Abstract
The aberrant regulation of B-cell receptor (BCR) signaling allows unwanted B cells to persist, thereby potentially leading to autoimmunity and B-cell malignancies. Casitas B-lineage lymphoma (Cbl) proteins suppress BCR signaling; however, the molecular mechanisms that control Cbl function in human B cells remain unclear. Here, we demonstrate that CIN85 (c-Cbl interacting protein of 85 kDa) is constitutively associated with c-Cbl, Cbl-b, and B-cell linker in B cells. Experiments using CIN85-overexpressing and CIN85-knockdown B-cell lines revealed that CIN85 increased c-Cbl phosphorylation and inhibited BCR-induced calcium flux and phosphorylation of Syk and PLCγ2, whereas it did not affect BCR internalization. The Syk phosphorylation in CIN85-overexpressing and CIN85-knockdown cells was inversely correlated with the ubiquitination and degradation of Syk. Moreover, CIN85 knockdown in primary B cells enhanced BCR-induced survival and growth, and increased the expression of BcLxL, A1, cyclin D2, and myc. Following the stimulation of BCR and Toll-like receptor 9, B-cell differentiation- associated molecules were up-regulated in CIN85-knockdown cells. Together, these results suggest that CIN85 is required for Cbl-mediated regulation of BCR signaling and for downstream events such as survival, growth, and differentiation of human B cells.
Introduction
B-cell receptor (BCR) signaling guides critical cell fate decisions in B cells during ontogeny.1,2 BCRs can generate tolerogenic signals to purge or silence B cells that bind to self-antigens, and immunogenic signals to expand B cells that are specific for foreign antigens. Thus, BCR signaling must be properly regulated at the various stages of B-cell development, as aberrant regulation of BCR signaling potentially leads to autoimmunity and B-cell malignancies.
On BCR ligation by antigens, the Src-family protein tyrosine kinase (PTK) Lyn and Syk are initially activated. Syk propagates the signal by phosphorylating downstream signaling molecules, causing the activation of critical signaling intermediates phosphoinositol 3-kinase (PI3K) and phospholipase C (PLC)γ2. PI3K activates Akt kinase, which is important for B-cell survival.3 PLCγ2 activation induces the release of intracellular Ca2+ and the activation of protein kinase C (PKC), which cause the activation of mitogen-activated protein kinases (MAPKs; ERK, JNK, and p38 MAPK) and of transcription factors, including NF-κB and NF-AT. These molecules regulate further downstream molecules that are responsible for determining B-cell fates such as survival, growth, and differentiation.1,2
Casitas B-lineage lymphoma (Cbl) proteins are E3 ubiquitin ligases that regulate signals of various receptors by promoting the ubiquitination of signaling components.4,5 Tyrosine phosphorylation of Cbl proteins is critical for their function.6 Mammalian Cbl proteins consist of 3 members, c-Cbl, Cbl-b, and Cbl-3, among which c-Cbl and Cbl-b are expressed in hematopoietic cells.7 In B cells, Cbl proteins associate with Syk and B-cell linker (BLNK), and negatively regulate BCR signaling.8,9 B cell-specific ablation of c-Cbl/Cbl-b proteins in mice causes aberrant BCR signaling as well as impaired B-cell anergy, culminating in the development of systemic lupus erythematosus (SLE)–like disease.10 In addition, c-Cbl is hypophosphorylated on tyrosine in advanced stages of chronic lymphocytic leukemia (CLL).11 These findings suggest that Cbl-mediated regulation of BCR signaling is critical for the fate decisions of self-reactive and malignant B cells.
Adaptors are noncatalytic molecules that integrate the spatial and temporal assembly of multiprotein complexes involved in the survival, growth, and differentiation of B cells. We previously showed that the B lymphocyte adaptor molecule of 32 kDa (Bam32)/DAPP1 regulates BCR signaling/internalization and B-cell survival.12,13 The SH3KBP1 (SH3-domain kinase-binding protein 1) gene, which is also known as CIN85 (c-Cbl interacting protein of 85 kDa), encodes an adaptor that is independently identified by several groups and contains 3 SH3 domains, a proline-rich region, and a coiled-coil domain.14-17 Early studies showed that in nonimmune cells, CIN85 regulates the clathrin-dependent internalization of receptor tyrosine kinases (RTKs) such as epidermal growth factor receptors (EGFRs).18,19 The formation of the ternary complex of CIN85, c-Cbl, and endophillin is critical for this process. In immune cells, however, little is known approximately the function of CIN85. CIN85 facilitates ligand-induced FcϵRI internalization in RBL-2H3 mast cells.20 In addition, it regulates FcϵRI signaling via Cbl-mediated regulation of Syk expression in RBL-2H3 cells.21 A recent study showed that CIN85 modulates c-Cbl–mediated down-regulation of FcγRIIa in human neutrophils.22 It is thus of interest to determine whether CIN85 regulates the signaling pathways of other multimeric immune receptors, such as the T- and B-cell receptors.
Here, we demonstrate that CIN85 is constitutively associated with c-Cbl, Cbl-b, and BLNK in human B cells. Gain-of-function and loss-of-function experiments revealed that CIN85 up-regulated c-Cbl phosphorylation and inhibited BCR-induced calcium flux and phosphorylation of Syk and PLCγ2, without affecting BCR internalization. CIN85 also promoted c-Cbl–dependent ubiquitination and degradation of Syk. Moreover, CIN85 knockdown in primary B cells caused enhanced BCR-induced survival and growth, and augmented BCR/TLR9-induced expression of B-cell differentiation-associated molecules. Collectively, these results suggest that CIN85 is required for Cbl-mediated regulation of BCR signaling and for downstream events such as the survival, growth, and differentiation of human B cells.
Methods
Reagents
Goat anti–human IgM and IgG/IgA/IgM F(ab′)2 fragments were purchased from Jackson ImmunoResearch Laboratories. Rabbit anti–human phospho-Zap-70 (Y319)/Syk (Y352), anti–human phospho-PLCγ2 (Y1217), anti–human phospho-Akt, anti–mouse Akt, anti–human phospho-JNK, anti–human phospho-ERK, and anti–human PLCγ2 pAbs as well as anti–human BclxL and Blimp-1 mAbs were purchased from Cell Signaling Technology. Mouse anti–human phospho-Btk, anti–human phospho-BLNK, anti–human c-Cbl, and anti–human Rac1 mAbs were from BD Immunocytometry. Mouse anti–human Cbl-b, anti–human Syk (4D10), anti–human BLNK, and anti-ubiquitin mAbs as well as rabbit anti–human c-Cbl and anti–mouse cyclin D2 pAbs were from Santa Cruz Biotechnology. Mouse anti-V5 mAb was from Invitrogen. Mouse anti-phosphotyrosine and anti–human CIN85 mAbs were from Upstate Biotechnology. Rabbit anti–human Vav2 mAb was from Epitomics. Sheep anti–human CD2AP pAb was from R&D Systems. Mouse anti–β-actin mAb was from Sigma-Aldrich.
B cell lines and primary B cells
The B lymphoma cell line BJAB was cultured in RPMI 1640 medium supplemented with 10% FCS. Human peripheral blood mononuclear cells, kindly provided by healthy volunteers, were separated from their buffy coats. Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. The Institutional Review Board of Kyushu University Hospital approved all research on human subjects. B cells were isolated with Dynabeads M450 CD19 and DETACHaBEAD CD19 (Dynal Biotech), according to the manufacturer's instructions. The isolated B cells exhibited greater than 99.5% viability on trypan blue exclusion and more than 95% purity on flow cytometry. Trace levels of phosphorylation of BCR-signaling molecules were observed in the B cells immediately after purification, probably because of mechanical stress.23 The cells were thus rested for a couple of hours before further analysis. The cells were cultured at a density of 1 × 106 cells/mL in a 96 flat-bottom microtiter plate in complete RPMI 1640 medium supplemented with 10% FCS.
Expression constructs and transfection
Constructs encoding V5-tagged wild-type (WT) and 3 SH3 domain-deleted mutants of human CIN85 (pEF1/V5-CIN85 and –CIN85-dSH3ABC) were previously described.24 The BJAB cells were transfected with the aforementioned constructs using a Gene Pulser apparatus (Bio-Rad Laboratories). The control cells were transfected with an empty vector. Stably transfected BJAB clones were selected in the presence of G418 (2 mg/mL) and screened with anti-V5 mAb.
RNA interference
The pSUPER-based strategy was adopted to knockdown hCIN85 expression. To generate CIN85 small-hairpin RNA (shRNA), a 19-nucleotide sequence (CAGCAATGACATTGACTTA) selected from human CIN85 cDNA was annealed and ligated into the pSUPER or GFP-pSUPER vector. A scrambled sequence (GTTACTAACGCGAATTAAC) was used as negative control. hCIN85 or the control shRNA vector was transfected into BJAB cells using a Gene Pulser apparatus, and stable hCIN85-knockdown BJAB clones were selected in the presence of puromycin (0.5 μg/mL). Transient transfections of primary B cells with the pSUPER-hCIN85 vector were performed using the Nucleofection kit from AMAXA Biosystems as previously described.23
Measurement of intracellular free calcium
B cells were washed with RPMI 1640 medium containing 10% FCS and adjusted to a concentration of 1 × 106 cells/mL. After incubation at 37°C for 15 minutes, 1 μg/mL of Fluo 4/AM (Dojindo) was added, and the cells were incubated for an additional 30 to 45 minutes with resuspension every 15 minutes. The cells were centrifuged and resuspended in RPMI 1640 at a density of 2 × 106 cells/mL and stimulated with anti-IgM (20 μg/mL). The fluorescence intensity of intracellular Fluo 4 was monitored and analyzed using flow cytometry.
Immunoprecipitation
Cells were lysed as described.13 Subsequently, protein G-Sepharose (Amersham Pharmacia Biotech) precleared lysates were incubated with anti-V5, -BLNK, -Syk, -Cbl, -Vav2 mAb, or -CD2AP pAb for 1 hour at 4°C and then immunoprecipitated with protein G-Sepharose overnight at 4°C. The precipitated proteins were resolved by 10% SDS-PAGE; transferred onto a Millipore Immobilon polyvinylidene difluoride membrane; and blotted with anti-phosphotyrosine (4G10), -V5, -c-Cbl, -Cbl-b, -BLNK, -Vav2, -Syk, or -ubiquitin mAbs, followed by incubation with secondary HRP-conjugated IgG (Jackson ImmunoResearch Laboratories) specific for the primary Ab. The blots were developed with an ECL Plus kit (Amersham Biosciences).
Western blot analysis
Nonstimulated or stimulated cells (1 × 106) were lysed as described.12 The lysates were then denatured in an equal volume of 2 × SDS sample buffer, resolved on a 10% SDS-PAGE gel, and electro-transferred to nitrocellulose membranes in non-SDS–containing transfer buffer (25mM Tris, 0.2M glycine, and 20% methanol; pH 8.5). Western blotting was performed with anti–phospho-Syk (1:2000), anti–phospho-PLCγ2 (1:2000), anti–phospho-BLNK (1:2000), anti–phospho-JNK (1:2000), anti–phospho-ERK (1:2000), anti–phospho-Akt (1:2000), anti-Akt (1:2000), anti-CIN85 (1:2000), anti–β-actin (1:2000), or anti-Vav2 (1:2000), followed by a 1:15 000 dilution of anti–rabbit or anti–mouse HRP-conjugated IgG. The blots were developed with the ECL plus kit (Amersham Biosciences). The chemiluminescence intensity was monitored using a Laser3000 (FujiFilm) instrument. We quantitated the band intensity of the proteins using ImageGauge Version 4.22 software (FujiFilm). The resulting values were expressed as fold changes in protein expression relative to the protein expression in unstimulated control cells.
Luciferase assays
Cells (1 × 107) were transfected by electroporation with the NF-AT-reporter construct, which was generously provided by Dr Shoichiro Miyatake (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). After 18 to 20 hours, cells were harvested and plated on 96-well plates at a density of 2 × 105/well. Triplicate cultures were incubated in the media alone with graded doses of anti-IgM or with 50nM PMA and 2.5μM ionomycin. After 6 hours, the cells were lysed in 50 μL reporter lysis buffer (Promega) for 15 minutes at room temperature. The luciferase activity was assayed by adding 20 μL luciferase substrate (Promega) to 50 μL lysate and immediately measuring the luminescence on a Lumat LB9507 luminometer (EG & G Berthold). To serve as a control for the transfection efficiency, the relative luciferase activity of the medium and cells stimulated with BCR was calculated relative to stimulation with PMA/ionomycin.
Flow cytometric analysis
BJAB cells were incubated on ice for 15 minutes with 20 μg/mL goat-unlabeled anti-IgM before they were washed with ice-cold medium and warmed at 37°C for the indicated time intervals. The cells were washed with ice-cold PBS containing 2% FBS and 0.2% sodium azide (Fisher Scientific) to stop internalization at the assigned time points and to remove the unbound Ab. The remaining surface BCRs were stained with FITC-labeled rabbit anti–goat Ig and quantified by flow cytometry. The data are presented as the percentage of surface BCR remaining.
Fluorescence microscopic analysis
BJAB cells were incubated with 10 μg/mL of unlabeled goat anti–human IgM sera (20 μg/mL) at 4°C for 30 minutes and warmed to 37°C for the indicated time periods. The cells were then fixed with 3.7% paraformaldehyde and permeabilized with PBS containing 1% BSA and 0.05% saponin (wash buffer). The cells were then incubated for 30 minutes with FITC-conjugated anti–goat IgG pAb (Jackson ImmunoResearch Laboratories) at 4°C. The stained cells were centrifuged onto slides and analyzed with inverted fluorescent microscopy (BZ-9000; Keyence).
Quantitative real-time PCR
The total RNA was extracted from the primary B cells using Isogen reagent (Nippon gene) and was treated with DNase I (Invitrogen) to remove contaminating genomic DNA. First-strand cDNA was synthesized using a QuantiTect reverse transcription kit (QIAGEN). Quantitative real-time PCR was performed in the ABI Prism 7700 Sequence Detector (Applied Biosystems). The reactions were performed in triplicate wells in 96-well plates. TaqMan target mixes for Cyclin D2, Myc, BCL2L1/BclxL, BCL2A1/A1, PRDM1/Blimp-1, and XBP1 were purchased from Applied Biosystems. 18S ribosomal RNA (rRNA) was separately amplified in the same plate as an internal control for variation in the amount of cDNA in PCR. The collected data were analyzed using the Sequence Detector software (Applied Biosystems). The data were expressed as the fold change in gene expression relative to the expression in the control cells.
Annexin V staining
After culture, cells (1-2 × 105) were washed twice with PBS and suspended in 85 μL binding buffer (MBL) containing Ca2+. The cell suspension supplemented with 10 μL annexin-V–FITC or annexin-V–PE (MBL) and 5 μg of propidium iodide (PI) or 1 μg of 7-ADD was incubated at room temperature for 15 minutes in the dark. Subsequently, binding buffer was added, and the fraction of early apoptotic cells was measured using flow cytometry.
BrdU assay
DNA synthesis was monitored by pulse-labeling cells for 2 hours with the thymidine analog 5-bromo-2′-deoxyuridine (BrdU). The cells were washed 3 times with PBS and fixed for 20 minutes at −20°C in an ethanol fixative (0.15mM glycine in 70% EtOH, pH 2.0). After rehydration in PBS, BrdU incorporation was detected by incubation with an anti-BrdU mAb for 1 hour at 37°C, followed by a rhodamine-conjugated anti–mouse antibody (1:500; Jackson ImmunoResearch Laboratories) and staining of the nucleus with 4′-6-diamidino-2-phenylindole for 1 hour. The proportion of BrdU-positive nuclei (BrdU labeling index) was assessed, based on a sample size of 500 cells per data point.
Statistical analysis
Statistical analysis was performed using the Student t test. P < .05 was considered statistically significant.
Results
CIN85 associates with Cbl and BLNK and regulates their phosphorylation
The tyrosine phosphorylation of signaling molecules is a critical event in BCR signaling.1,2 Because SH3 domains play an important role in the function of CIN85,25 we focused on tyrosine-phosphorylated molecules downstream of the BCR that could associate with the SH3 domains of CIN85. Specifically, we focused on the 2 molecules, BLNK and c-Cbl, that function as key positive and negative regulators of BCR signaling,1,10 respectively; both proteins can associate with the SH3 domains of CIN85.14,26
We first determined the association of Cbl and BLNK with CIN85 using WT and SH3-deleted CIN85-expressing B cell lines. Consistent with previous reports,14,26 WT CIN85 was constitutively associated with c-Cbl and BLNK, and these associations were increased after BCR stimulation (Figure 1A). Cbl-b was similarly associated with WT CIN85, albeit to a lesser extent. Although the association of WT CIN85 and BLNK appeared modest, the inverse immunoprecipitation of BLNK confirmed the association (Figure 1A). As expected, the association of Cbl and BLNK with CIN85 was abrogated in SH3-deleted CIN85-expressing B cells, suggesting that the SH3 domains of CIN85 are required for its association with Cbl and BLNK. Because the tyrosine phosphorylation of c-Cbl and BLNK is critical for their function,6,27 we next determined whether the overexpression of WT and SH3-deleted CIN85 affects BCR-induced phosphorylation of c-Cbl and BLNK. Compared with control cells, WT and SH3-deleted CIN85 sustained and inhibited c-Cbl phosphorylation, respectively (Figure 1B). In addition, WT and SH3-deleted CIN85 inhibited and enhanced BLNK phosphorylation, respectively (Figure 1B). These findings suggest that CIN85 associates with Cbl and BLNK and regulates their phosphorylation in an opposite manner.
CIN85 associates with Cbl and BLNK and regulates their phosphorylation in B cells. (A) BJAB cells stably expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-V5 or anti-BLNK mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–c-Cbl, anti–Cbl-b, anti-BLNK, or anti-V5 mAb. 5′c, immunoprecipitation of the cell lysates at 5 minutes with isotype control. (B) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti–c-Cbl mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-phosphotyrosine or anti–c-Cbl mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-BLNK or anti-BLNK mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls).
CIN85 associates with Cbl and BLNK and regulates their phosphorylation in B cells. (A) BJAB cells stably expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-V5 or anti-BLNK mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–c-Cbl, anti–Cbl-b, anti-BLNK, or anti-V5 mAb. 5′c, immunoprecipitation of the cell lysates at 5 minutes with isotype control. (B) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti–c-Cbl mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-phosphotyrosine or anti–c-Cbl mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′) 2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-BLNK or anti-BLNK mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls).
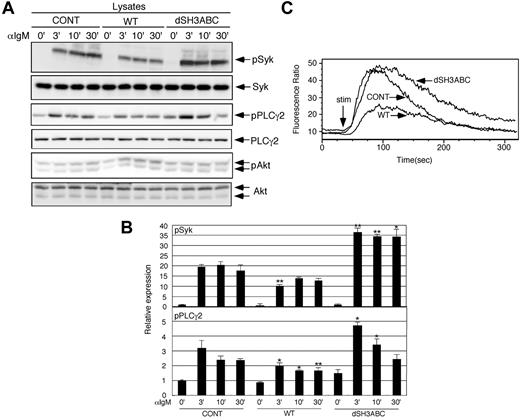
Forced CIN85 expression inhibits BCR-induced calcium flux and the phosphorylation of Syk and PLCγ2
We tested whether the overexpression of WT and SH3-deleted CIN85 affects early BCR signaling. Syk phosphorylation, which is one of the earliest events in BCR signaling, was inhibited in WT CIN85-expressing cells, whereas it was sustained in SH3-deleted CIN85-expressing cells (Figure 2A-B). Two enzymes, PLCγ2 and PI3K, function as critical mediators downstream of BCR signaling.1,2,28 WT and SH3-deleted CIN85 partially inhibited and enhanced BCR-induced phosphorylation of PLCγ2, respectively (Figure 2A-B). In contrast, the phosphorylation of Akt, which is a downstream molecule of PI3K, was not affected in WT or SH3-deleted CIN85-expressing cells (Figure 2A). Activated PLCγ2 converts PIP2 into IP3 and diacyl glycerol, of which PIP2 is critical for calcium flux in B cells.1,2,12 Consistent with the levels of PLCγ2 phosphorylation, the BCR-induced calcium flux was significantly inhibited in WT CIN85-expressing cells, whereas it was slightly sustained in SH3-deleted CIN85-expressing cells (Figure 2C). These results suggest that CIN85 inhibits BCR-induced calcium flux and the phosphorylation of Syk and PLCγ2, and that the SH3 domains of CIN85 are required for its inhibitory function.
Forced CIN85 expression inhibits BCR-induced calcium flux and phosphorylation of Syk and PLCγ2. (A-B) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′)2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb, anti-Syk mAb, anti–phospho-PLCγ2 pAb, anti-PLCγ2 pAb, anti–phospho-Akt pAb, or anti-Akt pAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Ca2+ influx in control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85. The intracellular free calcium levels in Fluo 4/AM-loaded cells were analyzed using flow cytometry after the cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM. The results shown are representative of 4 independent experiments.
Forced CIN85 expression inhibits BCR-induced calcium flux and phosphorylation of Syk and PLCγ2. (A-B) Control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85 were stimulated with 20 μg/mL of F(ab′)2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb, anti-Syk mAb, anti–phospho-PLCγ2 pAb, anti-PLCγ2 pAb, anti–phospho-Akt pAb, or anti-Akt pAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Ca2+ influx in control BJAB cells and stable transformants expressing either WT or SH3-deleted CIN85. The intracellular free calcium levels in Fluo 4/AM-loaded cells were analyzed using flow cytometry after the cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM. The results shown are representative of 4 independent experiments.
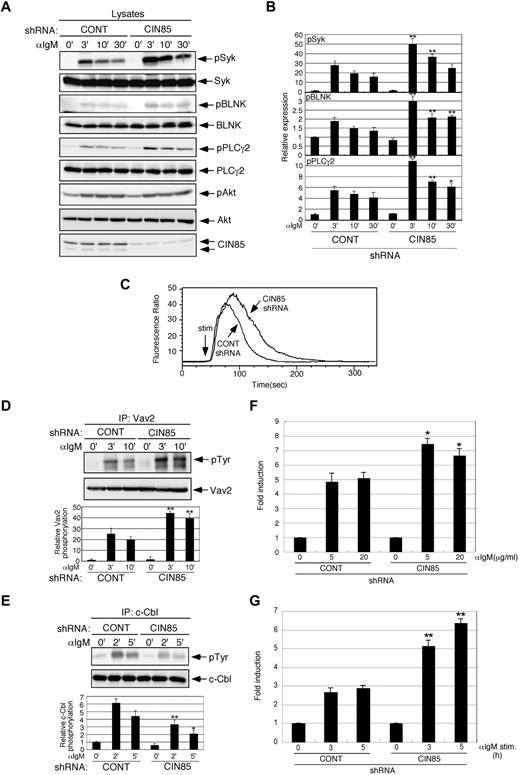
CIN85 knockdown enhances BCR-induced calcium flux and the phosphorylation of Syk, Vav2, and PLCγ2, leading to augmented NF-AT activation and CD69 expression
To elucidate the role of endogenously expressed CIN85 in BCR signaling, we generated CIN85-knockdown B cell lines. In contrast to the CIN85-overexpressing cells (Figures 1 and 2), CIN85-knockdown cells exhibited enhanced phosphorylation of Syk, BLNK, and PLCγ2 (Figure 3A-B). Akt phosphorylation was comparable between control and CIN85-knockdown cells (Figure 3A). Consistent with the levels of PLCγ2 phosphorylation, BCR-induced calcium flux was accentuated in CIN85-knockdown cells (Figure 3C). Vav2 positively regulates PLCγ2 activation in B cells.29 Vav2 phosphorylation was enhanced in CIN85-knockdown cells (Figure 3D). These BCR signaling profiles in CIN85-knockdown cells are reminiscent of those in c-Cbl/Cbl-b double-knockout B cells.10 The phosphorylation of c-Cbl was significantly inhibited in CIN85-knockdown cells (Figure 3E). BCR-induced calcium flux plays a crucial role in the activation of the transcription factor NF-AT, the disruption of which results in significant defects in B-cell function.30 BCR-induced NF-AT activation was enhanced in CIN85-knockdown cells (Figure 3F). In addition, BCR-induced up-regulation of the activation marker CD69 was pronounced in CIN85-knockdown cells (Figure 3G). These phenotypes in CIN85-knockdown cells were again similar to those observed in Cbl-deficient B cells.10 Given that CIN85 strongly associates with Cbl proteins (Figure 1A), these results suggest that CIN85 plays a vital role in Cbl-mediated regulation of BCR signaling.
CIN85 knockdown enhances BCR-induced calcium flux and phosphorylation of Syk, Vav2, and PLCγ2, leading to augmented NF-AT activation and CD69 expression. (A-B) Stable control and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′) 2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb, anti-Syk mAb, anti–phospho-BLNK mAb, anti-BLNK mAb, anti–phospho-PLCγ2 pAb, anti-PLCγ2 pAb, anti–phospho-Akt pAb, anti-Akt pAb, or anti-CIN85 mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Ca2+ influx in stable control and CIN85-knockdown BJAB cells. Intracellular free calcium levels in Fluo 4/AM-loaded cells were analyzed using flow cytometry after the cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM. The results shown are representative of 4 independent experiments. (D-E) Stable control and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-Vav2 or anti–c-Cbl mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-phosphotyrosine mAb, anti-Vav2 mAb, or anti–c-Cbl mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (F) Stable control and CIN85-knockdown BJAB cells transfected with the NF-AT luciferase reporter construct were stimulated with graded doses of F(ab′)2 goat anti–human IgM for 8 hours and lysed, and the luciferase activity was assayed using a luminometer. The relative luciferase activity of the medium and BCR-stimulated cells was expressed with respect to that of the PMA/ionomycin stimulation. The results were presented as the mean and SEM of triplicate cultures. One experiment representative of 4 independent experiments is shown (*P < .05 vs controls). (G) Stable control and CIN85-knockdown BJAB cells before and after stimulation with 20 μg/mL F(ab′)2 goat anti–human IgM (3 and 5 hours) were analyzed for surface expression of CD69. One experiment representative of 3 independent experiments is shown (**P < .01 vs controls).
CIN85 knockdown enhances BCR-induced calcium flux and phosphorylation of Syk, Vav2, and PLCγ2, leading to augmented NF-AT activation and CD69 expression. (A-B) Stable control and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′) 2 goat anti–human IgM for the indicated time periods. The cell lysates were subsequently separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb, anti-Syk mAb, anti–phospho-BLNK mAb, anti-BLNK mAb, anti–phospho-PLCγ2 pAb, anti-PLCγ2 pAb, anti–phospho-Akt pAb, anti-Akt pAb, or anti-CIN85 mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (C) Ca2+ influx in stable control and CIN85-knockdown BJAB cells. Intracellular free calcium levels in Fluo 4/AM-loaded cells were analyzed using flow cytometry after the cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM. The results shown are representative of 4 independent experiments. (D-E) Stable control and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-Vav2 or anti–c-Cbl mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-phosphotyrosine mAb, anti-Vav2 mAb, or anti–c-Cbl mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (*P < .05, **P < .01 vs controls). (F) Stable control and CIN85-knockdown BJAB cells transfected with the NF-AT luciferase reporter construct were stimulated with graded doses of F(ab′)2 goat anti–human IgM for 8 hours and lysed, and the luciferase activity was assayed using a luminometer. The relative luciferase activity of the medium and BCR-stimulated cells was expressed with respect to that of the PMA/ionomycin stimulation. The results were presented as the mean and SEM of triplicate cultures. One experiment representative of 4 independent experiments is shown (*P < .05 vs controls). (G) Stable control and CIN85-knockdown BJAB cells before and after stimulation with 20 μg/mL F(ab′)2 goat anti–human IgM (3 and 5 hours) were analyzed for surface expression of CD69. One experiment representative of 3 independent experiments is shown (**P < .01 vs controls).
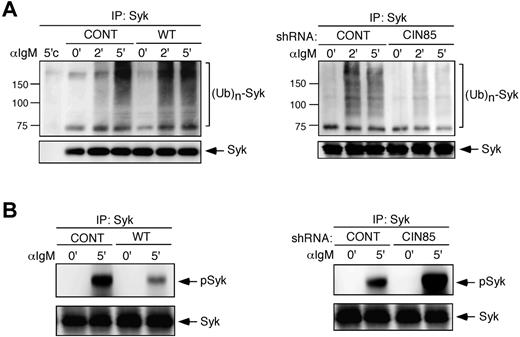
CIN85 promotes the ubiquitination and degradation of Syk in B cells
Cbl proteins function as E3 ubiquitin ligases and target PTK substrates, including Syk, for degradation.31,32 We thus tested whether CIN85 affects Syk ubiquitination in B cells. Syk ubiquitination was induced on BCR stimulation. Compared with control cells, Syk ubiquitination was increased in WT CIN85-expressing cells (Figure 4A). In contrast, an impairment in Syk ubiquitination was noted in CIN85-knockdown cells (Figure 4A). These results suggest that CIN85 positively regulates Cbl-mediated ubiquitination of BCR-signaling molecules including Syk. Despite the altered levels of Syk ubiquitination, the level of total Syk protein was not altered in the WT CIN85-expressing or CIN85-knockdown cells throughout the stimulation (Figures 2A and 3A). Because only a small pool of Syk is phosphorylated on stimulation and targeted for degradation in B cells,31 we tested the degree of Syk phosphorylation among the total Syk immunoprecipitate. The levels of phosphorylated Syk were reduced in WT CIN85-expressing cells but enhanced in CIN85-knockdown cells (Figure 4B), suggesting that CIN85 promotes Cbl-dependent loss of the phosphorylated pool of Syk in B cells.
CIN85 promotes Syk ubiquitination and degradation in B cells. (A) Control BJAB cells, stable transformants expressing WT CIN85, and CIN85- knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-Syk mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-ubiquitin or anti-Syk mAb. 5′'c, immunoprecipitation of the cell lysates at 5 minutes with isotype control. The molecular weight is indicated on the left side of the blots. (B) Control BJAB cells, stable transformants expressing WT CIN85, and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for 5 minutes. Immunoprecipitates with anti-Syk mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb or anti-Syk mAb.
CIN85 promotes Syk ubiquitination and degradation in B cells. (A) Control BJAB cells, stable transformants expressing WT CIN85, and CIN85- knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for the indicated time periods. Immunoprecipitates with anti-Syk mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti-ubiquitin or anti-Syk mAb. 5′'c, immunoprecipitation of the cell lysates at 5 minutes with isotype control. The molecular weight is indicated on the left side of the blots. (B) Control BJAB cells, stable transformants expressing WT CIN85, and CIN85-knockdown BJAB cells were stimulated with 20 μg/mL F(ab′)2 goat anti–human IgM for 5 minutes. Immunoprecipitates with anti-Syk mAb were separated on a 10% SDS-PAGE gel and analyzed by Western blotting with anti–phospho-Syk pAb or anti-Syk mAb.
CIN85 does not affect BCR internalization
CIN85 regulates Cbl-mediated internalization of the EGFR in several cell types other than B cells.18,19 To test whether CIN85 affects BCR internalization, we first monitored the levels of surface BCR expression after stimulation. Without stimuli, the levels of surface BCR were similar on CIN85 overexpression and CIN85 knockdown. In parental cells, BCR crosslinking caused a rapid decrease in surface BCR levels, suggesting that BCR was efficiently internalized after stimulation (Figure 5A; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). BCR internalization was not affected in the WT or SH3-deleted CIN85-expressing cells. Moreover, the absence of endogenous CIN85 did not affect BCR internalization (Figure 5B, supplemental Figure 1A). Next, we directly visualized BCRs in B cell lines using fluorescence microscopy. In control cells, the BCR complexes exhibited a slightly patchy distribution before stimulation, and within 3 minutes after stimulation, the BCRs formed polarized tight caps on the cell surface. After 10 minutes of BCR stimulation, a punctate pattern of internalized BCRs was clearly visualized (Figure 5C). Consistent with the findings obtained with flow cytometry (Figure 5A-B), the spatial and temporal distribution of BCR complexes in CIN85-overexpressing and CIN85-knockdown cells appeared similar to that in control cells (Figure 5C, supplemental Figure 1B-C). These findings suggest that CIN85 does not affect BCR internalization.
CIN85 does not affect BCR internalization. (A) BJAB cells (control and stable transformants expressing either WT or SH3-deleted CIN85) and (B) BJAB cells (control and CIN85-knockdown) were incubated at 4°C with F(ab′)2 goat anti–human IgM for 30 minutes. The cells were washed, warmed to 37°C for the indicated time intervals, stained at 4°C for 30 minutes with a FITC-labeled anti–goat IgG pAb, and analyzed by flow cytometry. The results are expressed as the percentage of surface BCRs remaining. The data are presented as the average and SEM of 3 independent experiments. (C) Control BJAB cells, stable transformants expressing either WT or SH3-deleted CIN85, and CIN85-knockdown BJAB cells were incubated at 4°C with 20 μg/mL F(ab′)2 goat anti–human IgM for 30 minutes. The cells were washed and warmed to 37°C for the indicated time periods. The cells were fixed, permeabilized, stained with a FITC-labeled anti–goat IgG pAb, and analyzed by fluorescence microscopy. The images shown are representative of 3 independent experiments.
CIN85 does not affect BCR internalization. (A) BJAB cells (control and stable transformants expressing either WT or SH3-deleted CIN85) and (B) BJAB cells (control and CIN85-knockdown) were incubated at 4°C with F(ab′)2 goat anti–human IgM for 30 minutes. The cells were washed, warmed to 37°C for the indicated time intervals, stained at 4°C for 30 minutes with a FITC-labeled anti–goat IgG pAb, and analyzed by flow cytometry. The results are expressed as the percentage of surface BCRs remaining. The data are presented as the average and SEM of 3 independent experiments. (C) Control BJAB cells, stable transformants expressing either WT or SH3-deleted CIN85, and CIN85-knockdown BJAB cells were incubated at 4°C with 20 μg/mL F(ab′)2 goat anti–human IgM for 30 minutes. The cells were washed and warmed to 37°C for the indicated time periods. The cells were fixed, permeabilized, stained with a FITC-labeled anti–goat IgG pAb, and analyzed by fluorescence microscopy. The images shown are representative of 3 independent experiments.
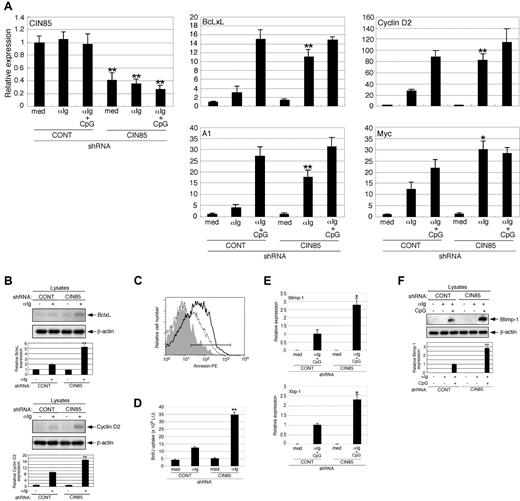
CIN85 knockdown enhances the survival, growth, and differentiation of primary B cells
BCR signaling plays a critical role in determining the survival, growth, and differentiation of B cells.1 It was thus of interest to test whether CIN85 affects B cell fate. A major obstacle, however, is that the survival, growth, and differentiation of B cell cannot be properly assessed in transformed B cells. We therefore sought to knock down CIN85 expression in human primary B cells. After introduction of the GFP-CIN85 knockdown vector, GFP-positive B cells were sorted and used for further experimentation. Under these conditions, we were able to knock down the CIN85 mRNA expression in B cells by 60%-80% (Figure 6A).
CIN85 knockdown enhances the survival, growth, and differentiation of primary B cells. (A) Control and CIN85-knockdown primary B cells were incubated for 24 hours in medium containing F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) or αIg plus CpG (1μM), and CIN85, BcLxL, A1, cyclin D2, and myc mRNA levels were quantified by real-time PCR. The data are normalized to the expression of 18S rRNA. The results shown are representative of 3 independent experiments (*P < .05, **P < .01 vs controls). (B) Control and CIN85-knockdown primary B cells were incubated for 24 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). The cell lysates were subsequently separated on a SDS-PAGE gel and analyzed by Western blotting with anti-BclxL mAb, anti-cyclin D2 pAb, or anti–β-actin mAb. The resulting values are expressed as fold changes in protein expression compared with nonstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls). (C) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). After culture, the cells were stained with PE-labeled annexin V and analyzed using flow cytometry. The percentages of annexin-positive cells are shown. A representative histogram of 3 independent experiments is shown. (D) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). After culture, the cells were pulsed with BrdU, and its incorporation was detected by incubation with anti-BrdU mAb, followed by rhodamine-conjugated anti–mouse Ab. A representative histogram of 3 independent experiments is shown (**P < .01 vs controls). (E) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) and CpG (1μM), and quantitation of Blimp-1 and Xbp-1 mRNA by real-time PCR was carried out. The data are normalized to the expression of 18S rRNA. The results shown are representative of 3 independent experiments (*P < .05 vs controls). (F) Control and CIN85-knockdown primary B cells were incubated for 48 hours with or without F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) in the absence or presence of CpG (1μM). The cell lysates were subsequently separated on a SDS-PAGE gel and analyzed by Western blotting with anti–Blimp-1 mAb or anti–β-actin mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls).
CIN85 knockdown enhances the survival, growth, and differentiation of primary B cells. (A) Control and CIN85-knockdown primary B cells were incubated for 24 hours in medium containing F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) or αIg plus CpG (1μM), and CIN85, BcLxL, A1, cyclin D2, and myc mRNA levels were quantified by real-time PCR. The data are normalized to the expression of 18S rRNA. The results shown are representative of 3 independent experiments (*P < .05, **P < .01 vs controls). (B) Control and CIN85-knockdown primary B cells were incubated for 24 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). The cell lysates were subsequently separated on a SDS-PAGE gel and analyzed by Western blotting with anti-BclxL mAb, anti-cyclin D2 pAb, or anti–β-actin mAb. The resulting values are expressed as fold changes in protein expression compared with nonstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls). (C) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). After culture, the cells were stained with PE-labeled annexin V and analyzed using flow cytometry. The percentages of annexin-positive cells are shown. A representative histogram of 3 independent experiments is shown. (D) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL). After culture, the cells were pulsed with BrdU, and its incorporation was detected by incubation with anti-BrdU mAb, followed by rhodamine-conjugated anti–mouse Ab. A representative histogram of 3 independent experiments is shown (**P < .01 vs controls). (E) Control and CIN85-knockdown primary B cells were incubated for 48 hours in the absence or presence of F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) and CpG (1μM), and quantitation of Blimp-1 and Xbp-1 mRNA by real-time PCR was carried out. The data are normalized to the expression of 18S rRNA. The results shown are representative of 3 independent experiments (*P < .05 vs controls). (F) Control and CIN85-knockdown primary B cells were incubated for 48 hours with or without F(ab′)2 goat anti–human IgG/IgA/IgM (αIg, 20 μg/mL) in the absence or presence of CpG (1μM). The cell lysates were subsequently separated on a SDS-PAGE gel and analyzed by Western blotting with anti–Blimp-1 mAb or anti–β-actin mAb. The resulting values are expressed as fold changes in protein expression compared with unstimulated control cells. The values are the mean ± SD of 3 independent experiments (**P < .01 vs controls).
We first tested whether CIN85 knockdown affects the expression of the B-cell survival-associated genes BcLxL and A1. Consistent with previous studies,33 BCR stimulation induced BcLxL and A1 mRNA expression in control cells. This induction was far more drastic in CIN85-knockdown B cells (Figure 6A). The costimulation of TLR9 with its ligand CpG enhances BCR-induced expression of B-cell survival genes.34 This enhancement was less evident in CIN85-knockdown cells than in control cells (Figure 6A), suggesting that CIN85 knockdown requires less costimulation for the full induction of B-cell survival genes. Consistent the findings for the transcript levels, the BCR-induced expression of BcLxL protein was more pronounced in CIN85-knockdown cells (Figure 6B). We also tested whether CIN85 knockdown affects BCR-induced death of B cells using the annexin-binding assay. The CIN85-knockdown cells exhibited less BCR-induced cell death (Figure 6C). We next tested whether CIN85 knockdown affects the expression of the B-cell growth-associated genes cyclin D2 and myc. Again, BCR-induced expression of these genes was more pronounced in CIN85-knockdown cells (Figure 6A), and costimulation with TLR9 did not enhance induction compared with the control cells. Consistent with the findings for the transcript levels, BCR-induced expression of cyclin D2 protein was more pronounced in CIN85-knockdown cells (Figure 6B). We also tested whether CIN85 knockdown affects B-cell growth using the BrdU uptake assay. Consistent with the expression levels of cyclin D2 and myc, CIN85 knockdown enhanced BCR-induced cell growth (Figure 6D). On activation, B cells undergo plasma cell differentiation along with the expression of critical differentiation-associated genes such as Blimp-1 and Xbp-1. Consistent with previous studies,35 BCR stimulation alone was not sufficient to induce the expression of Blimp-1 and Xbp-1 in human B cells (data not shown). However, the combined stimulation of BCR and TLR9 clearly induced the expression of these genes in control cells, whereas this induction was more pronounced in CIN85-knockdown B cells (Figure 6E). Consistent with the findings for the transcript levels, BCR stimulation alone did not induce detectable levels of Blimp-1 protein. However, the combined stimulation of BCR and TLR9 clearly induced the expression of the Blimp-1 protein in control cells, although this was more pronounced in CIN85-knockdown cells (Figure 6F). These results suggest that CIN85 is required for Cbl-mediated regulation of BCR signaling and downstream events such as the survival, growth, and differentiation of human B cells.
Discussion
We demonstrated here that CIN85 functions as a novel adaptor to regulate proximal BCR signaling. Gain-of-function and loss-of-function experiments revealed that CIN85 not only enhances BCR-induced c-Cbl phosphorylation but also inhibits BCR-induced calcium flux and the phosphorylation of Syk and PLCγ2. CIN85 promotes c-Cbl–dependent ubiquitination and degradation of Syk, which is a key upstream kinase that propagates BCR signaling by phosphorylating downstream molecules including PLCγ2. Because Cbl proteins directly associate with Syk and inhibit its function,6 it is probable that CIN85 acts as a critical scaffolding adaptor for Cbl proteins and is indispensable for Cbl-mediated regulation of Syk activation in B cells.
Consistent with our findings, a critical role of CIN85 in Cbl-mediated regulation of Syk activation was recently shown in FcϵRI signaling in mast cells.21 In mast cells, CIN85 enhances c-Cbl-mediated ubiquitination and the degradation of Syk protein.21 In B cells, however, CIN85 overexpression significantly increased Syk ubiquitination (Figure 4), but CIN85 knockdown did not alter the total levels of Syk protein throughout stimulation (Figure 3A), as previously shown in c-Cbl/Cbl-b double-knockout B cells.10 This apparent discrepancy in Syk degradation between mast cells and B cells could be explained by the findings of Rao et al,31 who showed that on BCR stimulation, only a small portion of Syk is phosphorylated and then degraded by c-Cbl. Rao et al also showed that c-Cbl does not directly affect the catalytic activity of Syk.31 Consistent with these findings, our study showed that CIN85 promotes c-Cbl–mediated ubiquitination and degradation of the phosphorylated pool of Syk (Figure 4A-B).
What, then, are the possible mechanisms by which CIN85 enhances BCR-induced c-Cbl phosphorylation in B cells? Src-family PTKs and Syk are proposed to phosphorylate c-Cbl on tyrosines.6 We previously showed that CIN85 directly interacts with the SH3 domain of Src-family PTKs including Lyn.17 In addition, CIN85 directly associates with BLNK, PLCγ and Vav, all of which are direct Syk interactors,17,36 and thus, CIN85 is indirectly associated with Syk via binding to BLNK, PLCγ, and Vav. In view of these findings, it seems probable that CIN85 acts as a key scaffolding adaptor that permits the spatial proximity of Src-family PTKs, Syk, and Cbl proteins and thus facilitates their phosphorylation of Cbl proteins.
Although CIN85 appears to function in concert with Cbl proteins to regulate BCR signaling, an additional mechanism is possible. Previous in vitro binding experiments showed that CIN85 directly binds to Src-family tyrosine kinases, PLCγ, p85 PI3K,Vav, Btk, and SHIP, all of which are involved in BCR signaling, through its SH3 domains and proline-rich region.15,24,25 In addition, a recent study showed that the SH3 domains of CIN85 could uniquely bind to ubiquitin.37 Thus, after various BCR-signaling molecules are ubiquitinated by Cbl proteins on stimulation, the competition between canonical SH3 ligands and ubiquitin binding to CIN85 may affect BCR signaling in a temporal and spatial manner. Therefore, it is probable that CIN85 also directly regulates BCR signaling by a Cbl-independent mechanism.
A recent study using liquid chromatography-coupled tandem mass spectrometry showed that 3 SH3 domains of CIN85 could recruit protein molecules required for the proper formation and function of coated vesicles.25 Similarly, early studies showed a characteristic feature of CIN85 in the formation of clathrin-coated vesicles during the internalization of RTKs such as EGFRs in nonimmune cells.18,19 Brain-specific CIN85-deficient mice manifest impaired internalization of D2 dopamine receptors, which belong to the 7-transmembrane G protein-coupled receptor superfamily.38 In addition, CIN85 facilitates ligand-induced FcϵRI internalization in RBL-2H3 mast cell lines.20 Because BCR internalization is regulated via a clathrin-dependent pathway,39 it was of interest to determine whether CIN85 regulates BCR internalization. Our study, however, shows that CIN85 does not affect BCR internalization (Figure 5, supplemental Figure 1). These data are somewhat surprising, given that Cbl proteins control BCR internalization by a ubiquitin-dependent mechanism.10,40 However, the role of Cbl proteins in BCR ubiquitination and internalization is still rather controversial. The HECT family member Itch, but not c-Cbl, is an E3 ubiquitin ligase that is involved in BCR ubiquitination.41 In addition, the ubiquitination of Igβ, which is a component of BCR, does not facilitate BCR internalization but is required for the sorting of early endosomes and for trafficking into late endosomes,41 which suggests that BCR ubiquitination is more critical at the later stage of its trafficking. Because our imaging analysis (Figure 5C) cannot clearly distinguish the spatial distribution of early and late endosomes, it is of great interest to test whether CIN85 affects postendocytotic BCR trafficking. A recent study showed that in human neutrophils, CIN85 modulates c-Cbl–mediated down-regulation of FcγRIIa in the later stages of receptor trafficking without affecting the internalization of this receptor.22
During the submission of this paper, 2 studies were published that, in contrast to our findings, showed that CIN85 positively regulates BCR signaling in mouse and chicken B cells.42,43 These studies found that CIN85 associates with BLNK and regulates BCR-induced NF-κB activation. However, the detailed profiles of BCR signaling differ between the 2 studies: the BCR-induced phosphorylation of BLNK and PLCγ2 and the calcium flux are significantly decreased on the loss of CIN85 in chicken B cells, whereas they are apparently normal in CIN85-deficient mouse B cells.42,43 It should be noted that the former study did not actually use CIN85-deficient cells; rather, it used cells expressing a mutant BLNK that failed to bind to CIN85 or its homolog CD2AP.42 Although these findings are intriguing, it is rather surprising that Cbl-mediated function of CIN85 in B cells was barely investigated in these studies. As previously mentioned, it is becoming evident that Cbl proteins play a critical role in the function of CIN85 in immune cells.20-22 In addition, BCR-induced association of CIN85 with c-Cbl was recently shown even in mouse B cells.44 We thus find that in human B cells, CIN85 negatively regulates BCR signaling via a Cbl-dependent mechanism. Our data obtained using CIN85-knockdown primary B cells also support this hypothesis. The molecular reason underlying the apparent discrepancy between our study and the aforementioned ones42,43 remains unclear. One possibility, however, is that the relative contribution of CIN85-binding partners varies depending on the source of B cells used. In human B cells, Cbl proteins seem to preferentially associate with CIN85 over BLNK (Figure 1A). Notably, we found that CD2AP seems to preferentially associate with BLNK over c-Cbl in human B cells (supplemental Figure 2). It is therefore of potential interest to compare the roles of CIN85 and CD2AP in the function of human B cells.
BCR signals play a pivotal role in the survival, growth, and differentiation of B cells.1,2 Under physiologic conditions, BCR signaling is fine-tuned by positive and negative regulators and is generally insufficient for the full activation of B cells, rendering them susceptible to apoptosis and anergy. However, when the negative regulation of BCR signaling is compromised, unwanted B cells could grow and survive, thereby potentially leading to autoimmunity and B-cell malignancies. This study showed that CIN85 knockdown in primary B cells causes full activation of B cells and enhances BCR-induced survival and growth via the increased expression of BcLxL, A1, cyclin D2, and myc (Figure 6). Given that Cbl proteins are critical for B-cell anergy,10 CIN85 may cooperate with Cbl proteins to function as a key negative regulator for BCR signaling and to maintain self-tolerance. It is thus of interest to determine whether the expression and/or function of CIN85 could be altered in human autoimmune diseases such as SLE. Surprisingly, CLL cells from advanced-stage patients exhibit hypophosphorylation of c-Cbl,11 as seen in CIN85-knockdown cells. The manipulation of CIN85 expression may therefore provide a novel strategy to control aberrant cell growth and survival in B-cell malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Editage for proofreading the English used in this paper.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.N. and K.A.).
Authorship
Contribution: H.N. and K.A. designed and performed the research, analyzed the data, and wrote the paper; S.J.-T., Y.K., T.S., K.N., S.-i.O., H. Tsuzuki, Y.I., Y.A., H.I., S.S., E.B., H. Tsukamoto, and T.H. performed the research; and T.T. provided cDNA constructs and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroaki Niiro, Dept of Medicine and Biosystemic Science, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: hniiro@med.kyushu-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal