Abstract
Diseases that cause hemolysis or myonecrosis lead to the leakage of large amounts of heme proteins. Free heme has proinflammatory and cytotoxic effects. Heme induces TLR4-dependent production of tumor necrosis factor (TNF), whereas heme cytotoxicity has been attributed to its ability to intercalate into cell membranes and cause oxidative stress. We show that heme caused early macrophage death characterized by the loss of plasma membrane integrity and morphologic features resembling necrosis. Heme-induced cell death required TNFR1 and TLR4/MyD88-dependent TNF production. Addition of TNF to Tlr4−/− or to Myd88−/− macrophages restored heme-induced cell death. The use of necrostatin-1, a selective inhibitor of receptor-interacting protein 1 (RIP1, also known as RIPK1), or cells deficient in Rip1 or Rip3 revealed a critical role for RIP proteins in heme-induced cell death. Serum, antioxidants, iron chelation, or inhibition of c-Jun N-terminal kinase (JNK) ameliorated heme-induced oxidative burst and blocked macrophage cell death. Macrophages from heme oxygenase-1 deficient mice (Hmox1−/−) had increased oxidative stress and were more sensitive to heme. Taken together, these results revealed that heme induces macrophage necrosis through 2 synergistic mechanisms: TLR4/Myd88-dependent expression of TNF and TLR4-independent generation of ROS.
Introduction
The term programmed cell death was used for many years as a synonym of apoptosis, whereas necrosis in the opposite extreme was considered an abrupt and uncontrolled type of cell death. However, recent evidence clearly shows that several nonapoptotic cell death modes including autophagy, pyroptosis, and necrosis also involve elaborate molecular circuitry.1,2 This scenario was originally revealed in a study showing that depending on the cell type, tumor necrosis factor (TNF) could trigger different cellular fates including survival, apoptosis, and necrosis.3 On blockage of protein synthesis or NF-κB, activation of death cytokine receptors of the TNF superfamily triggers caspase-dependent apoptosis, whereas simultaneous inhibition of caspase reorients the cell death to necrosis.4-7 Receptor-interacting protein 1 (RIP1, also known as RIPK1) regulates survival and cell death fates. Mice deficient in Rip1 present extensive apoptosis, dying early after birth. The increased sensitivity to TNF-mediated cell death in Rip1−/− cells correlates with a failure to activate NF-κB.8 Recent work shows that necrotic cell death is highly regulated by the RIP1 and RIP3 kinases (also known as RIPK3).6,7,9-11 Programmed necrosis can be initiated by several stimuli including DNA damage, oxidative stress, infection, and activation of pattern recognition receptors.1,2,12-17
Intra or extra vascular hemolysis, rhabdomyolysis, and extensive cell damage cause the release of large quantities of hemeproteins. The oxidation of some hemeproteins including hemoglobin and myoglobin can release the heme moiety promoting further oxidation and cellular stress.18 Highly cytotoxic free heme is removed by plasma proteins such as hemopexin and albumin, and subsequently catabolized by heme oxygenase (HO-1); this leads to generation of CO, iron, and biliverdin, which is then converted to bilirrubin.18 Exposure of astrocytes to heme, as a model of hemorrhagic injury, causes cell death with characteristics of programmed necrosis including the loss of plasma membrane integrity and inhibition by necrostatin-1 and antioxidants.19 In endothelial cells, heme causes a cell death with morphologic and biochemical characteristics of apoptosis.20 Heme also sensitizes several nonhematopoietic cell types to undergo cell death triggered by TNF.21 This cell death has the hallmark of apoptosis including membrane blebbing, caspase activation, nuclear shrinking, and fragmentation, as well as formation of apoptotic bodies and chromatin condensation.21 Interestingly, treatment with a pan caspase inhibitor did not fully rescue cell death induced by heme and TNF, suggesting that at least some of the cells are not dying by a caspase-dependent cell death. The cytotoxic effect of heme is counteracted by HO-1. Heme is an inducer of HO-1 and several studies demonstrated that HO-1 protects cells from death.22-27 The cytoprotective effect of HO-1 has been attributed to its essential role in controlling heme levels as well as iron homeostasis and oxidative stress.18 Mice deficient in HO-1 (Hmox1−/−) exhibit iron overload, increased cell death, and tissue inflammation.23,27,28 Cultured embryonic fibroblasts from Hmox1−/− mice had increased oxidative stress and were more sensitive to heme and H2O2.23 Moreover, Hmox1−/− mice have reduced macrophage numbers in the spleen and liver because of cell death.27 This increased macrophage cell death was confirmed in vitro on erythrophagocytosis, suggesting that exposure to heme is the cause of the increased macrophage death observed in Hmox1−/− mice in vivo.
Reactive oxygen species (ROS) affect cellular physiology in multiple ways. In programmed necrosis, ROS can trigger death, act as second messengers in the signaling pathways of death receptors, or as effectors capable of promoting peroxydation of lipids, proteins and DNA.1,2 The generation of free radicals by the Fenton reaction has been considered the major form of ROS generation by heme and an important mechanism of heme-induced cytotoxicity.18,21,29,30 However, we observed that generation of ROS by heme also relies on activation of specific signaling pathways.31,32 Heme affects cytokine and lipid mediator production by macrophages, in part through superoxide dismutase and 5-lypoxigenase.33,34 Moreover, heme activates Toll-like receptor 4 (TLR4)–induced TNF production in macrophages.35 Interestingly, heme induces ROS in a TLR4-independent manner. Hence, heme induces macrophage necrosis through 2 distinct and complementary signaling pathways.
Methods
Reagents
Heme was purchased from Frontier Scientific. It was dissolved in NaOH (0.1M) diluted in RPMI and filtered just before. Stock solutions of porphyrins were prepared in the dark to avoid free radical generation. LPS 0111:B4 from Escherichia coli was obtained from Invivogen. Recombinant mouse TNF was obtained from Peprotech. Apocynin, N-acetyl-L-cysteine (NAC), deferoxamine, N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone (zVAD-fmk), SP600125 (JNK-inhibitor) were obtained from Sigma-Aldrich. The 5-(and-6)-chloromethyl-2′-7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) was obtained from Molecular Probes. The 5-(Indol-3-ylmethyl)- (2-thio-3-methyl) hydantoin (Necrostatin-1) was obtained from Enzo Life Sciences. RPMI 1640 medium, penicillin-streptomycin, and FCS were from Invitrogen.
Macrophages and NIH3T3 Rip1-deficient fibroblasts
C57BL/6 (WT) and Tlr4−/− were obtained from the animal facility at the Universidade Federal do Rio de Janeiro (Rio de Janeiro, Brasil). C57BL/6, Myd88−/−, Trif−/− and Rip3−/− were obtained from the animal facility at the University of Massachusetts Medical School. Tibias and femurs of WT and Hmox1−/− were obtained from the animal facility at the Instituto Gulbenkian de Ciência (Oeiras-Portugal). Peritoneal exudate cells were harvested by lavage from C57BL/6 mice (4-6 weeks) 4 days after intraperitoneal injection of 2 mL of 3% thioglycollate (VETEC). Briefly, cells were collected by lavage with 5 mL of cold RPMI, washed twice by centrifugation at 400g for 10 minutes at 4°C, and suspended in RPMI with 10% FCS. Cell viability was consistently > 95% in trypan blue exclusion test. Peritoneal macrophages were plated in 96 tissue culture plates at 2 × 105 cells per well in medium (RPMI plus 10% FCS). Cells were kept at a 2 hour incubation time at 37°C 5% CO2 and nonadherent cells were removed by washing with RPMI. The remaining adherent cells, 98% macrophages as determined by FACS staining, after overnight incubation at RPMI 10% FCS, 37°C in 5% CO2 were stimulated in RPMI or RPMI plus 10% FCS. Bone marrow-derived macrophages (BMM) were prepared using tibia and femur from 6- to 8-week-old mice. Bone marrow was obtained flushing bones with 5 mL of cold sterile RPMI. The differentiation medium was RPMI supplemented with 20% heat-inactivated FCS and 30% L929 cell supernatant. Initially, bone marrow cells were resuspended in 10 mL of differentiation medium at an initial density of 0.5 × 106 cells/mL, then seeded in 100 mm petri dish (BD Biosciences) at 37°C in humidified 5% CO2 for 3 days and added more 10 mL of differentiation medium. After 4 days, cells were washed with cold RPMI, resuspended, counted, and seeded at a density of 106 cells/mL for all experiments. Before any experiments, cells were cultivated for 24 hours. The experiments were approved by the UFRJ institutional animal welfare committee (protocol IMPPG-011).
LDH-based toxicity assay
To measure plasma membrane integrity, we assayed serum lactate dehydrogenase (LDH) levels and calculated percentages of LDH release to the medium. LDH activity was measured spectrophotometrically using a commercial kit (Doles) or LDH Cytotoxicity Detection Kit (Promega). After stimulation, 25 μL of the supernatant were transferred to an enzymatic assay plate and 100 μL of LDH substrate plus 5 μL ferric alum were added and incubated for 3 minutes at 37°C, protected from light. We added 6 μL of NAD and incubated for 6 minutes at 37°C, protected from light. The reaction was stopped with 100 μL of stop solution and the absorbance was recorded at 490 nm using a microELISA plate reader (490 nm). The percentage of LDH release was calculated by ([LDH] sample × 100)/total [LDH]. [LDH] sample was the LDH level of the sample (released in medium) and total [LDH] was the LDH content in the wells after addition of lysis solution (Triton 0.9%).
Transmission electron microscopy
Cells that adhered to culture flasks were fixed for 60 minutes at room temperature in 2.5% glutraraldehyde, 5mM CaCl2, 3.7% sucrose, in 0.1M cacodylate buffer, pH 7.2. After washing in the same buffer, cells were gently scraped off with a rubber policeman, centrifuged, and postfixed with 1% OsO4, 0.8% potassium ferricianide, 5mM CaCl2 in 0.1M cacodylate buffer. Samples were washed twice in the same buffer, dehydrated in acetone and embedded in PolyBed 812. Ultrathin sections were observed stained with uranyl acetate and lead citrate and observed in a FEI Morgagni transmission electron microscope operating at 80 kV.
Measurement of intracellular ROS
The determination of intracellular oxidative formation was based on the oxidation of 2μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) to yield an intracellular-trapped fluorescent compound. Cells were left untreated or were pretreated with FCS, NAC, apocynin, deferoxamine, or SP600125 and stimulated with heme for 60 minutes. After the treatments, macrophages or fibroblasts (106 cells/tube) were incubated with CM-H2DCFDA for 30 minutes at 37°C with 5% CO2. Cytosolic ROS was measured by flow cytometry using FACScan flow cytometer (BD Biosciences).
Western blotting
Thioglycolate-elicited macrophages were stimulated with 30μM heme in serum-free RPMI 1640 medium for the indicated times. Cells were harvested by scraping, washed with cold phosphate-buffered saline (PBS), and lysed in RIPA buffer (150mM NaCl, 20mM Tris-Cl pH 8.0, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate and 1.0% NP-40 supplemented with 1× complete protease inhibitors (Roche) and 5mM iodoacetamide) for 15 minutes with rotation at 4°C. Cell lysates were spun down at 12 000g for 10 minutes. The resulting cell lysates (50 μg) were resolved by SDS-PAGE. Western blots were performed with antibodies against RIP3 (ProSci) and RIP1 (BD Pharmingen). For phosphatase treatment, cell lysates were incubated with 1000 or 30 units of Lambda phosphatase (λPP) and calf intestinal alkaline phosphatase (CIP), respectively, at 37°C for 3 hours. For the analysis of phosphorylated JNK on stimulation with heme, macrophages were treated with lysis buffer (50mM Tris-HCl, pH 7.6, 150mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1mM EDTA, 1mM PMSF, 5 mg/mL aprotinin, 5 mg/mL leupeptin, 5 mg/mL pepstatin, 1mM NaF, 1mM sodium orthovanadate) on ice for 10 minutes. Four-fold concentrated SDS sample buffer was added to cell lysates (50 μg), boiled for 5 minutes, and subjected to electrophoresis on a 10% SDS-polyacrylamide gel, and then transferred onto a nitrocellulose sheet. Protein molecular weight standards (Bio-Rad) were run concurrently. The sheet was blocked in Tris-buffered saline (TBS) with Tween 20 (0.1%) containing 3% bovine serum albumin (BSA) for 1 hour at room temperature (RT). Blots were then probed 48 hours at 4°C with rabbit anti-phosphoJNK (1:1000). Bound primary antibody was detected using goat anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology), diluted 1:5000, followed by incubation with the chemiluminescent substrate Luminol (Santa Cruz Biotechnology) and the membrane was exposed to X-ray film (Kodak). The same membrane was probed to detect the loading control protein β-actin with rabbit anti–β-actin (1:1000; Sigma-Aldrich).
Measurement of TNF production
The concentration of TNF in each sample was determined using a commercially available kit from Peprotech performed according to the manufacturer's instructions.
Statistical analysis
Differences between the groups were analyzed using a student t test or 1-way ANOVA with Newman-Keuls multiple comparison test (Prism 5.0). Values were expressed as means ± SEM and differences between values were considered significant at P ≤ .05.
Results
Heme induces cell Death in macrophages
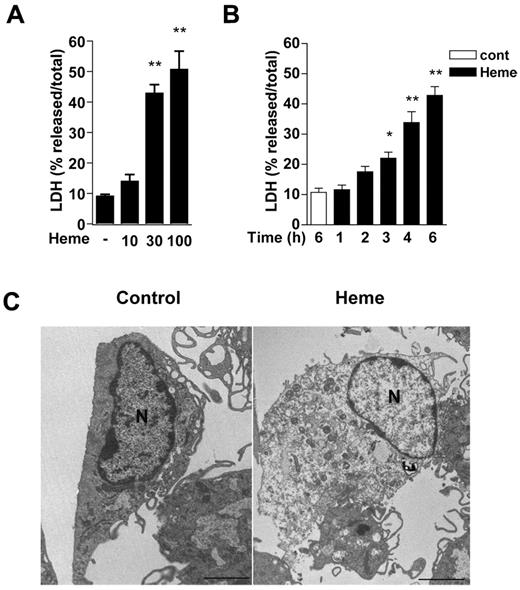
We treated macrophages with increasing concentrations of heme to determine its cytotoxic mechanisms. Heme at 30μM and 100μM were cytotoxic to macrophages, as determined by the release of LDH in the supernatants of cells (Figure 1A). This cytotoxic effect of heme was observed after 3 hours of treatment and peaked at 6 hours (Figure 1B). Heme-induced cell death was confirmed using MTT and trypan blue assays (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Moreover, heme-treated macrophages exhibited plasma membrane rupture, formation of numerous cytoplasmic vacuoles, swelling organelles, and intact nuclei membrane (Figure 1C). These results indicate that heme caused an early macrophage cell death with loss of plasma membrane integrity and morphologic features characteristic of necrosis.
Heme causes macrophage cell death with early loss of plasma membrane integrity and morphologic features of necrosis. Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were left untreated or stimulated with heme in the absence of serum. Macrophage cell death was dependent on heme concentrations (μM) at 6 hours after stimulation (A), and occurs as early as 3 hours after treatment with heme at 30μM (B). After the indicated time of stimulation with heme, the supernatants were collected for LDH determination. Data are representative of 5 independent experiments performed in triplicates and represent mean ± SEM (*P < .01; **P < .001 compared with control group). (C) Representative transmission electron micrographs of control and heme-treated macrophages (30μM) after 6 hours of stimulation. Scale bars = 2 μm.
Heme causes macrophage cell death with early loss of plasma membrane integrity and morphologic features of necrosis. Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were left untreated or stimulated with heme in the absence of serum. Macrophage cell death was dependent on heme concentrations (μM) at 6 hours after stimulation (A), and occurs as early as 3 hours after treatment with heme at 30μM (B). After the indicated time of stimulation with heme, the supernatants were collected for LDH determination. Data are representative of 5 independent experiments performed in triplicates and represent mean ± SEM (*P < .01; **P < .001 compared with control group). (C) Representative transmission electron micrographs of control and heme-treated macrophages (30μM) after 6 hours of stimulation. Scale bars = 2 μm.
Cell death induced by heme requires TNF receptor 1 (TNFR1) and TLR-4/MyD88-dependent TNF
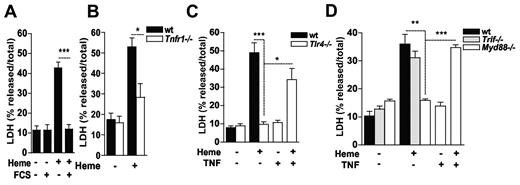
TNF can trigger both apoptosis as well as necrosis.1-4 We have previously shown that heme induces TNF production by macrophages in the absence of serum in a TLR4-dependent manner.35 The presence of serum abolished the cytotoxic effect of heme (Figure 2A). The putative role of heme-induced TNF in macrophage cytotoxicity was analyzed with the use of macrophages from Tnfr1−/− mice. We observed a significant reduction of heme-induced macrophage cell death in Tnfr1−/− cells (Figure 2B). Moreover, Tlr4−/− macrophages also exhibited reduced cytotoxic response to heme (Figure 2C). Addition of recombinant murine TNF to heme-treated Tlr4−/− macrophages restored cell death. By contrast, neither heme nor TNF alone were cytotoxic to Tlr4−/− macrophages. Activation of TLR4 by LPS signals through both TRIF- and MyD88-dependent pathways.36 Trif−/− macrophages were sensitive to the cytotoxic effects of heme whereas macrophages from Myd88−/− mice were resistant (Figure 2D). Inclusion of recombinant TNF reversed the resistance of Myd88−/− macrophages to heme. Together, these results indicate that heme exerts 2 synergistic responses to induce macrophage death: (1) It induces TNF secretion in a TLR4/MyD88-dependent manner, and (2) sensitizes macrophages to TNF-induced death in a TLR4/MyD88-independent manner.
Macrophage cell death induced by heme is dependent on autocrine TNF production and TNFR1 signaling. (A) Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were treated for 6 hours with heme (30μM) in the absence or in the presence of 10% FCS. Bone marrow-derived macrophages (2 × 105/well) from WT and Tnfr1−/− (B), WT and Tlr4−/− (C), WT, Trif−/− and Myd88−/− (D) were left untreated (−) or stimulated with heme at 30μM and/or TNF at 0.5 ng/mL (+) for 6 hours and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001).
Macrophage cell death induced by heme is dependent on autocrine TNF production and TNFR1 signaling. (A) Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were treated for 6 hours with heme (30μM) in the absence or in the presence of 10% FCS. Bone marrow-derived macrophages (2 × 105/well) from WT and Tnfr1−/− (B), WT and Tlr4−/− (C), WT, Trif−/− and Myd88−/− (D) were left untreated (−) or stimulated with heme at 30μM and/or TNF at 0.5 ng/mL (+) for 6 hours and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001).
RIP proteins are crucial to the cytotoxic effect of heme
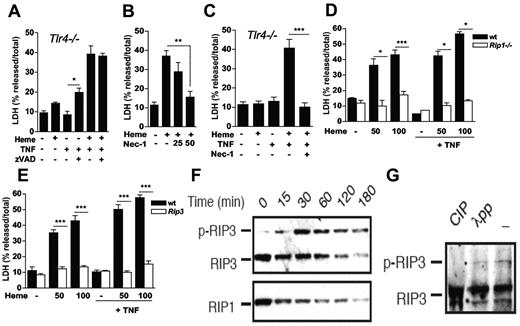
To gain insight into the mechanism of heme-induced cell death, we treated macrophages with zVAD-fmk, a pancaspase inhibitor that protect cells from apoptosis.4 Treatment with zVAD-fmk did not protect Tlr4−/− macrophages against heme plus TNF-induced cell death (Figure 3A). In contrast, treatment of WT macrophages with Nec-1 protected macrophages from heme-induced cell death (Figure 3B). We also treated Tlr4−/− macrophages with Nec-1 and found that it protected macrophages from heme plus TNF-induced death (Figure 3C). To confirm the involvement of RIP1 on heme cytotoxicity, we used NIH3T3 Rip1−/− fibroblasts.8 Although heme caused cell death in a dose-dependent manner in WT fibroblasts, Rip1−/− cells were resistant to the cytotoxic effects of heme (Figure 3D). The cotreatment with TNF did not reverse the resistance of Rip1−/− cells to heme. RIP3 has been recently shown to be essential in the signaling pathway triggered by TNF leading to cell death.9-11 The lack of RIP3 protected macrophages from heme-induced cell death and Rip3−/− macrophages were also resistant to exogenous TNF plus heme (Figure 3E). During programmed necrosis, RIP1 and RIP3 form a tight complex that results in RIP3 phosphorylation.9 Heme induced the appearance of a slow migrating RIP3 signal on Western blot as early as 30 minutes of stimulation (Figure 3G). Treatment with CIP resulted in disappearance of this slow migrating RIP3 signal, indicating that it is indeed phosphorylated RIP3 (Figure 3H). Total cellular level of RIP1, and to a lesser extent RIP3, was reduced over time in response to heme. Taken together, these results show that heme induced RIP1/RIP3-dependent programmed necrosis in peritoneal macrophages.
Heme-induced cell death requires the RIP proteins. (A) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or stimulated with heme at 30μM, z-VAD-fmk at 10μM and/or TNF at 0.5 ng/mL (+) for 6 hours and supernatants were collected for LDH determination. (B) Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were treated for 6 hours with heme at 30μM (+) in the presence or absence of necrostatin-1 (Nec-1) at 25 and 50μM. (C) Experiment performed as in (A) with Nec-1 at 50μM. (D) Wt and Rip1−/− NIH-3T3 fibroblasts and (E) peritoneal macrophages from WT and Rip3−/− were left untreated (−) or stimulated with heme at 50 or 100μM in the absence or presence of TNF at 0.5 ng/mL for 6 hours and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001). Heme induced RIP3 phosphorylation. (F) Peritoneal macrophages from C57BL/6 mice were stimulated with heme (30μM) for the indicated times. (G) Cell lysates from samples treated with heme for 60 minutes were treated with CIP or lambda phosphatase (λPP) for 3 hours. Whole cell extracts were submitted to SDS-PAGE and RIP3 phosphorylation and RIP1 were detected by immunoblotting.
Heme-induced cell death requires the RIP proteins. (A) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or stimulated with heme at 30μM, z-VAD-fmk at 10μM and/or TNF at 0.5 ng/mL (+) for 6 hours and supernatants were collected for LDH determination. (B) Peritoneal macrophages (2 × 105/well) from C57Bl/6 mice were treated for 6 hours with heme at 30μM (+) in the presence or absence of necrostatin-1 (Nec-1) at 25 and 50μM. (C) Experiment performed as in (A) with Nec-1 at 50μM. (D) Wt and Rip1−/− NIH-3T3 fibroblasts and (E) peritoneal macrophages from WT and Rip3−/− were left untreated (−) or stimulated with heme at 50 or 100μM in the absence or presence of TNF at 0.5 ng/mL for 6 hours and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001). Heme induced RIP3 phosphorylation. (F) Peritoneal macrophages from C57BL/6 mice were stimulated with heme (30μM) for the indicated times. (G) Cell lysates from samples treated with heme for 60 minutes were treated with CIP or lambda phosphatase (λPP) for 3 hours. Whole cell extracts were submitted to SDS-PAGE and RIP3 phosphorylation and RIP1 were detected by immunoblotting.
Essential role of ROS to heme-induced cell death
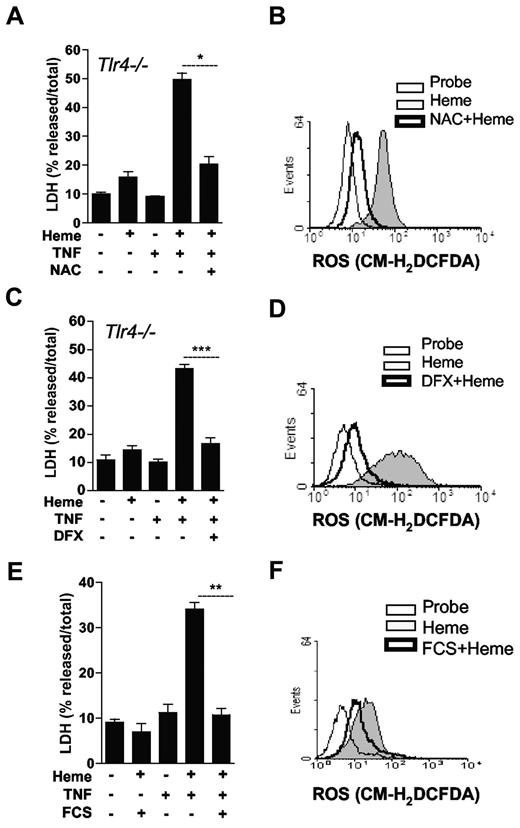
ROS is considered essential to several biologic effects of heme. We recently observed that heme induces ROS in a TLR4-independent manner.35 To characterize the involvement of ROS on heme-induced cell death, we treated macrophages with inhibitors of ROS generation. To bypass a possible inhibitory effect of antioxidants on heme-induced TNF production, we used macrophages from Tlr4−/− mice and added recombinant TNF to the cell cultures. Treatment with NAC blocked macrophage cell death induced by heme plus TNF (Figure 4A) and inhibited heme-induced ROS generation (Figure 4B). Similarly, iron chelation using deferoxamine abrogated cell death induced by heme plus TNF (Figure 4C), and reduced the generation of ROS induced by heme (Figure 4D). Addition of TNF did not revert the protective effect of serum on heme-induced macrophage death (Figure 4E). To determine whether the protective effect of serum was because of inhibition of ROS generation, we measured the production of ROS in the presence and absence of serum. The presence of 10% serum caused a significant reduction of ROS (Figure 4F). Considering the critical role of ROS in the heme-induced cell death and the participation of RIP1 on ROS generation induced by TNF,37,38 we tested whether Nec-1 affected the generation of ROS induced by heme. Treatment with Nec-1 did not interfere with the generation of ROS by heme (supplemental Figure 2A). We also used Rip1−/− cells to determine the role of RIP proteins on heme-induced ROS. Similar to treatment of WT cells with Nec1, heme-induced ROS production was not affected by the absence of Rip1 (supplemental Figure 2B). These results indicate that ROS and RIP proteins are essential for heme-induced cell death.
ROS are essential to the cytotoxic effect of heme. (A-C) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or pretreated for 1 hour with NAC at 10mM or pretreated for 2 hours with deferoxamine (DFX) at 2mM, stimulated for 6 hours with heme at 30μM, and/or TNF at 0.5 ng/mL (+) and supernatants were collected for LDH determination. (B-D) Peritoneal macrophages (106/mL) were pretreated as in (A-C) and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). (E) Macrophages from C57Bl/6 mice were left untreated or stimulated with heme at 30μM, and/or TNF at 0.5 ng/mL in the presence or absence of FCS at 10%. After 6 hours supernatants were collected for LDH determination. (F) Peritoneal macrophages (106/mL) were stimulated for 1 hour with heme (30μM) in the absence or presence of FCS at 10% and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ 0,05; ***P ≤ .0001).
ROS are essential to the cytotoxic effect of heme. (A-C) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or pretreated for 1 hour with NAC at 10mM or pretreated for 2 hours with deferoxamine (DFX) at 2mM, stimulated for 6 hours with heme at 30μM, and/or TNF at 0.5 ng/mL (+) and supernatants were collected for LDH determination. (B-D) Peritoneal macrophages (106/mL) were pretreated as in (A-C) and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). (E) Macrophages from C57Bl/6 mice were left untreated or stimulated with heme at 30μM, and/or TNF at 0.5 ng/mL in the presence or absence of FCS at 10%. After 6 hours supernatants were collected for LDH determination. (F) Peritoneal macrophages (106/mL) were stimulated for 1 hour with heme (30μM) in the absence or presence of FCS at 10% and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ 0,05; ***P ≤ .0001).
Protective role of HO-1 against heme cytotoxic effect
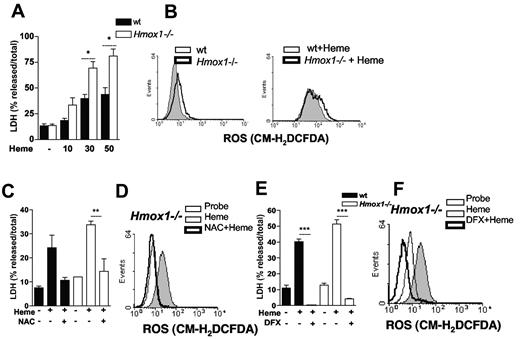
The cytoprotective effect of HO-1 is in part because of its ability to catabolize heme and to reduce cellular oxidative stress.18 Exposure of Hmox1−/− macrophages to heme caused higher level of cell death compared with WT macrophages (Figure 5A). For example, 90% of LDH was released into the culture media of Hmox1−/− macrophages treated with 50μM heme compared with 40% in similarly treated WT macrophages. Hmox1−/− macrophages produced higher amounts of ROS both in resting conditions as well as on stimulation with heme (Figure 5B). NAC, deferoxamine, or serum, protected Hmox1−/− macrophages from heme-induced cell death (Figures 5C-E; supplemental Figure 3A). The protection observed with these treatments correlated with a significant inhibition of heme-induced ROS on Hmox1−/− macrophages (Figure 5D-F; supplemental Figure 3B). These results suggest that iron-dependent ROS generation is essential to the cytotoxic effect of heme, and that HO-1 protects macrophages from heme-induced cytotoxicity through inhibition of ROS generation.
HO-1 protects against heme-induced macrophage cell death. (A) BMM (2 × 105/well) from WT and Hmox1−/− mice were left untreated (−) or stimulated with increasing concentrations of heme (μM) for 6 hours and supernatants were collected for LDH determination. (B) BMM (106/mL) from WT and Hmox1−/− mice were pretreated as in panel A and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). (C) BMM from WT and Hmox1−/− mice were pretreated with NAC at 10mM for 1 hour and stimulated with heme (30μM) for 6 hours and supernatants were collected for LDH determination. (D) BMM macrophages (106/mL) from WT and Hmox1−/− mice were pretreated as in panel C and stimulated for 1 hour with heme (30μM) and ROS generation was determined. (E) BMM from WT and Hmox1−/− mice were pretreated with deferoxamine (DFX) at 2mM for 2 hours and stimulated with heme (30μM) for 6 hours and supernatants were collected for LDH determination. (F) BMM macrophages (106/mL) from WT and Hmox1−/− mice were pretreated as in panel E and stimulated for 1 hour with heme (30μM) and ROS generation was determined. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001).
HO-1 protects against heme-induced macrophage cell death. (A) BMM (2 × 105/well) from WT and Hmox1−/− mice were left untreated (−) or stimulated with increasing concentrations of heme (μM) for 6 hours and supernatants were collected for LDH determination. (B) BMM (106/mL) from WT and Hmox1−/− mice were pretreated as in panel A and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated by flow cytometry using the probe CM-H2DCFDA (2μM). (C) BMM from WT and Hmox1−/− mice were pretreated with NAC at 10mM for 1 hour and stimulated with heme (30μM) for 6 hours and supernatants were collected for LDH determination. (D) BMM macrophages (106/mL) from WT and Hmox1−/− mice were pretreated as in panel C and stimulated for 1 hour with heme (30μM) and ROS generation was determined. (E) BMM from WT and Hmox1−/− mice were pretreated with deferoxamine (DFX) at 2mM for 2 hours and stimulated with heme (30μM) for 6 hours and supernatants were collected for LDH determination. (F) BMM macrophages (106/mL) from WT and Hmox1−/− mice were pretreated as in panel E and stimulated for 1 hour with heme (30μM) and ROS generation was determined. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (*P ≤ .05; **P ≤ .001; ***P ≤ .0001).
JNK is involved in the cytotoxic effect of heme
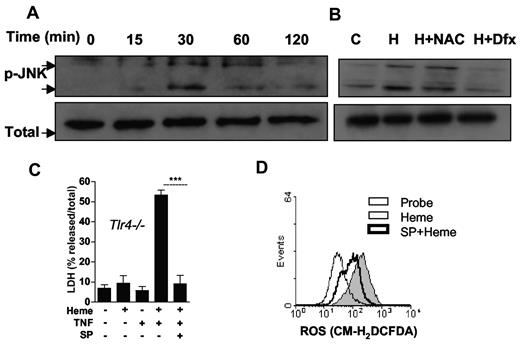
It has been demonstrated that JNK is activated by ROS, and that JNK in turn controls ROS generation. In addition, JNK plays an important role in programmed necrosis.2,39-41 Treatment of macrophages with heme induced JNK phosphorylation that peaked after 30 minutes (Figure 6A). Importantly, NAC and deferoxamine inhibited the heme-induced JNK phosphorilation (Figure 6B). We therefore tested the role of JNK in heme-induced cell death using macrophage cultures from Tlr4−/− mice supplemented with TNF. Treatment with a selective inhibitor of JNK abrogated the cell death induced by heme plus TNF (Figure 6C). Moreover, inhibition of JNK caused a significant reduction of ROS generation induced by heme (Figure 6D). These results suggest that ROS facilitates JNK activation in a positive feedback manner to promote macrophage cell death.
JNK phosphorylation induced by heme is involved in macrophage cell death. (A) Macrophages from C57Bl/6 mice were stimulated with heme (30μM) in the time intervals indicated. (B) Macrophages were left untreated or pretreated for 1 hour with NAC at 10mM or 2 hours with DFX at 2mM and stimulated with heme (30μM). Cell extracts were submitted to SDS-PAGE and JNK phosphorylation was detected by immunoblotting. Detection of β-actin was used as loading control. (C) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or pretreated for 1 hour with SP600125 20μM and stimulated for 6 hours with heme at 30μM, and/or TNF at 0.5 ng/mL (+) and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (***P ≤ .0001). (D) Macrophages (106/mL) were pretreated as in (C) and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated. The results are representative of 3 different experiments with similar results.
JNK phosphorylation induced by heme is involved in macrophage cell death. (A) Macrophages from C57Bl/6 mice were stimulated with heme (30μM) in the time intervals indicated. (B) Macrophages were left untreated or pretreated for 1 hour with NAC at 10mM or 2 hours with DFX at 2mM and stimulated with heme (30μM). Cell extracts were submitted to SDS-PAGE and JNK phosphorylation was detected by immunoblotting. Detection of β-actin was used as loading control. (C) BMM (2 × 105/well) from Tlr4−/− mice were left untreated (−) or pretreated for 1 hour with SP600125 20μM and stimulated for 6 hours with heme at 30μM, and/or TNF at 0.5 ng/mL (+) and supernatants were collected for LDH determination. Data are representative of 3 independent experiments performed in triplicates and represent mean ± SEM (***P ≤ .0001). (D) Macrophages (106/mL) were pretreated as in (C) and stimulated for 1 hour with heme (30μM) and ROS generation was evaluated. The results are representative of 3 different experiments with similar results.
Discussion
In this study, we analyzed the mechanisms involved in heme-induced macrophage cell death. Heme caused a dose and time-dependent macrophage cell death with an early increase of LDH in the supernatants and morphologic features of necrosis. The coordinated nature of this necrotic cell death was revealed by the following facts: the lack of serum or the genetic deficiency of Hmox-1 increased the susceptibility to heme, whereas TNF, RIP proteins (RIP1 and RIP3), ROS, and JNK but not caspases, were all required for heme-induced cell death. Together, these results indicate that heme causes programmed necrosis of macrophages.
During hemolytic and hemorrhagic disorders the concentrations of heme can reach up to 50μM in the circulation or even higher concentrations in restricted tissue areas.18 We observed significant cytotoxicity at 30μM of heme in macrophages that was abrogated by serum. Heme also induced TNF production at these concentrations in the absence of serum.35 Serum contains hemopexin, albumin, and lipoproteins that are scavengers of heme.18 A recent study demonstrated that hemopexin inhibits the sensitizing effect of heme to TNF-induced hepatocyte cell death.42 This beneficial effect of hemopexin was attributed to its ability to block the generation of ROS induced by heme plus TNF. Similarly, we observed that serum inhibited the generation of ROS by heme in macrophages and blocked its cytotoxic effect, even when exogenous TNF was added. Interestingly, serum protected Hmox1−/− macrophages from heme-induced death. Thus, the protective effect of serum is probably because of inhibition of both TNF as well as ROS triggered by heme.
The activation of TNFR1 by TNF in the absence of caspase activation is a well-characterized route responsible for programmed necrosis.1,2 The resistance of Tnfr1−/− macrophages to heme indicated the participation of TNF in heme-induced cytotoxicity. We observed that macrophages deficient in Tlr4 or Myd88, but not Trif, were resistant to heme-induced cell death. However, exogenous TNF restored heme-induced cytotoxicity. Thus, TLR4/MyD88-dependent production of TNF was essential to the cytoxicity of heme. It has been shown that LPS-induced activation of TLR4 causes RIP1-mediated necrosis in macrophages only when NF-κB and caspase 8 are concomitantly suppressed.15 Although RIP1 and TRIF are required for TLR4 and TLR3 induced NF-κB activation,43,44 heme-induced cell death requires RIP1 and RIP3, but not TRIF. A recent study demonstrated that on caspase inhibition, LPS and poly(I:C) cause a TRIF/RIP3-dependent, and TNF/NF-κB-independent, macrophage programmed necrosis.45 Taken together, these results indicate that heme cytotoxicity is different from LPS-induced macrophage necrosis and requires TNF, TNFR1, and the RIP proteins, occurring even in the absence of caspase inhibitors.
Treatment with NAC and iron chelation with deferoxamine protected macrophages from heme-induced death. These treatments also blocked macrophage necrosis when exogenous TNF was added. Thus, heme-induced ROS and TNF production were mediated by distinct pathways. At high concentrations, H2O2 triggers necrosis that is dependent on lysosomal membrane permeabilization and reactive hydroxyl radicals generated through Fenton-type reaction.12,13,46 In L929 fibroblasts, deferoxamine has been shown to protect against H2O2 but not TNF-induced death.46 The role of RIP and JNK in H2O2-induced cell death is controversial. H2O2 causes cell death of L929 cells independent of RIP1 or JNK.46 In mouse embryonic fibroblasts (MEFs), these pathways were shown to be important for H2O2-induced necrosis in some reports,47 but not others.48 The role of JNK in TNF-induced necrosis also appears to depend on the cell type and the culture conditions used.37,48,49 Heme induced JNK phosphorylation, and inhibition of JNK protected macrophages against heme plus TNF-induced cell death and caused a significant inhibition of ROS. The mechanism by which JNK influences programmed necrosis is not completely understood. The ability of JNK activation to promote the degradation of ferritin could increase the labile iron pool and consequently enhance ROS generation.41 JNK also has been implicated in the generation of high amounts of ROS by mitochondria.39 The abrogation of heme-induced JNK phosphorylation by antioxidants and the involvement of JNK on ROS production by heme suggest that JNK is induced by ROS and further promotes ROS generation. These results are reminiscent of the roles of JNK and ROS in TNF-induced necrosis.48
Several organisms have evolved the heme oxygenase system to control heme quantities through its enzymatic degradation.18 Hmox-1−/− mice display reduced numbers of macrophages in the spleen and liver, probably because of defects on erythrocyte recycling, heme degradation, and iron overload.27 In fact, it was shown that macrophages from Hmox1−/− mice died after phagocytosis of red blood cells in vitro.27 Moreover, the increased survival of monocytic cell lines to TNF-induced cell death is dependent of HO-1 expression.50 We observed an increased susceptibility of Hmox1−/− macrophages to free heme. HO-1 has important antioxidant functions and over-expression of HO-1 is cytoprotective because of reduction of ROS.21,42 Treatment of Hmox1−/− macrophages with NAC or deferoxamine abrogated heme-induced ROS generation and cell death by necrosis, indicating that an imbalance in oxidative stress response might be responsible for the increase susceptibility of Hmox1−/− macrophages to heme.
In conclusion, our study demonstrates that heme causes macrophage cell death with characteristics of programmed necrosis. The cytotoxic effect of free heme requires TNF, RIP proteins, ROS, and JNK. A central role for necrotic cell death has been demonstrated in pathologic conditions such as ischemia reperfusion injury and cerebral damage followed by a hemorrhagic episode.1,2,7 In these conditions, high amounts of free heme can be found in the lesions that could exacerbate tissue injury. In addition, strong evidence indicates that free heme participates in the pathogenesis of malaria and sepsis.18,21,32,42,51 Macrophage cell death induced by heme might also affect the ability to control intracellular infections. Thus, it is tempting to speculate that blocking the pathways involved in the cytotoxic effects of heme will benefit as adjunctive therapy in the treatment of these pathologies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. Bozza (Fundaçäo Oswaldo Cruz, Brazil) and P. Oliveira (Universidade Federal do Rio de Jeneiro [UFRJ]) for providing reagents and M. Soares (Instituto Gulbenkian de Ciêrca, Portugal) for providing bone marrow from Hmox1−/− and the corresponding WT controls.
The study received financial support from Conselho Nacional de Pesquisa (CNPq), Fundaçao de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), INCTDengue, Coordenaçao de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Secretaria Nacional de Ciencia Tecnologia e Innovacion de Panama (SENACYT).
Authorship
Contribution: G.B.F. and M.T.B. designed the research; G.B.F., L.S.A., R.d.O., F.F.D., D.R., M.J.D.R, P.L.F., and T.S.-P. performed the research; G.B.F., L.S.A., R.d.O., F.F.D., M.J.D.R., P.L.F., T.S.-P., M.K., D.G., F.K.M.C., and M.T.B. analyzed the data; and M.T.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcelo T. Bozza, Departamento de Imunologia, Instituto de Microbiologia, CCS Bloco D 36, UFRJ, Avenida Carlos Chagas Filho, 373 Cidade Universitaria, Rio de Janeiro, RJ, 21941-902 Brazil; e-mail: mbozza@micro.ufrj.br.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal