cell disorders that are unrecognized and of uncertain significance. Six of 2560 presumably healthy infants had abnormal screens. Many of these may have been premature infants, which have occasionally been an issue in TREC screening pilots, or they may have an undiagnosed and either important or trivial immune deficiency. The design of this study does not permit an answer to this critical question. At this rate, some screening programs would potentially be required to followup thousands of infants each year. Addressing this question is essential before the full deployment of expanded immune deficiency screening is possible.
As public health comes to immune deficiency, it brings a new way of thinking about our approach to care. Most work in immune deficiency to this point has appropriately focused on the understanding of biology, but newborn screening opens a new window on the field. Now we need to think just as carefully about the way we deliver care as we do about the biology underlying it.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal