Abstract
Prostaglandin E2 (PGE2) is a lipid mediator that acts by ligating 4 distinct G protein–coupled receptors, E prostanoid (EP) 1 to 4. Previous studies identified the importance of PGE2 in regulating macrophage functions, but little is known about its effect on macrophage maturation. Macrophage maturation was studied in vitro in bone marrow cell cultures, and in vivo in a model of peritonitis. EP2 was the most abundant PGE2 receptor expressed by bone marrow cells, and its expression further increased during macrophage maturation. EP2-deficient (EP2−/−) macrophages exhibited enhanced in vitro maturation compared with wild-type cells, as evidenced by higher F4/80 expression. An EP2 antagonist also increased maturation. In the peritonitis model, EP2−/− mice exhibited a higher percentage of F4/80high/CD11bhigh cells and greater expression of macrophage colony-stimulating factor receptor (M-CSFR) in both the blood and the peritoneal cavity. Subcutaneous injection of the PGE2 analog misoprostol decreased M-CSFR expression in bone marrow cells and reduced the number of peritoneal macrophages in wild-type mice but not EP2−/− mice. The suppressive effect of EP2 ligation on in vitro macrophage maturation was mimicked by a selective protein kinase A agonist. Our findings reveal a novel role for PGE2/EP2/protein kinase A signaling in the suppression of macrophage maturation.
Introduction
Mononuclear phagocytes play a vital role in innate immune defense1 and in disease states such as diabetes,2 atherosclerosis,3 and emphysema.4 The mononuclear phagocyte system encompasses both circulating peripheral blood monocytes and tissue macrophages.5 It is generally believed that monocytes and macrophages originate from a common myeloid precursor in the bone marrow.6 Tissue macrophages may derive from either local proliferation or from recruitment of peripheral monocytes. Although local proliferation may play a role under steady-state conditions, repopulation of resident tissue macrophages in the setting of inflammation is more dependent on the recruitment of precursor cells from the blood.7 After emigrating from the blood to the tissues, monocytes continue the process of maturation and undergo the final differentiation steps necessary to become a macrophage.8 Macrophage maturation also has been studied in vitro, with a commonly used protocol consisting of bone marrow cells cultured in the presence of macrophage colony-stimulating factor (M-CSF). This in vitro approach results in a high yield of relatively homogenous primary macrophages that are not conditioned by the tissue microenvironment.
Prostaglandin E2 (PGE2) is a lipid mediator derived from the metabolism of arachidonic acid by cyclooxygenase (COX). It is the most abundant prostanoid in most tissues, and it is a well-known regulator of numerous physiologic and pathophysiologic processes, including blood flow,9 parturition,10 fever, and cancer and inflammation.11 PGE2 exerts its actions through 4 different types of G protein–coupled receptors called E prostanoid (EP) receptors 1 to 4. PGE2 has diverse effects on differentiation, migration, and activation of immune cells, including lymphocytes, dendritic cells, and macrophages. Its effects on macrophage functions have been amply documented to be mainly suppressive,12,13 reflecting an increase in the levels of the intracellular second messenger cAMP generated on ligation of EP2 and EP4 receptors.14 By contrast, there is little known about the role of PGE2/cAMP in macrophage maturation. Questions of how PGE2 affects macrophage maturation, whether endogenously generated PGE2 modulates this process, the role of specific EP receptors in such effects, and whether PGE2 influences macrophage maturation in vivo remain unanswered.
Here, we have used pharmacologic and genetic approaches to determine the role of PGE2/EP2 signaling in the regulation of macrophage maturation in vitro and in vivo. We report that PGE2/EP2 signaling suppresses macrophage maturation, and we have characterized key afferent and efferent mechanisms for this effect. Because PGE2 production is altered by both disease states and medications, and because maturation status influences macrophage functions, these findings have broad implications.
Methods
Animals
Mice harboring a targeted deletion of both alleles of the ptger2 encoding the EP2 receptor were originally generated by Dr Richard Breyer (Vanderbilt University).15 These 6- to 8-week-old males EP2-deficient (EP2−/−) mice, bred on a C57BL/6 background, and age-matched, males C57BL/6 wild-type (WT; EP2+/+) mice were purchased from The Jackson Laboratory and bred in the University of Michigan Unit for Laboratory Animal Medicine. Animals were treated according to National Institutes of Health guidelines for the use of experimental animals, with the approval of the University of Michigan Committee for the Use and Care of Animals.
Reagents
Dulbecco modified Eagle medium without phenol red, RPMI-1640, and penicillin/streptomycin solution were purchased from Invitrogen. Aspirin was from Sigma-Aldrich. AH6809, misoprostol, and PGE2 were from Cayman Chemicals. Ono-AE3-208 was a kind gift from Ono Pharmaceuticals. The protein kinase A (PKA)–specific cAMP analog N6-benzoyladenosine-3′,5′-cyclic monophosphate and the Epac-1–specific cAMP analog 8-4-chlorophenylthio-2′-O-methyladenosine-3′,5′-cyclicmonophosphate were obtained from Biolog Life Science Institute. TRIzol was purchased from Invitrogen, and primers for quantitative real-time RT-PCR were obtained from Integrated DNA Technologies.
Cell harvest and culture
Isolation of bone marrow cells and peritoneal macrophages was performed as described previously.16 Blood leukocytes were collected from the inferior vena cava and transferred to 15-mL tubes. Clotting was prevented by addition of EDTA. Erythrolysis was performed with 0.8% ammonium chloride lysis buffer for 10 minutes at room temperature and stopped by addition of RPMI-1640. Cells were washed with RPMI-1640 and suspended in PBS, 2mM EDTA, and 0.5% FCS for flow cytometry staining. To study maturation of bone marrow–derived macrophages, 2 × 106 bone marrow cells were cultured as described previously16 for 6 days in 35-mm-diameter Petri dishes in 30% L929 cell supernatant in RPMI-1640 containing 20% FCS, l-glutamine, and penicillin/streptomycin. After 3 days, the cell culture was supplemented with new medium totaling 50% of original volume.
Quantitative PCR and RNA isolation
Cells were suspended in 1 mL of TRIzol, and RNA was extracted as described previously.17 RNA was amplified by quantitative (q) RT-PCR performed with a SYBR Green PCR kit (Applied Biosystem) on an ABI Prism 7300 thermocycler (Applied Biosystems). Relative gene expression was determined by the ΔCT method, and β-actin was used as reference gene. Primer efficiency tests were performed on all primers and ranged from 97% to 107%.
Flow cytometry
For staining for flow cytometric analysis, cells were resuspended in PBS, 2mM EDTA, and 0.5% FCS. Fc receptor–mediated and nonspecific antibody binding was blocked by addition of excess CD16/CD32 (BD Biosciences Pharmingen). Staining was performed at 4°C in the dark for 15 minutes. During maturation experiments, when cells were collected each day for 6 days, samples were stabilized with 1% paraformaldehyde and analyzed on the same day. The following monoclonal antibodies were used at appropriate dilutions for staining: CD11c, CD11b, GR-1 (all from BD Biosciences Pharmingen), CD115 (BioLegend), and F4/80 (eBioscience). An FACSCalibur flow cytometer (BD Biosciences) was used for flow cytometric characterization of cell populations, and data were analyzed with WinMDi and FlowJo Version 7.6.4 software (TreeStar).
Peripheral blood count
Determination of peripheral blood white blood cell counts was performed by the University of Michigan Unit for Laboratory Animal Medicine's Animal Diagnostic Laboratory using a Hemavet cell analyzer (Drew Scientific).
ELISA
The quantification of murine CCL2 protein (R&D Systems) from peritoneal lavage and PGE2 (Enzo) from cell cultures was performed by commercially available ELISA kits following the instructions of the manufacturers.
In vivo thioglycollate treatment
In vivo misoprostol treatment
To determine the effects of a stable PGE2 analog on peritonitis, mice were injected subcutaneously with 200 μL of saline containing 50 μg of misoprostol in 0.5% DMSO 2 hours before and 10 hours after intraperitoneal thioglycollate; control mice received 200 μL saline containing 0.5% DMSO alone.
Data analysis
All data are displayed as mean values ± SEM from 3 to 8 independent experiments, each using a different mouse. Statistical differences among treatment groups were estimated by ANOVA with Tukey posthoc test for multiple comparisons, or by paired Student t test, as appropriate, using Prism 5.0 (GraphPad Software). A P value < .05 is considered statistically significant.
Results
EP2 is the most highly expressed PGE2 receptor during in vitro macrophage maturation
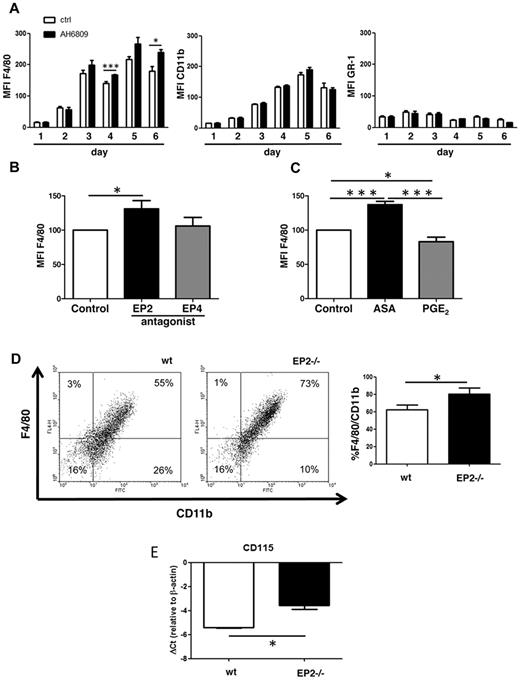
As an in vitro model of macrophage maturation, freshly flushed bone marrow cells were cultured for up to 6 days in the presence of 30% L929 murine fibroblast supernatant, a source of M-CSF and other growth factors. Cell supernatant was collected every day of the culture, and PGE2 concentration was measured by ELISA. The PGE2 level in cell supernatants exceeded that in cell-free medium at every day of culture, but its level did not change during macrophage maturation (Figure 1A). Cells started to adhere to the bottom of cell culture plates ∼ day 3, and expression of EP1 to EP4 was determined by qRT-PCR analysis of both the adherent cell fraction on days 3 to 6 (Figure 1B) and of pooled adherent plus nonadherent cells on days 1 to 6 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). EP2 was the most highly expressed of the receptors in adherent cells, and its expression peaked at day 4, when mRNA levels exceeded those of EP1 and EP4 by 16-fold and that of EP3 by 64-fold (Figure 1B). Because adherence is itself a characteristic of mature macrophages,19 this kinetic pattern suggests the possible relevance of EP2 in the process of maturation. EP4, whose mRNA expression transiently disappeared at day 1 (supplemental Figure 1) exhibited an increase in expression only after the increase in adherence ensued.
PGE2 and its receptors during macrophage maturation. (A) PGE2 is present during macrophage differentiation. Freshly flushed bone marrow cells were cultured for up to 6 days in the presence of 30% L929 supernatant; after 3 days, cultures were replenished with new medium totaling 50% of original volume. Day 0 indicates 30% L929 supernatant alone in the absence of cells. Each day of culture, an aliquot of cell supernatant was aspirated and analyzed for PGE2 concentration by ELISA. Data are expressed as the mean ± SEM from 6 to 8 experiments, each using cells from a single mouse. (B) EP2 is most highly expressed among EP receptors during macrophage maturation. At each of days 3 to 6 of cell culture (as described in panel A), adherent cells were collected and analyzed for the expression of EP receptors with qRT-PCR. Data presented in all panels are expressed as the mean ± SEM from 3 to 4 experiments, each using cells from a single mouse (*P < .05, # indicates significant [P < .05] difference from all other conditions).
PGE2 and its receptors during macrophage maturation. (A) PGE2 is present during macrophage differentiation. Freshly flushed bone marrow cells were cultured for up to 6 days in the presence of 30% L929 supernatant; after 3 days, cultures were replenished with new medium totaling 50% of original volume. Day 0 indicates 30% L929 supernatant alone in the absence of cells. Each day of culture, an aliquot of cell supernatant was aspirated and analyzed for PGE2 concentration by ELISA. Data are expressed as the mean ± SEM from 6 to 8 experiments, each using cells from a single mouse. (B) EP2 is most highly expressed among EP receptors during macrophage maturation. At each of days 3 to 6 of cell culture (as described in panel A), adherent cells were collected and analyzed for the expression of EP receptors with qRT-PCR. Data presented in all panels are expressed as the mean ± SEM from 3 to 4 experiments, each using cells from a single mouse (*P < .05, # indicates significant [P < .05] difference from all other conditions).
Lack of PGE2 signaling through EP2 promotes macrophage maturation in vitro
Based on its abundance (Figure 1B), EP2 is the receptor most likely to mediate any biologic actions of PGE2 during the process of macrophage maturation. Both pharmacologic and genetic approaches were used to determine the importance of the PGE2/EP2 signaling axis in in vitro maturation. First, bone marrow cells were plated in the absence and presence of the EP2 antagonist AH6809; this agent was replenished at day 3 along with fresh medium. Macrophage maturation was assessed on the basis of surface expression of CD11b (myeloid lineage marker) and F4/80 (macrophage marker). As expected with this protocol, CD11b and F4/80 increased in a time-dependent manner in untreated cells, consistent with macrophage maturation (Figure 2A). By contrast, the neutrophil marker Gr-1 was expressed at day 1 but did not significantly change throughout 6 days of culture. Cells treated with the EP2 antagonist exhibited significantly higher expression of F4/80, whereas CD11b and GR-1 did not change (Figure 2A). In contrast to the effect of the EP2 antagonist, an EP4 antagonist (Ono-AE3-208) added to parallel wells in the same experiments exhibited no effect on F4/80 expression (Figure 2B), highlighting the specific importance of EP2 signaling in regulating macrophage maturation. Addition of the COX inhibitor aspirin, which blocks PGE2 production, increased F4/80 expression, whereas the addition of exogenous PGE2 decreased it (Figure 2C). Although L929 supernatant itself contains PGE2, the effect of aspirin on F4/80 expression implicates the contributions of autocrine PGE2 derived from macrophage COX metabolism in restraining macrophage maturation. The importance of EP2 was validated using bone marrow cells from EP2−/− mice. The percentage of mature (CD11bpos/F4/80pos) macrophages at day 6 in culture was significantly higher in EP2−/− mice than in WT controls (Figure 2D). Compared with WT cells, day 6 macrophages from EP2−/− mice likewise exhibited significantly increased expression of M-CSFR (also known as CD115; Figure 2E), a key transducer of macrophage maturation. These differences in F4/80 and M-CSFR expression between EP2−/− and WT bone marrow–derived macrophages were not present at day 1 (data not shown), indicating that these changes are associated with maturation. Together, these data demonstrate that endogenous and exogenous PGE2/EP2 signaling suppresses in vitro macrophage maturation.
EP2 antagonism/deficiency promotes macrophage maturation. (A) EP2 antagonism promotes macrophage maturation. Freshly flushed bone marrow cells were cultured for 6 days with 30% L929 supernatant in the absence (Ctrl) or presence of EP2 antagonist AH6809 at 10μM; after 3 days, the culture was replenished with new medium containing AH6809, totaling 50% of original volume. Each day of the culture, cells were collected, stained, and analyzed for the markers indicated. Mean fluorescent intensity (MFI) is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, ***P < .001). (B) EP4 antagonism does not promote macrophage maturation. Freshly flushed bone marrow cells were cultured with the EP2 antagonist AH6809 at 10μM, or with 1μM EP4 antagonist Ono-AE3-208; control cells were cultured with DMSO. Cells were stained with F4/80-APC and CD11b-FITC and analyzed after 6 days of culture as described in panel A. Control containing DMSO was set as 100%. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05). (C) Effects of aspirin (ASA) and exogenous PGE2 on macrophage maturation. Freshly flushed bone marrow cells were cultured with aspirin at 200μM, or with 1μM PGE2; control cells were cultured with DMSO. Cells were stained with F4/80-APC and CD11b-FITC and analyzed as described in panel A. Control containing DMSO was set at 100%. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, ***P < .001). (D) EP2 deficiency promotes macrophage maturation. Freshly flushed bone marrow cells isolated from EP2−/− and WT mice (wt) were cultured in 30% L929 cell supernatant as described in panel A. The dot-plot shown is representative of 3 experiments, each using cells from a different mouse. The percentage of CD11bpos/F4/80pos cells in both genotypes was compared using paired t test (*P < .05). (E) CD115 (M-CSFR) is up-regulated in EP2−/− mice during in vitro macrophage maturation. After 6 days of culture, bone marrow–derived cells were collected and analyzed for the expression of CD115 with qRT-PCR. Data are expressed as the mean ± SEM from 3 experiments, each using cells from a different mouse (*P < .05).
EP2 antagonism/deficiency promotes macrophage maturation. (A) EP2 antagonism promotes macrophage maturation. Freshly flushed bone marrow cells were cultured for 6 days with 30% L929 supernatant in the absence (Ctrl) or presence of EP2 antagonist AH6809 at 10μM; after 3 days, the culture was replenished with new medium containing AH6809, totaling 50% of original volume. Each day of the culture, cells were collected, stained, and analyzed for the markers indicated. Mean fluorescent intensity (MFI) is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, ***P < .001). (B) EP4 antagonism does not promote macrophage maturation. Freshly flushed bone marrow cells were cultured with the EP2 antagonist AH6809 at 10μM, or with 1μM EP4 antagonist Ono-AE3-208; control cells were cultured with DMSO. Cells were stained with F4/80-APC and CD11b-FITC and analyzed after 6 days of culture as described in panel A. Control containing DMSO was set as 100%. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05). (C) Effects of aspirin (ASA) and exogenous PGE2 on macrophage maturation. Freshly flushed bone marrow cells were cultured with aspirin at 200μM, or with 1μM PGE2; control cells were cultured with DMSO. Cells were stained with F4/80-APC and CD11b-FITC and analyzed as described in panel A. Control containing DMSO was set at 100%. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, ***P < .001). (D) EP2 deficiency promotes macrophage maturation. Freshly flushed bone marrow cells isolated from EP2−/− and WT mice (wt) were cultured in 30% L929 cell supernatant as described in panel A. The dot-plot shown is representative of 3 experiments, each using cells from a different mouse. The percentage of CD11bpos/F4/80pos cells in both genotypes was compared using paired t test (*P < .05). (E) CD115 (M-CSFR) is up-regulated in EP2−/− mice during in vitro macrophage maturation. After 6 days of culture, bone marrow–derived cells were collected and analyzed for the expression of CD115 with qRT-PCR. Data are expressed as the mean ± SEM from 3 experiments, each using cells from a different mouse (*P < .05).
EP2−/− mice exhibit increased macrophage maturation in vivo
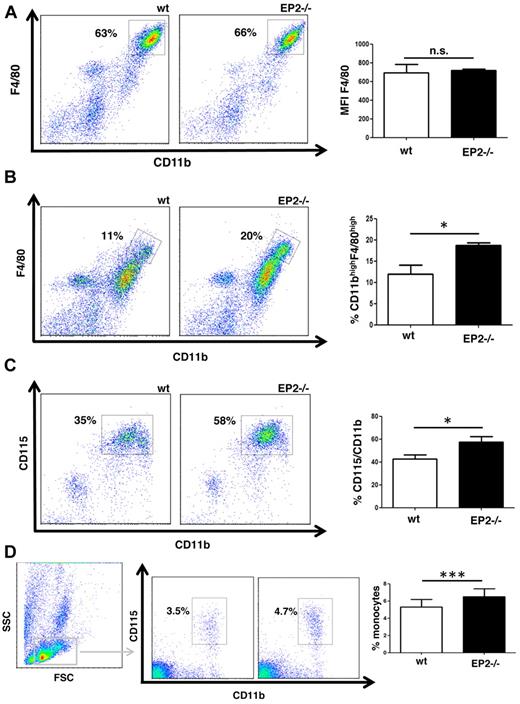
Data presented in Figure 2D and E indicated that bone marrow–derived macrophages from EP2−/− mice are more mature (exhibit greater CD11b/F4/80 double positivity) than those from WT controls. By contrast, no such difference between the 2 genotypes was observed in resident peritoneal macrophages (Figure 3A). Moreover, WT and EP2−/− mice had similar number of CD11bpos/F4/80pos cells in the bone marrow and number of monocytes in the blood (data not shown). Likewise, resident alveolar macrophages from EP2−/− and WT mice demonstrated similar expression of F4/80 and the alveolar macrophage-specific maturation marker CD11c (data not shown). These observations suggested that, in contrast to the in vitro maturation model, under steady-state conditions in vivo, the degree of maturation in long-lived tissue macrophages was not affected by the lack of PGE2/EP2 signaling. However, the importance of PGE2/EP2 signaling in a dynamic model of macrophage maturation that occurs under in vivo inflammatory conditions remained to be defined. Mature peritoneal macrophages, like bone marrow–derived macrophages, can be defined as F4/80posCD11bpos cells.19,20 This allows direct comparison of in vitro and in vivo data. Moreover, it has been shown that in the model of thioglycollate peritonitis, newly recruited and maturing macrophages originate from blood monocytes, whose precursor resides in the bone marrow.21 Thus, studying each of these compartments permitted us to follow macrophage maturation under dynamic rather than steady-state conditions.
EP2−/− mice exhibit increased macrophage maturation in a model of thioglycollate peritonitis. (A) Maturation of peritoneal macrophages under steady-state conditions does not differ between EP2−/− and WT mice. Peritoneal cells were isolated by lavage from naive WT and EP2−/− mice and stained for the markers indicated. The dot-plot shown is representative of 4 experiments, each using cells from a different mouse. (B-C) EP2−/− mice have higher numbers of mature macrophages (CD11bhigh/F4/80high) and cells expressing CD115 in the peritoneal cavity in a model of peritonitis. EP2−/− and WT mice were injected intraperitoneally with thioglycollate. Peritoneal cells were isolated by lavage 4 days later and stained with CD11b-FITC, F4/80-APC, and CD115-PE as described in panel A. The dot-plots shown are representative of 3 to 4 experiments, each using a separate mouse. The percentage of CD11bhigh/F4/80highcells (B) and CD11bpos/CD115pos cells (C) was compared between both genotypes using a paired t test (*P < .05). (D) EP2−/− mice have a higher number of blood monocytes during peritonitis. Blood leukocytes were obtained from EP2−/− and WT mice 4 days after intraperitoneal injection of thioglycollate and stained as described in panels B and C. Blood monocytes were identified as a low side scatter (SSC) cell population showing cell surface expression of CD11b and CD115. The dot-plot shown is representative of 4 experiments, each using a different mouse. Percentages of CD11bpos/CD115pos cells in both genotypes were compared using paired t test (*P < .05; n.s. indicates not significant).
EP2−/− mice exhibit increased macrophage maturation in a model of thioglycollate peritonitis. (A) Maturation of peritoneal macrophages under steady-state conditions does not differ between EP2−/− and WT mice. Peritoneal cells were isolated by lavage from naive WT and EP2−/− mice and stained for the markers indicated. The dot-plot shown is representative of 4 experiments, each using cells from a different mouse. (B-C) EP2−/− mice have higher numbers of mature macrophages (CD11bhigh/F4/80high) and cells expressing CD115 in the peritoneal cavity in a model of peritonitis. EP2−/− and WT mice were injected intraperitoneally with thioglycollate. Peritoneal cells were isolated by lavage 4 days later and stained with CD11b-FITC, F4/80-APC, and CD115-PE as described in panel A. The dot-plots shown are representative of 3 to 4 experiments, each using a separate mouse. The percentage of CD11bhigh/F4/80highcells (B) and CD11bpos/CD115pos cells (C) was compared between both genotypes using a paired t test (*P < .05). (D) EP2−/− mice have a higher number of blood monocytes during peritonitis. Blood leukocytes were obtained from EP2−/− and WT mice 4 days after intraperitoneal injection of thioglycollate and stained as described in panels B and C. Blood monocytes were identified as a low side scatter (SSC) cell population showing cell surface expression of CD11b and CD115. The dot-plot shown is representative of 4 experiments, each using a different mouse. Percentages of CD11bpos/CD115pos cells in both genotypes were compared using paired t test (*P < .05; n.s. indicates not significant).
Four days after intraperitoneal thioglycollate injection, ∼ 90% of cells in the peritoneal cavity were mononuclear cells and no difference was observed between WT and EP2−/− mice, as determined from microscopic inspection of modified Wright-Giemsa–stained cytospins (supplemental Figure 2). Likewise, the percentage of F4/80pos/CD11bpos cells was not significantly different between the 2 genotypes (data not shown). Interestingly, however, compared with WT mice, cells in the peritoneal cavities of EP2−/− mice had significantly higher expression of F4/80, as assessed by mean fluorescent intensity (supplemental Figure 3A). This difference in F4/80 expression between WT and EP2−/− mice was explained by higher numbers of mature macrophages in the latter, reflected by gating on a F4/80high/CD11bhigh cell population (Figure 3B). In addition, EP2−/− mice had significantly more cells expressing M-CSFR compared with WT (Figure 3C). Defined by CD11bpos/CD115pos as described previously,22 a modest but significant increase in the percentage of blood monocytes in EP2−/− mice compared with WT mice also was observed 4 days after thioglycollate injection (Figure 3D). This difference in percentage of blood monocytes was validated by Coulter counter blood analysis (supplemental Figure 3B). However, neither total peripheral blood monocyte nor total white blood cell numbers differed between the 2 genotype (supplemental Figure 3C). In summary, EP2−/− mice in this in vivo model of peritonitis exhibited a greater number of mature macrophages in the peritoneal cavity as well as macrophage precursors in the blood in association with higher expression of M-CSFR on mononuclear cells, implicating the endogenous role of PGE2 in the process of macrophage maturation.
Misoprostol suppresses in vivo macrophage maturation
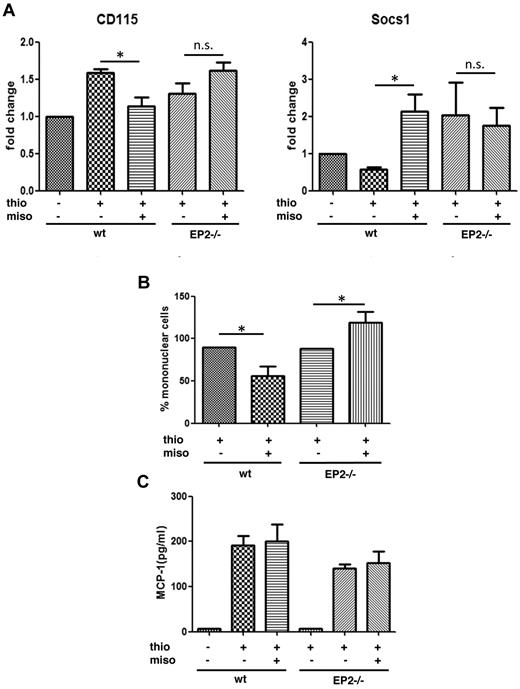
Monocytes originate from the bone marrow; circulate in the blood; and, especially under inflammatory conditions, serve as a reservoir of myeloid precursor cells for renewal of tissue macrophage populations.7 To better understand the role of PGE2/EP2 signaling in the in vivo macrophage maturation process, misoprostol—a stable but receptor-nonselective PGE2 analog—was administered subcutaneously using a modification of a previously published protocol.23 Specifically, misoprostol was administered 2 hours before and 10 hours after intraperitoneal thioglycollate injection. Potential receptor selectivity of any actions of misoprostol were then evaluated by determining its actions in EP2−/−, compared with WT, mice. Bone marrow cells were isolated 24 hours after thioglycollate injection and expression of M-CSFR (CD115) and suppressor of cytokine signaling-1 (SOCS1) was analyzed by qRT-PCR. SOCS1 is a protein that negatively regulates MCSF-R signaling24 and is a critical controller of numerous macrophage functions25 as well as monocyte to macrophage differentiation.26 As seen in Figure 4A, thioglycollate treatment increased transcripts for M-CSFR in bone marrow cells of WT animals and misoprostol substantially blunted this increase. By contrast, misoprostol failed to attenuate the thioglycollate-induced increase in M-CSFR in EP2−/− mice. Conversely, SOCS1 expression in bone marrow cells was down-regulated by thioglycollate but substantially up-regulated by misoprostol treatment in WT animals. No such up-regulation was elicited by misoprostol in EP2−/− mice. These data suggest the importance of PGE2/EP2 in the suppression of expression as well as signaling of MSCF-R in bone marrow cells. The effects of misoprostol on peritoneal macrophage accumulation 4 days after thioglycollate also were examined. The number of mononuclear cells in the peritoneal cavity of mice treated with misoprostol was significantly decreased in WT but not in EP2−/− mice (Figure 4B). In fact, misoprostol treatment significantly increased the number of mononuclear cells in EP2−/− mice. Because misoprostol is a PGE2 analog that can ligate all EP receptors, this finding presumably reflects the unmasking in the absence of EP2 of opposing actions mediated via other receptors. Notably, misoprostol failed to inhibit peritoneal production of CCL2 (MCP-1), the major chemokine mediating macrophage recruitment in the peritonitis model (Figure 4C). The fact that MCSF levels in serum did not differ between the 2 genotypes (data not shown) suggests that the observed differences in macrophage maturation instead reflect the importance of the differences in M-CSFR expression on macrophages and macrophage precursors. These data suggest that misoprostol, via EP2, inhibits in vivo macrophage maturation, rather than recruitment signals.
In vivo effects of misoprostol on macrophage maturation during peritonitis. (A) Misoprostol down-regulates CD115 and up-regulates SOCS1 in bone marrow cells of WT mice but not EP2−/− mice in a model of thioglycollate peritonitis. Two hours before and 10 hours after intraperitoneal thioglycollate injection, mice were injected subcutaneously with 200 μL of saline containing either 50 μg of the nonselective PGE2 analog misoprostol in 0.5% DMSO or DMSO alone. After 24 hours, WT and EP2−/− mice were killed, and bone marrow cells were isolated and analyzed for the expression of CD115 and SOCS1 with qRT-PCR. Data shown are expressed as the mean ± SEM from 6 experiments, each using a different mouse (*P < .05). (B) Misoprostol attenuates macrophage accumulation in the peritoneal cavity in a model of thioglycollate peritonitis. Mice were treated as described in panel A. After 4 days, peritoneal cells of EP2−/− and WT mice were lavaged and counted. The percentage of mononuclear cells was determined from modified Wright-Giemsa–stained cytospins. Data are expressed as the mean ± SEM from 8 experiments, each using a different mouse. (C) Misoprostol fails to attenuate CCL2 (MCP-1) production. Mice were treated as described in panel A. After 24 hours, the peritoneal cavities of EP2−/− and WT mice were lavaged with 1 mL of lavage buffer, and the supernatant was analyzed for MCP-1 using ELISA. Data are expressed as the mean ± SEM from 6 experiments, each using a different mouse. Thio indicates thioglycollate and miso, misoprostol.
In vivo effects of misoprostol on macrophage maturation during peritonitis. (A) Misoprostol down-regulates CD115 and up-regulates SOCS1 in bone marrow cells of WT mice but not EP2−/− mice in a model of thioglycollate peritonitis. Two hours before and 10 hours after intraperitoneal thioglycollate injection, mice were injected subcutaneously with 200 μL of saline containing either 50 μg of the nonselective PGE2 analog misoprostol in 0.5% DMSO or DMSO alone. After 24 hours, WT and EP2−/− mice were killed, and bone marrow cells were isolated and analyzed for the expression of CD115 and SOCS1 with qRT-PCR. Data shown are expressed as the mean ± SEM from 6 experiments, each using a different mouse (*P < .05). (B) Misoprostol attenuates macrophage accumulation in the peritoneal cavity in a model of thioglycollate peritonitis. Mice were treated as described in panel A. After 4 days, peritoneal cells of EP2−/− and WT mice were lavaged and counted. The percentage of mononuclear cells was determined from modified Wright-Giemsa–stained cytospins. Data are expressed as the mean ± SEM from 8 experiments, each using a different mouse. (C) Misoprostol fails to attenuate CCL2 (MCP-1) production. Mice were treated as described in panel A. After 24 hours, the peritoneal cavities of EP2−/− and WT mice were lavaged with 1 mL of lavage buffer, and the supernatant was analyzed for MCP-1 using ELISA. Data are expressed as the mean ± SEM from 6 experiments, each using a different mouse. Thio indicates thioglycollate and miso, misoprostol.
Roles of PKA and Epac in in vitro macrophage maturation
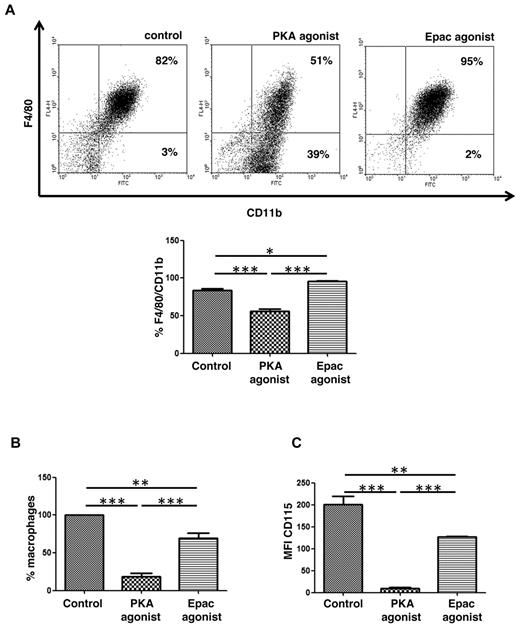
PGE2/EP2 signaling increases intracellular cAMP concentration, and cAMP-dependent PKA and guanine nucleotide exchange protein directly activated by cAMP (Epac) are 2 effectors of cAMP.14 To further investigate the downstream mechanisms by which PGE2/EP2 signaling restrains in vitro macrophage maturation, the effects of cAMP analogs that selectively activate PKA or Epac were studied. Agonists and their concentrations used were based on our previous studies in macrophages, where we also have shown that Epac-1, but not Epac-2, was expressed in macrophages.27 Bone marrow cells were plated in 30% L929 cell supernatant in the absence or presence of PKA or Epac agonists that were replenished with the addition of fresh medium at day 3 of culture. After 6 days of culture, PKA agonist-treated cultures demonstrated significantly fewer F4/80pos/CD11bpos cells than did control cultures (Figure 5A), and the effect of PKA agonist was similar in WT and EP2−/− mice (data not shown). Interestingly, cells cultured with the Epac agonist manifested the opposite effect, showing an increased number of F4/80pos/CD11bpos cells compared with control (Figure 5A). Moreover, PKA agonist decreased the number of adherent cells by ∼ 80% (Figure 5B). To exclude the possibility that the PKA agonist effect was simply because of a reduction in cell adherence, floating cells also were analyzed for the expression of CD11b and F4/80. As was the case for adherent cells, the floating cell fraction of cultures treated with PKA agonist also contained significantly fewer F4/80pos/CD11bpos cells (24.5%) than did control (39%) or Epac agonist-treated (34.5%) cultures (average values of 2 independent experiments), demonstrating that the PKA agonist indeed inhibited macrophage maturation. In addition, PKA agonist-treated cells exhibited a dramatic reduction in cell surface M-CSFR, whereas Epac agonist-treated cells exhibited a much more modest reduction (Figure 5C). Thus, the effects of PGE2/EP2 signaling on macrophage maturation and M-CSFR expression are closely mimicked by the PKA agonist but not the Epac agonist. The fact that the suppression of macrophage maturation by PGE2 is less robust than that by PKA agonist is probably explained by the opposing action of Epac, which is also activated by PGE2-driven cAMP, on macrophage maturation.
Role of PKA and Epac in in vitro macrophage maturation. (A) PKA inhibits, whereas Epac promotes, macrophage maturation. Freshly flushed bone marrow cells isolated from EP2−/− and WT mice were cultured in 30% L929 cell supernatant in the presence of Epac or PKA agonist (500μM each). After 3 days, cell culture was supplemented with new medium containing Epac or PKA agonist totaling 50% of original volume; and after 6 days, cells were collected and stained with CD11b-FITC and F4/80-APC. The dot-plot shown is from a single experiment representative of 3 independent experiments, each using a different mouse. Percentages of CD11bpos/CD115pos cells in different conditions were compared. Data are expressed as the mean ± SEM from 3 experiments, each using a separate mouse (*P < .05, **P < .01, ***P < .001). (B) PKA agonist reduces numbers of macrophages during in vitro macrophage maturation. Cells were cultured as described in panel A. After 6 days of culture floating cells were washed away, and adherent cells were counted by light microscopy. Data are expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, **P < .01, ***P < .001). (C) PKA agonist diminishes M-CSFR (CD115) expression on the cell surface. Cells were cultured as described in panels A-B. After 6 days of culture, adherent CD11bpos/CD115pos cells were stained with CD115-PE. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (**P < .01, ***P < .001). PKA agonist N6-benzoyladenosine-3′,5′-cyclic monophosphate (6-Bnz-cAMP) and Epac agonist 8-4-chlorophenylthio-2′-O-methyladenosine-3′,5′-cyclicmonophosphate (8-pCPT-2′-O-Me-cAMP).
Role of PKA and Epac in in vitro macrophage maturation. (A) PKA inhibits, whereas Epac promotes, macrophage maturation. Freshly flushed bone marrow cells isolated from EP2−/− and WT mice were cultured in 30% L929 cell supernatant in the presence of Epac or PKA agonist (500μM each). After 3 days, cell culture was supplemented with new medium containing Epac or PKA agonist totaling 50% of original volume; and after 6 days, cells were collected and stained with CD11b-FITC and F4/80-APC. The dot-plot shown is from a single experiment representative of 3 independent experiments, each using a different mouse. Percentages of CD11bpos/CD115pos cells in different conditions were compared. Data are expressed as the mean ± SEM from 3 experiments, each using a separate mouse (*P < .05, **P < .01, ***P < .001). (B) PKA agonist reduces numbers of macrophages during in vitro macrophage maturation. Cells were cultured as described in panel A. After 6 days of culture floating cells were washed away, and adherent cells were counted by light microscopy. Data are expressed as the mean ± SEM from 3 experiments, each using a different mouse (*P < .05, **P < .01, ***P < .001). (C) PKA agonist diminishes M-CSFR (CD115) expression on the cell surface. Cells were cultured as described in panels A-B. After 6 days of culture, adherent CD11bpos/CD115pos cells were stained with CD115-PE. MFI is expressed as the mean ± SEM from 3 experiments, each using a different mouse (**P < .01, ***P < .001). PKA agonist N6-benzoyladenosine-3′,5′-cyclic monophosphate (6-Bnz-cAMP) and Epac agonist 8-4-chlorophenylthio-2′-O-methyladenosine-3′,5′-cyclicmonophosphate (8-pCPT-2′-O-Me-cAMP).
Discussion
Local PGE2 has been shown to modulate numerous functions of mature leukocytes.28 For example, in macrophages PGE2 inhibits production of TNF-α29 and IL-12;30 decreases MHC II expression, which alters antigen presentation capacity;31 and suppresses ingestion and killing of microbes.12,32 These suppressive functions have mainly been linked to the ligation of EP2 receptors, EP4 receptors, or both.33 An inhibitory effect of PGE2 on proliferation of myeloid progenitor cells in agar cultures was described in 1979.34,35 A concentration of PGE2 as low as 10−10M was reported to inhibit macrophage-colony proliferation on agar cultures.34 Subsequently, PGE2 has been shown to suppress differentiation of dendritic cells via EP236 or EP437 receptors. In addition to these local actions, an endocrine effect of PGE2 is suggested by the finding that PGE2 either elaborated by keratinocytes or released from subcutaneously implanted time-release pellets influenced the characteristics of bone marrow–derived cells.38 Here, we demonstrate that both endogenous and exogenous PGE2 inhibits maturation of macrophage precursors in the bone marrow. This occurs via an EP2/cAMP/PKA pathway and is able to limit the accumulation of mature tissue macrophages recruited in response to an in vivo inflammatory stimulus.
Maturation is crucial for effective macrophage-mediated functions such as immune surveillance. Although the function of F4/80 remains obscure decades after its first description,39 it is generally considered the best available murine mononuclear phagocyte marker.40 Because F4/80 is highly expressed by resident peritoneal macrophages and is up-regulated during in vitro differentiation of bone marrow–derived macrophages, we were able to use it as a marker of maturation both in vitro and in vivo. In the in vivo circumstance, mononuclear phagocyte maturation must be distinguished from recruitment. In a peritonitis model it has been demonstrated that CCL2 is the main chemokine driving monocyte recruitment, and mice lacking CCL241 or its receptor CCR242 manifested decreased numbers of F4/80-positive cells in the peritoneal cavity after thioglycollate-induced peritonitis. We observed no effect of in vivo misoprostol on CCL2 levels in the peritoneum after thioglycollate injection, and preliminary experiments (data not shown) likewise noted that it had no effect on CCR2 expression in bone marrow cells. Therefore, it is likely that the reduced accumulation of mature macrophages with misoprostol treatment in the peritonitis model is independent of chemokine-mediated cell recruitment and instead reflects impaired maturation.
Regulation of macrophage maturation is believed to be mediated by colony-stimulating factors (CSFs), including M-CSF and granulocyte-CSF (G-CSF), granulocyte macrophage–CSF (GM-CSF), and IL-3.43 The central importance of M-CSF in this regard is well established. Expression of the high-affinity receptor M-CSFR, also known as CD115, increases during macrophage maturation,43 and in a variety of in vitro and in vivo models it has been shown that administration of M-CSF leads to increases in blood monocytes as well as peritoneal macrophages.43 In our study, both bone marrow–derived macrophages and freshly recruited peritoneal macrophages from EP2−/− mice exhibited an increased level of M-CSFR compared with cells from WT animals. To our knowledge, however, this study is the first to report an increase in M-CSFR transcripts and a decrease in SOCS1 transcripts in bone marrow cells of mice subjected to thioglycollate peritonitis. Moreover, in vivo administration of misoprostol reduced M-CSFR and increased SOCS1 levels in bone marrow precursor cells. Together, these data strongly suggest that down-regulation of M-CSFR represents an important mechanism by which EP2 signaling in response to either endogenous or exogenous PGE2 suppresses macrophage maturation.
Interestingly, macrophages stimulated with M-CSF have been reported to increase PGE2 production,44 suggesting the presence of an autocrine loop similar to that described in TLR signaling, in which PGE2 is produced on lipopolysaccharide stimulation and in turn, this prostanoid via EP2/cAMP/PKA modifies macrophage responses to lipopolysaccharide.45,46 Beyond its ability to regulate expression of M-CSFR, PGE2 also may control the ability of this receptor to transmit signals,24 via its up-regulation of SOCS1 in bone marrow precursors. Because M-CSF also activates a breadth of macrophage functions, including phagocytosis, production of reactive oxygen species, chemotaxis, and microbial killing,43 PGE2 regulation of M-CSFR probably has functional implications beyond maturation. Indeed, our group has reported previously that alveolar macrophages from EP2−/− mice manifested an increased capacity for phagocytosis32 ; the possible role of enhanced M-CSFR signaling in this phenomenon remains to be evaluated.
EP2 was the most abundantly expressed PGE2 receptor on bone marrow cells and maturing macrophages, and both genetic and pharmacologic data in vitro and in vivo argue that this receptor, rather than EP4, mediates the suppressive actions of PGE2 on macrophage maturation. The suppressive effect on macrophage maturation was mirrored by the addition of a selective PKA agonist. PKA has been implicated previously as mediating PGE2-induced suppression of macrophage functions such as bacterial killing and cytokine synthesis.27,46 Bone marrow cells treated with a selective PKA agonist displayed significantly decreased macrophage maturation and almost lacked M-CSFR on the cell surface. Interestingly, a selective Epac agonist increased macrophage maturation, albeit not by increasing M-CSFR. The mechanism by which Epac activation enhanced macrophage maturation remains to be explored. However, an action that is independent of M-CSF is not without precedent, because even mice lacking M-CSF, G-CSF, and GM-CSF generate macrophages and mount an inflammatory response in thioglycollate-induced peritonitis.47 In this context, it is worth noting as well that the expression of Epac was reported to increase during monocyte to macrophage maturation and that Epac became functional only in fully matured macrophages.48
In summary, we have shown using both pharmacologic and genetic approaches that PGE2, through EP2 and PKA, suppresses macrophage maturation. This is a reflection of actions exerted on bone marrow precursors that include reduced expression of M-CSFR and result in reduced numbers of blood monocytes and recruited tissue macrophages in an in vivo peritonitis model. This form of regulation is pertinent to the actions of both endogenous as well as exogenous PGE2. PGE2 levels are often elevated in disease states such as infection, inflammation, and cancer.49 Furthermore, EP2 expression can be altered in disease states.50 Finally, commonly used medications, including nonsteroidal anti-inflammatory drugs and glucocorticoids, are well known to inhibit PGE2 biosynthesis. Thus, the dynamics of macrophage maturation would be expected to be modulated in association with disease states or treatments in which PGE2 production or responses are altered.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Teresa Murphy, Sally Przybranowski, and Casey Lewis for technical assistance and the members of the Peters-Golden laboratory for helpful input.
This work was supported by Deutsche Forschungsgemeinschaft (German Research Foundation, Z.Z.), an American Lung Association senior research fellowship (K.O.), and National Institutes of Health grants HL058897 (M.P.-G.), HL103777-01 (C.H.S.), and HD057176 (D.M.A.).
National Institutes of Health
Authorship
Contribution: Z.Z. designed the research, performed experiments, analyzed data, and wrote the paper; K.O. performed experiments and analyzed data; C.H.S. and D.M.A. designed the research and analyzed data; and M.P.-G. designed research, supervised the work, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Peters-Golden, 6301 MSRB III, 1150 W Medical Center Dr, Ann Arbor, MI 48109-5642; e-mail: petersm@umich.edu.

![Figure 1. PGE2 and its receptors during macrophage maturation. (A) PGE2 is present during macrophage differentiation. Freshly flushed bone marrow cells were cultured for up to 6 days in the presence of 30% L929 supernatant; after 3 days, cultures were replenished with new medium totaling 50% of original volume. Day 0 indicates 30% L929 supernatant alone in the absence of cells. Each day of culture, an aliquot of cell supernatant was aspirated and analyzed for PGE2 concentration by ELISA. Data are expressed as the mean ± SEM from 6 to 8 experiments, each using cells from a single mouse. (B) EP2 is most highly expressed among EP receptors during macrophage maturation. At each of days 3 to 6 of cell culture (as described in panel A), adherent cells were collected and analyzed for the expression of EP receptors with qRT-PCR. Data presented in all panels are expressed as the mean ± SEM from 3 to 4 experiments, each using cells from a single mouse (*P < .05, # indicates significant [P < .05] difference from all other conditions).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/10/10.1182_blood-2011-08-374207/4/m_zh89991286890001.jpeg?Expires=1769079765&Signature=llJR1yMPxaP7zuUNlN6ulDOmQxLhW2yYkdTiJE75cM7DWY1Gbyhy47LLvLjjimvz3SaFJqMGJ~58QaRVe5yMVByczTZwFJWwDJayiGvkpEOj45kyLFueKVSguwbejgaC7Dg-Ro9BtyePEFISxfd7bV-JkqvwXE9yyaD8D0eLpY6lXAIIF4vUH7MKjvMURCz~ug~k~qpm8-A~copGqvn4BzYI1YzmhVn9mBL1~bOun4gf8cSZ8G5UaVPvZTZu7wxflr6Cb2H4HHp~mjpdh6HR4XASayCa5f03tS4atBMeu02I9NEbNrMT6YfOzbC1ScI7~tcsmpXCInKdGwfYnzNn7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal