Abstract
Chronic GVHD (cGVHD) is a main cause of late death and morbidity after allogeneic hematopoietic cell transplantation, but its pathogenesis remains unclear. We investigated the roles of Th subsets in cGVHD with the use of a well-defined mouse model of cGVHD. In this model, development of cGVHD was associated with up-regulated Th1, Th2, and Th17 responses. Th1 and Th2 responses were up-regulated early after BM transplantation, followed by a subsequent up-regulation of Th17 cells. Significantly greater numbers of Th17 cells were infiltrated in the lung and liver from allogeneic recipients than those from syngeneic recipients. We then evaluated the roles of Th1 and Th17 in cGVHD with the use of IFN-γ–deficient and IL-17–deficient mice as donors. Infusion of IFN-γ−/− or IL-17−/− T cells attenuated cGVHD in the skin and salivary glands. Am80, a potent synthetic retinoid, regulated both Th1 and Th17 responses as well as TGF-β expression in the skin, resulting in an attenuation of cutaneous cGVHD. These results suggest that Th1 and Th17 contribute to the development of cGVHD and that targeting Th1 and Th17 may therefore represent a promising therapeutic strategy for preventing and treating cGVHD.
Introduction
GVHD is a result of immune attack of host tissues, such as the skin, gut, liver, and lung, by donor T cells in transplants.1,2 On the basis of the differences in clinical manifestations and histopathology, GVHD can be divided into acute and chronic types. Chronic GVHD (cGVHD) is the main cause of late death and morbidity after allogeneic hematopoietic stem cell transplantation.3-5 cGVHD often presents with clinical manifestations that resemble those observed in autoimmune diseases, such as systemic lupus erythematosus, Sjögren syndrome, lichen planus, and scleroderma. It has traditionally been assumed that the predominant cytokines produced during acute GVHD are Th1 cytokines, whereas those produced during cGVHD are Th2 cytokines. Although recent studies have suggested that cGVHD could be caused by cytokines secreted by Th1 cells,6 Th17 cells,7 or autoantibodies,8 or both, the immune mechanisms leading to the development of cGVHD are not completely understood.
Th17 cells are a third subset of polarized effector T cells characterized by their expression of proinflammatory cytokine IL-17 and other cytokines.9 IL-17 belongs to a family of 6 members: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F. Of these, IL-17A and IL-17F are the best characterized cytokines and form heterodimers. IL-17 plays an important role in the control and clearance of various pathogens.9 In addition, Th17 cells have been implicated in allograft rejection of solid organs and several autoimmune diseases.10,11 Although a number of studies have addressed how Th17 cells contribute to GVHD12 and have reported that Th17 cells are sufficient but not necessary to induce acute GVHD,13,14 the functional role of Th17 in cGVHD is unclear.
Retinoic acid, the active metabolite of vitamin A, has multiple effects on cell differentiation and survival by ligating the receptors from 2 families, retinoic acid receptors (RARs) and retinoid X receptors, each of which exists in multiple isoforms.15 All-trans-retinoic acid (ATRA) has been reported to inhibit IFN-γ synthesis by Thl cells and to suppress the differentiation of Th17 cells by down-regulating the orphan nuclear receptor RORγt, a key regulator of Th17 differentiation.16-19 Am80 is a novel RARα/β-specific synthetic retinoid that shows ∼ 10-fold more potent biologic activity than ATRA by binding to RARα and RARβ but not to RARγ.20 Am80 also inhibits IL-6 signaling20,21 and reduces the severity and progression of inflammatory disease models.20-23
In the present study, we used the B10.D2 (H-2d) into BALB/c (H-2d) MHC-compatible, multiple minor histocompatibility Ag (miHA)–incompatible model of cGVHD to address the contribution of Th1/Th17 cells and the effects of retinoids on cGVHD with the use of IFN-γ−/− mice and IL-17−/− mice as donors. We also tested the hypothesis that the administration of Am80 ameliorates cGVHD by reducing the levels of Th1 and Th17 inflammatory cytokines and the fibrosis factor TGF-β.
Methods
Mice
Female B10.D2 (H-2d) mice were purchased from Japan SLC. BALB/c (H-2d) recipient mice were purchased from Charles River Japan. IL-17A–deficient (IL-17−/−) mice with the BALB/c background were generated previously.24 IFN-γ–deficient (IFN-γ−/−) mice were purchased from The Jackson Laboratory. IL-17−/− and IFN-γ−/− mice with the B10.D2 background were backcrossed for 8-10 generations from the original knockout mice. All experiments involving animals were performed according to the regulations of the Institutional Animal Care and Research Advisory Committee, Okayama University Advanced Science Research Center.
BM transplantation
Mice received transplants according to the standard protocols described previously.25 Briefly, BALB/c mice received a single dose of 6.75 Gy x-ray total body irradiation. Recipient mice were injected with 2 × 106 spleen T cells and 8 × 106 T cell–depleted BM (TCD-BM) cells from B10.D2 donors. T-cell depletion and purification were performed with anti-CD90.2 Microbeads, pan T-cell isolation kit, and CD25 isolation kit and an AutoMACS system (Miltenyi Biotec) according to the manufacturer's instructions. Donor cells were injected intravenously into the recipients on day 0.
Evaluation of cGVHD
After BM transplantation (BMT), animals were weighed every 3 days and scored for skin manifestations of GVHD. The following scoring system was used25 : healthy appearance, 0; skin lesions with alopecia < 1 cm2 in area, 1; skin lesions with alopecia 1-2 cm2 in area, 2; skin lesions with alopecia > 2 cm2 in area, 3. In addition, animals were assigned 0.3 points each for skin disease (lesions or scaling) on the ears, tails, and paws. The minimum score was 0, and the maximum score was 3.9.
Tissue histopathology
Shaved skin from the interscapular region (∼ 2 cm2), the left lung, liver, and colon specimens of recipients were fixed in 10% formalin, embedded in paraffin, sectioned, mounted on slides, and stained with H&E. Slides were scored by a pathologist blind to experimental group (K.T.) on the basis of dermal fibrosis, fat loss, inflammation, epidermal interface changes, and follicular drop-out (0-2 for each category; the maximum score was 10).25 Lung, liver, and colon slides were scored by a pathologist blind to the experimental group (T.T.). Lung slides were scored according to periluminal infiltrates, pneumonitis, and the extent of injury (0-3 for each category), and the maximum score was 9.26 Liver slides were scored according to bile duct injury and inflammation (0-4 for each category), and the maximum score was 8.27 Colon slides were scored according to crypt apoptosis and inflammation (0-4 for each category), and the maximum score was 8.27
Intracellular cytokine staining and cytokine analysis
Organs from mice were removed, processed into single-cell suspensions, and stimulated in vitro with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 100 ng/mL ionomycin (Sigma-Aldrich) at 37°C for 3 hours. Cells were then incubated with GolgiStop (BD PharMingen) for an additional 2 hours. mAbs conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin-chlorophyll protein complexes, allophycocyanin, or Alexa Fluor 488 were used to assess the cell populations and were purchased from BD PharMingen or eBioscience. Cells were analyzed on a FACSCalibur flow cytometer with CellQuest software (both from Becton Dickinson) or MACS Quant flow cytometer (Miltenyi Biotec) with FlowJo software (TreeStar); both were housed in the Central Research Laboratory, Okayama University Medical School. Total peripheral lymph node (PLN) cells were adjusted to 1 × 106/mL in cultures. Supernatants were removed, and cytokine levels were measured with a BD Cytometric Bead Array (CBA) or by ELISA (R&D Systems) according to the respective manufacturer's protocol.
IFN-γ neutralization
Anti–mouse IFN-γ mAbs for in vivo experiments were prepared from mouse ascites from clones R4-6A2. Mice were treated intraperitoneally with anti–IFN-γ mAbs or rat IgG (160 μg/mouse; Sigma-Aldrich) on days 0, 5, 10, and 15 after BMT.
Administration of ATRA and Am80
Recipients were orally administered ATRA (200 μg/mouse; Wako), Am80 (1.0 mg/kg body weight; Nippon Shinyaku), or vehicle solutions daily from day 0.
Real-time RT-PCR
Total RNA was isolated from homogenized ear tissue with the use of an RNeasy mini kit (QIAGEN). cDNA synthesis was initiated by application of oligo dT primers and TaqMan Reverse Transcription Reagents (Applied Biosystems). Target cDNA levels were quantified by real-time PCR. The TaqMan Universal PCR Master Mix and the following Assay-on-Demand mouse gene-specific fluorescently labeled TaqMan MGB probes were used in an ABI Prism 5300 sequence detection system (Applied Biosystems): Mm01178820_m1 (TGF-β1). The mRNA expression of individual genes was normalized relative to GAPDH with the use of the equation dCt =Cttarget − CtGAPDH. The samples were obtained at room temperature using light microscopy (BX51; Olympus) with an objective lens (10×/0.40 NA, or 20×/0.70 NA; Olympus) and a camera (DP-70; Olympus). The images were acquired with image processing software (DP2-BSW Version 1.2; Olympus).
Statistical analyses
Group comparisons of skin cGVHD scores and pathology scores were performed using the Mann-Whitney U test or Kruskal-Wallis test. Cell populations, cytokine levels, mean weights, and gene expression data were analyzed with the unpaired 2-tailed Student t test. In all analyses, P < .05 was taken to indicate statistical significance.
Results
Th17 cells are increased in lymphoid organs during cGVHD development
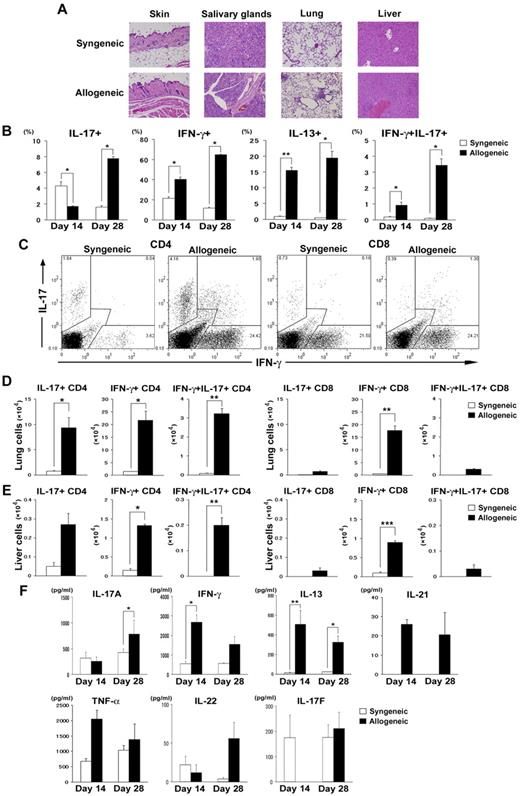
We first assessed the kinetics of Th1/Th2/Th17 cytokine production of donor T cells generated during cGVHD. We used the most common cGVHD model: the MHC-compatible, multiple miHA-incompatible allogeneic BMT model (B10.D2 into BALB/c). Sublethally irradiated (6.75 Gy) BALB/c mice were transplanted with 2 × 106 B10.D2 spleen T cells and 8 × 106 B10.D2 TCD-BM cells. Ly9.1 was used as a marker to distinguish donors from recipients; B10.D2 and BALB/c are negative and positive for Ly9.1, respectively. Flow cytometric analysis of the spleens and PLNs on days 14 and 28 indicated that donor chimerism as determined by the negativity for Ly9.1 was > 95%. The allogeneic recipients showed pathologic damage to the skin, salivary glands, lung, and liver, as reported previously (Figure 1A).25,27 Cells isolated from PLNs were harvested on days 14 and 28 after BMT and analyzed for cytokine expression. In the early phase (day 14), IL-17+ T cells were detected more frequently in the PLNs of recipients of syngeneic BMT, whereas in the late phase (day 28), IL-17+ T cells in allogeneic recipients increased and were detected significantly more frequently than in syngeneic recipients (Figure 1B). We detected consistently higher percentages of donor T cells expressing IFN-γ and IL-13 in PLNs from allogeneic recipients than from syngeneic recipients (Figure 1B). Intracellular staining showed that most of the IL-17–producing cells were CD4+ T cells (Figure 1C) and that IFN-γ/IL-17 double-positive cells (Th1/Th17 cells) were exclusively detected in allogeneic recipients (Figure 1B-C). As allogeneic recipients developed GVHD-induced lymphopenia on day 28; absolute numbers of IFN-γ+ T and IL-17+ T cells in PLNs from allogeneic recipients were not greater than those from syngeneic recipients (IFN-γ+ T, 51.8 ± 17.5 × 104 vs 49.4 ± 4.2 × 104, P = .92; IL-17+ T, 5.9 ± 2.2 × 104 vs 6.9 ± 0.59 × 104, P = .16). Numbers of Th1 and Th17 cells from allogeneic recipients were significantly greater than those from syngeneic recipients in the lung (Figure 1D) and liver (Figure 1E). Cells isolated from PLNs of allogeneic recipients secreted significantly greater amounts of IL-17, IFN-γ, and IL-13 after stimulation with PMA and ionomycin (Figure 1F) or without stimulation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These cytokine levels were also elevated in serum from allogeneic recipients 28 days after BMT (supplemental Figure 2). To confirm that our observations were not strain dependent or model dependent, we performed similar experiments in the DBA/2 into BALB/c model of cGVHD. We confirmed the up-regulated Th1 and Th17 responses in this model (supplemental Figure 3).
Th17 cells are increased in lymphoid organs during the late phase of cGVHD. Sublethally irradiated (6.75 Gy) BALB/c mice were transplanted with 2 × 106 spleen T cells plus 8 × 106 TCD-BM from WT B10.D2 mice (allogeneic group; black bars). The syngeneic group (white bars) received a transplant of the same dose of splenocytes and TCD-BM from BALB/c mice. (A) Histopathology of skin, salivary glands, lung, and liver of syngeneic and allogeneic recipients 35 days after BMT. (B) The percentages of donor-derived CD3+ T cells expressing IL-17, IFN-γ, IL-13, and IFN-γ/IL-17 on days 14 and 28 are shown. (C) Representative staining for intracellular IFN-γ and IL-17 on CD4+ and CD8+ T cells on day 28 for syngeneic and allogeneic mice. (D-E) Absolute numbers of IL-17–, IFN-γ–, and IFN-γ/IL-17–producing CD4+ and CD8+ T cells in recipient lung (D) and liver (E). (F) PLN cells from syngeneic and allogeneic recipients on days 14 and 28 were stimulated with PMA and ionomycin in vitro. Five hours later, the supernatants were collected to determine cytokine levels by ELISA or CBA. Graphs indicate the levels of cytokines secreted per 1 × 106 total stimulated PLN cells. Three to 6 mice per group were used. The means (± SE) of each group are shown. Data are from 1 representative of ≥ 2 independent experiments. *P < .05, **P < .01, and ***P < .005.
Th17 cells are increased in lymphoid organs during the late phase of cGVHD. Sublethally irradiated (6.75 Gy) BALB/c mice were transplanted with 2 × 106 spleen T cells plus 8 × 106 TCD-BM from WT B10.D2 mice (allogeneic group; black bars). The syngeneic group (white bars) received a transplant of the same dose of splenocytes and TCD-BM from BALB/c mice. (A) Histopathology of skin, salivary glands, lung, and liver of syngeneic and allogeneic recipients 35 days after BMT. (B) The percentages of donor-derived CD3+ T cells expressing IL-17, IFN-γ, IL-13, and IFN-γ/IL-17 on days 14 and 28 are shown. (C) Representative staining for intracellular IFN-γ and IL-17 on CD4+ and CD8+ T cells on day 28 for syngeneic and allogeneic mice. (D-E) Absolute numbers of IL-17–, IFN-γ–, and IFN-γ/IL-17–producing CD4+ and CD8+ T cells in recipient lung (D) and liver (E). (F) PLN cells from syngeneic and allogeneic recipients on days 14 and 28 were stimulated with PMA and ionomycin in vitro. Five hours later, the supernatants were collected to determine cytokine levels by ELISA or CBA. Graphs indicate the levels of cytokines secreted per 1 × 106 total stimulated PLN cells. Three to 6 mice per group were used. The means (± SE) of each group are shown. Data are from 1 representative of ≥ 2 independent experiments. *P < .05, **P < .01, and ***P < .005.
IL-17−/− donor T cells ameliorate cGVHD
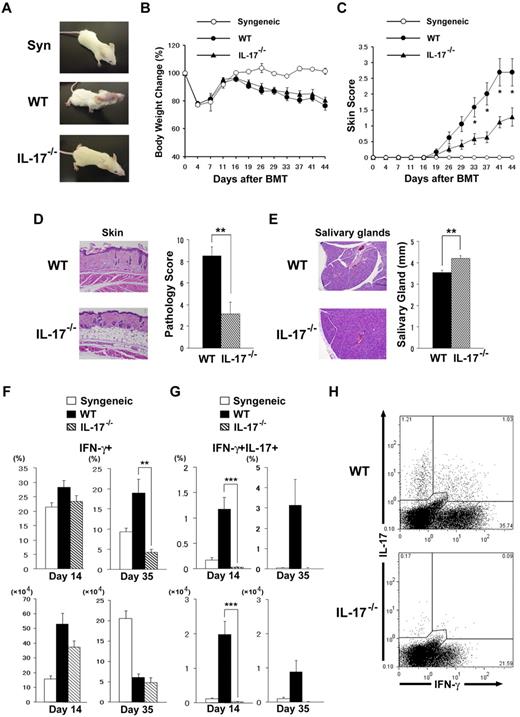
We next used IL-17−/− mice with the B10.D2 background as donors to evaluate whether Th17 contributes to cGVHD. On transfer of IL-17−/− B10.D2 donor T cells into allogeneic BMT models, weight loss was mild and fur loss was clearly ameliorated in comparison to that seen in recipients of wild-type (WT) T cells (Figure 2A-B). Clinical cGVHD severity was assessed with a standard scoring system (see “Methods”). Allogeneic IL-17−/− BMT recipients showed significantly less skin cGVHD than WT controls (P < .05; Figure 2C). Histopathologic examination of the skin showed significantly reduced cGVHD pathology in recipients of IL-17−/− donors (3.17 ± 1.09 vs 8.50 ± 0.84; P < .01; Figure 2D). A dry mouth is one of the distinctive features of cGVHD, and lymphocytic inflammation, fibrosis, and atrophy of acinar tissue were observed in the salivary glands of WT BMT recipients. Histopathologic examination of the salivary glands showed reduced cGVHD pathology in the recipients of IL-17−/− donors (Figure 2E). Atrophy of the salivary glands as determined by their size was significantly reduced in recipients of IL-17−/− donors (4.21 ± 0.13 vs 3.54 ± 0.11; P < .01; Figure 2E). No significant differences were observed in pathology scores of the lung, liver, or colon between recipients of IL-17−/− and WT donors (lung, 2.6 ± 1.04 vs 0.8 ± 0.44, P = .19; liver, 1.5 ± 0.87 vs 1.83 ± 0.37, P = .75; colon, 1.6 ± 0.36 vs 2.8 ± 0.33, P = .06). Thus, IL-17−/− BMT recipients showed less cGVHD in the skin and salivary glands than did the WT controls. Flow cytometric analysis of the PLNs in the early phase (day 14) showed no differences in frequency of IFN-g+ cells between IL-17−/− and WT recipients, whereas recipients of IL-17−/− showed fewer IFN-γ+ cells in the late phase (day 35, 4.3% ± 0.8% vs 18.9% ± 3.5%; P = .01; Figure 2F). As allogeneic WT recipients developed more severe GVHD-induced lymphopenia on day 35 than IL-17−/− recipients, absolute numbers of IFN-g+ cells in PLNs from allogeneic WT recipients were not greater than those from IL-17−/− recipients (IFN-γ+ T cells, 6.08 ± 0.87 × 104 vs 4.83 ± 1.23 × 104; P = .48). As expected, IFN-γ/IL-17 double-positive cells were not detected in recipients of IL-17−/− donors on days 14 and 35 (Figure 2G-H). No differences were observed in the IL-13+ cells or Foxp3+ cells between the groups (data not shown). These data suggest that donor IL-17 contributes to the pathogenesis of cGVHD.
IL-17−/− donor T cells ameliorate cGVHD. Sublethally irradiated BALB/c recipients were transplanted from WT, IL-17−/− B10.D2, or syngeneic BALB/c donors. (A) Gross observation of the skin lesions from recipients of syngeneic, WT, and IL-17−/− donors 28 days after BMT. The recipients were analyzed for body weight (B) and cGVHD skin scores (C); data from 2 independent experiments were combined (n = 14 per group). Pathology score of skin (D) and the longest diameter of the salivary gland (E) on day 35 of BMT are shown. Four to 6 recipients were examined in each group. (F-G) PLN cells of the recipients of syngeneic (white bar), WT (black bar), or IL-17−/− (striped bar) donors were stained for intracellular IFN-γ and IL-17 on days 14 and 35 after BMT. The percentages and absolute numbers of IFN-γ+ cells (F) and IFN-γ+/IL-17+ cells (G) are shown. Data from 2 replicated experiments were combined (n = 6-11 per group). (H) Representative staining for intracellular IFN-γ and IL-17 on CD4+ T cells of WT or IL-17−/− mice on day 35 is shown. Data represent the means ± SEs. *P ≤ .05, **P ≤ .01, and ***P ≤ .001.
IL-17−/− donor T cells ameliorate cGVHD. Sublethally irradiated BALB/c recipients were transplanted from WT, IL-17−/− B10.D2, or syngeneic BALB/c donors. (A) Gross observation of the skin lesions from recipients of syngeneic, WT, and IL-17−/− donors 28 days after BMT. The recipients were analyzed for body weight (B) and cGVHD skin scores (C); data from 2 independent experiments were combined (n = 14 per group). Pathology score of skin (D) and the longest diameter of the salivary gland (E) on day 35 of BMT are shown. Four to 6 recipients were examined in each group. (F-G) PLN cells of the recipients of syngeneic (white bar), WT (black bar), or IL-17−/− (striped bar) donors were stained for intracellular IFN-γ and IL-17 on days 14 and 35 after BMT. The percentages and absolute numbers of IFN-γ+ cells (F) and IFN-γ+/IL-17+ cells (G) are shown. Data from 2 replicated experiments were combined (n = 6-11 per group). (H) Representative staining for intracellular IFN-γ and IL-17 on CD4+ T cells of WT or IL-17−/− mice on day 35 is shown. Data represent the means ± SEs. *P ≤ .05, **P ≤ .01, and ***P ≤ .001.
Donor Th1 differentiation is responsible for the development of cGVHD
To test whether donor Th1 differentiation is responsible for cGVHD, we used IFN-γ−/− mice with the B10.D2 background as donors. BMT from IFN-γ−/− donors compared with WT donors significantly improved the clinical cGVHD score (P < .05; Figure 3A). Histopathologic examination of the skin showed significantly reduced cGVHD pathology in recipients of IFN-γ−/− donors (4.75 ± 0.54 vs 7.80 ± 0.52; P = .02; Figure 3B). Salivary gland atrophy was also reduced in recipients of IFN-γ−/− donors (3.81 ± 0.05 vs 2.87 ± 0.19; P < .05; Figure 3C). No significant differences were observed in pathology scores of the lung, liver, or colon between recipients of IFN-γ−/− and WT donors (lung, 2.4 ± 0.61 vs 3.2 ± 0.52, P = .4; Figure 3B; liver, 1.0 ± 0.4 vs 1.6 ± 0.32, P = .21; colon, 0.75 ± 0.21 vs 1.6 ± 0.67, P = .36). Intracellular staining of PLNs showed no differences in IL-13– or IL-17–producing cells between IFN-γ−/− and WT recipients (data not shown), although significantly greater numbers of Foxp3+ cells were detected in the IFN-γ−/− recipients (day 35; P < .05; Figure 3D). To examine whether an increase in numbers of Treg cells was responsible for the reduced cGVHD in the absence of donor IFN-γ−/−, mice were injected with whole T cells or CD25-depleted T cells from donors. As shown in Figure 3E, depletion of CD25+ cells from the donor inoculum exacerbated skin scores (P < .05). However, CD25-depleted T cells from IFN-γ−/− mice caused less severe skin GVHD than those from WT mice (P < .05). These findings suggest that IFN-γ contributes to the pathogenesis of cGVHD by both Treg-independent and -dependent pathways. Next, we evaluated the role of IFN-γ in the development of skin cGVHD by administering anti–IFN-γ mAbs to recipients of WT or IL-17−/− donors. Anti–IFN-γ mAb treatment significantly reduced skin scores and pathology scores in recipients of WT donors (Figure 3F-G). Recipients of IL-17−/− donors again showed reduced skin scores, and treatment with anti–IFN-γ mAbs further reduced skin scores (Figure 3H). These findings suggest that IFN-γ contributes to cGVHD pathogenesis.
Donor Th1 differentiation and IFN-γ production are responsible for exacerbated cGVHD. (A-D) Sublethally irradiated BALB/c recipients were transplanted from WT or IFN-γ−/− B10.D2 donors. Clinical skin cGVHD scores (A), pathology score of skin and lung (B), and the longest diameter of the salivary gland (C) on day 35 after BMT are shown. Four to 6 recipients were examined in each group. Data are from 1 representative of 3 independent experiments. (D) PLN cells of the recipients on day 35 were stained for intracellular Foxp3. The percentages and the absolute number of CD4+Foxp3+ Treg cells are shown. Four to 6 recipients were examined in each group. Data are from 1 representative of 2 independent experiments. (E) Sublethally irradiated BALB/c recipients were transplanted 8 × 106 TCD-BM cells plus 2 × 106 total spleen T cells or CD25-depleted T cells from WT or IFN-γ−/− B10.D2 donors. The skin cGVHD scores are shown (n = 6 per group). Data are from 1 representative of ≥ 2 independent experiments. (F-H) Sublethally irradiated BALB/c recipients were transplanted from WT or IL-17−/− B10.D2 donors. The recipients were injected with anti–IFN-γ mAbs or rat IgG (160 μg/mouse) on days 0, 5, 10, and 15 after BMT and were assessed for the clinical signs of cGVHD every 3 days. The clinical skin cGVHD scores (F), histopathology, and pathology score of the skin (G) on day 35 of BMT from WT donors. Four mice per group were used. Data are from 1 representative of ≥ 2 repeated experiments. (H) The clinical skin cGVHD scores after BMT from WT or IL-17−/− donors are shown. Six mice per group were used. Data are from 1 representative of 2 independent experiments. The means (± SEs) of each group are shown; *P < .05.
Donor Th1 differentiation and IFN-γ production are responsible for exacerbated cGVHD. (A-D) Sublethally irradiated BALB/c recipients were transplanted from WT or IFN-γ−/− B10.D2 donors. Clinical skin cGVHD scores (A), pathology score of skin and lung (B), and the longest diameter of the salivary gland (C) on day 35 after BMT are shown. Four to 6 recipients were examined in each group. Data are from 1 representative of 3 independent experiments. (D) PLN cells of the recipients on day 35 were stained for intracellular Foxp3. The percentages and the absolute number of CD4+Foxp3+ Treg cells are shown. Four to 6 recipients were examined in each group. Data are from 1 representative of 2 independent experiments. (E) Sublethally irradiated BALB/c recipients were transplanted 8 × 106 TCD-BM cells plus 2 × 106 total spleen T cells or CD25-depleted T cells from WT or IFN-γ−/− B10.D2 donors. The skin cGVHD scores are shown (n = 6 per group). Data are from 1 representative of ≥ 2 independent experiments. (F-H) Sublethally irradiated BALB/c recipients were transplanted from WT or IL-17−/− B10.D2 donors. The recipients were injected with anti–IFN-γ mAbs or rat IgG (160 μg/mouse) on days 0, 5, 10, and 15 after BMT and were assessed for the clinical signs of cGVHD every 3 days. The clinical skin cGVHD scores (F), histopathology, and pathology score of the skin (G) on day 35 of BMT from WT donors. Four mice per group were used. Data are from 1 representative of ≥ 2 repeated experiments. (H) The clinical skin cGVHD scores after BMT from WT or IL-17−/− donors are shown. Six mice per group were used. Data are from 1 representative of 2 independent experiments. The means (± SEs) of each group are shown; *P < .05.
Am80 inhibits donor Th1 and Th17 cells both in vitro and in vivo
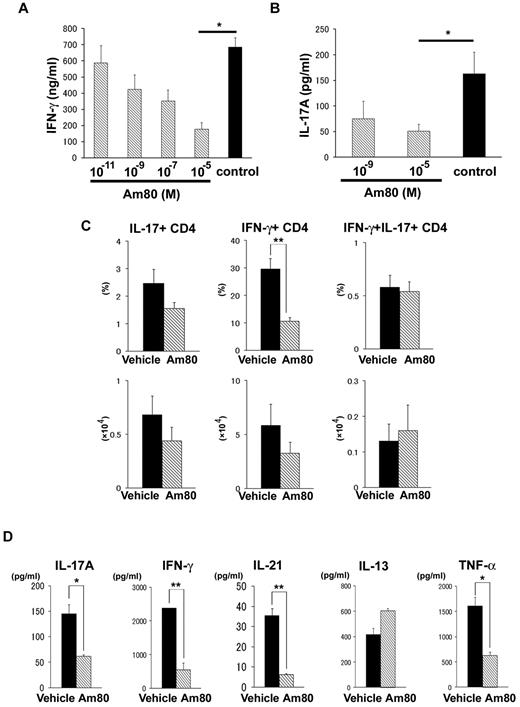
ATRA has been reported to suppress the differentiation of Th17 cells with a reciprocal induction of Treg cells.28 Am80, a novel RARα/β-specific synthetic retinoid, has a biologic activity ∼ 10 times more potent than that of ATRA20 and directly inhibits Th1 cytokine production.20,22,29 Therefore, we hypothesized that ATRA or Am80 down-regulates both Th1 and Th17 differentiation in donor T cells, resulting in attenuation of cGVHD. To clarify whether retinoids directly inhibit the production of cytokines, PLNs were isolated from mice 14 days after allogeneic BMT and cultured with Am80 for 24 hours to determine cytokine production. Am80 inhibited IFN-γ (Figure 4A) and IL-17 (Figure 4B) production in a dose-dependent manner. Next, BMT recipients were orally administered Am80 at a dose of 1.0 mg/kg of body weight or vehicle daily from day 0 of BMT, and cytokine expression was assessed in PLNs harvested on day 35. We detected significantly fewer IFN-γ+ T cells in Am80-administered recipients (Figure 4C). In addition, PLNs from Am80-treated recipients produced significantly less IFN-γ after stimulation with PMA and ionomycin (P < .01; Figure 4D). No difference was observed in the percentage of IL-17–producing donor cells, although PLN cells from Am80-treated recipients produced significantly less IL-17 (P < .05) and IL-21 (P < .01) after stimulation with PMA and ionomycin (Figure 4D). Taken together, these data suggest that Am80 down-regulates both Th1 and Th17 cells in vitro and in vivo.
Am80 inhibits donor Th1 and Th17 cells in vitro and in vivo. Sublethally irradiated BALB/c recipients were transplanted from WT B10.D2 donors. (A-B) PLN cells from recipients (n = 3-6 per group) on day 14 were treated with Am80 or vehicle solution for 24 hours, the supernatants were collected, and ELISA was performed to determine the cytokine levels. Graphs represent the levels of cytokines secreted per 1 × 106 whole stimulated PLN cells. The data are from 1 representative of ≥ 3 independent experiments. (C-D) After BMT, recipients (n = 4-6 per group) were administered oral Am80 (1.0 mg/kg of body weight) or vehicle solution daily from day 0. PLNs of the recipients were stained for intracellular IFN-γ and IL-17. (C) The percentage and absolute number of IFN-γ+ and IL-17+–producing CD4+ T cells. Data are from 1 representative of ≥ 2 repeated experiments. (D) PLN cells from recipients (n = 3-6 per group) treated with Am80 or vehicle on day 16 were stimulated with PMA and ionomycin. Five hours later, the supernatants were collected to determine cytokine levels by CBA. Graphs represent the levels of cytokines secreted per 1 × 106 whole stimulated PLN cells. The data are from 1 representative of ≥ 3 independent experiments. The means (± SEs) of each group are shown; *P < .05 and **P < .01.
Am80 inhibits donor Th1 and Th17 cells in vitro and in vivo. Sublethally irradiated BALB/c recipients were transplanted from WT B10.D2 donors. (A-B) PLN cells from recipients (n = 3-6 per group) on day 14 were treated with Am80 or vehicle solution for 24 hours, the supernatants were collected, and ELISA was performed to determine the cytokine levels. Graphs represent the levels of cytokines secreted per 1 × 106 whole stimulated PLN cells. The data are from 1 representative of ≥ 3 independent experiments. (C-D) After BMT, recipients (n = 4-6 per group) were administered oral Am80 (1.0 mg/kg of body weight) or vehicle solution daily from day 0. PLNs of the recipients were stained for intracellular IFN-γ and IL-17. (C) The percentage and absolute number of IFN-γ+ and IL-17+–producing CD4+ T cells. Data are from 1 representative of ≥ 2 repeated experiments. (D) PLN cells from recipients (n = 3-6 per group) treated with Am80 or vehicle on day 16 were stimulated with PMA and ionomycin. Five hours later, the supernatants were collected to determine cytokine levels by CBA. Graphs represent the levels of cytokines secreted per 1 × 106 whole stimulated PLN cells. The data are from 1 representative of ≥ 3 independent experiments. The means (± SEs) of each group are shown; *P < .05 and **P < .01.
Administration of Am80 ameliorates cGVHD
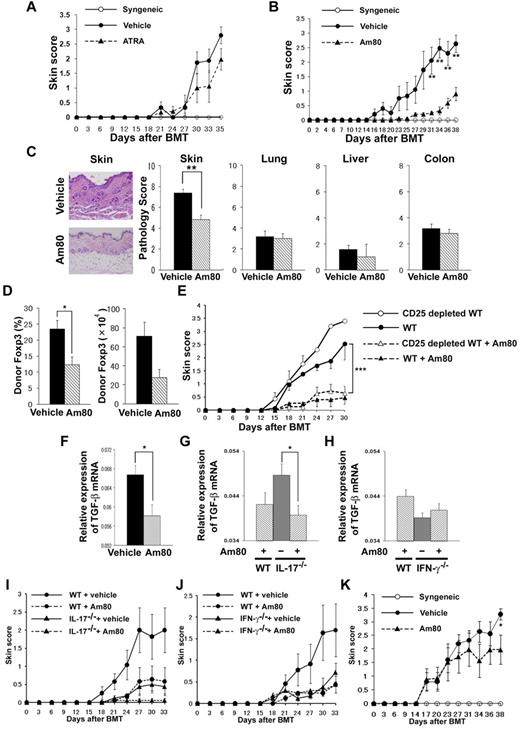
Next, we examined whether ATRA or Am80 can down-regulate cGVHD. BALB/c recipients were orally administered ATRA (200 μg/mouse) or Am80 from day 0 of BMT. We found that ATRA tended to decrease the clinical cGVHD score (Figure 5A), whereas Am80 significantly ameliorated the clinical score compared with controls (P = .01; Figure 5B). Histopathologic examination of the skin on day 16 showed significantly reduced cGVHD damage in Am80-treated animals (day 16, 4.8 ± 0.4 vs 7.4 ± 0.4; P < .01; Figure 5C), No differences were observed in pathology scores of the lung, liver, or colon between the 2 groups (Figure 5C). Because it has been reported that Am80 can induce Treg cells,29 we quantified the frequency of Foxp3-expressing CD4+ T cells in the PLNs after BMT. Recipients administered Am80 showed a decreased frequency of Foxp3+ cells (day 17, 12.3% ± 2.5% vs 23.5% ± 2.6%; P = .02; Figure 5D). Foxp3 mRNA expression of the target organ (the ear) was also decreased in the Am80 recipients (data not shown). To confirm that the effects of Am80 are independent of Treg cells, mice were injected with whole T cells or CD25-depleted T cells from donors. As shown in Figure 5E, depletion of CD25+ cells from the donor inoculum did not exacerbate skin cGVHD in Am80-treated mice, thus suggesting that the effects of Am80 treatment are not associated with Treg cells.
Administration of Am80 ameliorates cGVHD. (A-D) Sublethally irradiated BALB/c recipients were transplanted from WT B10.D2 donors. The recipients received daily administration of ATRA (200 μg/mouse; A), Am80 (1.0 mg/kg of body weight; B), or vehicle solution orally after BMT and were assessed for clinical signs of cGVHD every 3 days. The skin cGVHD scores are shown. (C) Representative histopathology of skin and pathology score of skin, lung, liver, and colon in each group (n = 5-6 per group) on day 16 after BMT are shown. (D) PLN cells of the recipients on day 16 were stained for intracellular Foxp3. The percentages and absolute numbers of CD4+Foxp3+ Treg cells are shown. Data are from 1 representative of ≥ 2 independent experiments. (E) Sublethally irradiated BALB/c recipients were transplanted with 8 × 106 TCD-BM cells plus 2 × 106 total spleen T cells or CD25-depleted T cells from WT or IFN-γ−/− B10.D2 donors. After BMT, recipients were given Am80 or vehicle solution. The skin cGVHD scores are shown. There were 6 recipients in each group; the data are from 1 representative of ≥ 2 independent experiments. (F-K) Sublethally irradiated BALB/c recipients were transplanted from WT (F), IL-17−/− (G), and IFN-γ−/− (H) donors. After BMT, recipients were given Am80 or vehicle solution. TGF-β mRNA expression in the ears on day 35 after BMT (F-H) and skin cGVHD scores (I-J) are shown. Data are from 1 representative of ≥ 2 independent experiments (n = 5 per group). (K) The skin cGVHD scores of BMT recipients treated with Am80 or vehicle solution orally daily after day 21 of BMT; data from 3 independent experiments were combined (n = 12-14 per group). *P < .05, **P < .01, and ***P < .005.
Administration of Am80 ameliorates cGVHD. (A-D) Sublethally irradiated BALB/c recipients were transplanted from WT B10.D2 donors. The recipients received daily administration of ATRA (200 μg/mouse; A), Am80 (1.0 mg/kg of body weight; B), or vehicle solution orally after BMT and were assessed for clinical signs of cGVHD every 3 days. The skin cGVHD scores are shown. (C) Representative histopathology of skin and pathology score of skin, lung, liver, and colon in each group (n = 5-6 per group) on day 16 after BMT are shown. (D) PLN cells of the recipients on day 16 were stained for intracellular Foxp3. The percentages and absolute numbers of CD4+Foxp3+ Treg cells are shown. Data are from 1 representative of ≥ 2 independent experiments. (E) Sublethally irradiated BALB/c recipients were transplanted with 8 × 106 TCD-BM cells plus 2 × 106 total spleen T cells or CD25-depleted T cells from WT or IFN-γ−/− B10.D2 donors. After BMT, recipients were given Am80 or vehicle solution. The skin cGVHD scores are shown. There were 6 recipients in each group; the data are from 1 representative of ≥ 2 independent experiments. (F-K) Sublethally irradiated BALB/c recipients were transplanted from WT (F), IL-17−/− (G), and IFN-γ−/− (H) donors. After BMT, recipients were given Am80 or vehicle solution. TGF-β mRNA expression in the ears on day 35 after BMT (F-H) and skin cGVHD scores (I-J) are shown. Data are from 1 representative of ≥ 2 independent experiments (n = 5 per group). (K) The skin cGVHD scores of BMT recipients treated with Am80 or vehicle solution orally daily after day 21 of BMT; data from 3 independent experiments were combined (n = 12-14 per group). *P < .05, **P < .01, and ***P < .005.
TGF-β is a critical mediator of fibrosis in cGVHD skin lesions.30 TGF-β mRNA expression was decreased in the ear of the Am80 recipients (day 17, P = .02; Figure 5F). We then assessed TGF-β mRNA expression in recipients of IL-17−/− or IFN-γ−/− donors treated with Am80. Am80 further reduced skin scores and TGF-β expression in recipients of IL-17−/− donors (Figure 5G-I) but not in recipients of IFN-γ−/− donors (Figure 5H,J). These results suggest that the effects of Am80 are more dependent on IFN-γ than on IL-17.
Finally, we examined whether Am80 could be used for the treatment of cGVHD. Am80 was orally administered to mice from day 21 of BMT, when mice had developed clinical signs of cGVHD. Am80 significantly improved clinical scores (P = .016; Figure 5K).
Discussion
The results of the present study showed that Th1 and Th17 cells contribute to cGVHD with the use of a MHC-compatible, miHA-incompatible model of cGVHD. In addition, we demonstrated that Am80 down-regulates both Th1 and Th17 cells in vitro and in vivo, resulting in attenuation of cGVHD.
For many years, the best defined subsets of effector T cells of the CD4+ Th lineage were the Th1 and Th2 cells. A third subset of CD4+ effector cells was identified and named Th17 cells, because the signature cytokine that they produce is IL-17.31 Although the role of Th17 in acute GVHD has been evaluated by several groups with inconsistent results,32-35 few studies have addressed the role of Th17 in cGVHD. Initially, cGVHD was hypothesized to be a Th2-mediated disease on the basis of the results in a nonirradiated P→F1 model of cGVHD. cGVHD in this model is mediated by host B-cell autoantibody production stimulated by donor Th2 cells. Th1 polarization of donor T cells activates donor CD8+ CTLs to kill host B cells, resulting in amelioration of cGVHD.36 However, the relevance of this model is unclear in clinical BMT in which host B cells are eliminated by conditioning. Such different effector mechanisms between the models may be associated with distinct requirement of Th subsets for cGVHD between the studies. In the present study, we assessed the kinetics of Th1, Th2, and Th17 cells during the development of cGVHD in the B10.D2→BALB/c model. Th1 and Th2 responses were up-regulated early after BMT, followed by a subsequent up-regulation of Th17 cells. Significantly greater numbers of Th17 cells were detected in the lung and liver from allogeneic recipients than in those from syngeneic recipients. We then evaluated the role of Th17 in cGVHD with the use of IL-17−/− mice as several groups had used,32-34,37,38 although interpretation of the results deserves caution because the Th17 lineage is uniquely regulated by RORγt,13,14 and other cytokines such as IL-21 and IL-22 produced by Th17 cells may also contribute to Th17-mediated GVHD. On transfer of IL-17−/− B10.D2 donor T cells, cGVHD was significantly ameliorated compared with that in recipients of WT T cells, suggesting that Th17 contributes to cGVHD in this model. In particular, Th17 plays a significant role in skin cGVHD. This agrees with the recent observation by Hill et al37 that donor pretreatment with G-CSF induces Th17 differentiation of donor T cells and induces skin GVHD after peripheral blood stem cell transplantation. In an adoptive transfer model of autoimmune cGVHD, Th17 cells infiltrated target tissues.39 However, a subsequent study showed the absence of donor Th17 cells did not abrogate GVHD pathology,38 in contrast to our results. In the absence of donor IL-17, Th1 responses were preserved in that study but were reduced in our study. Such difference in Th1 responses may produce different outcomes between the studies. In mouse models of acute GVHD, Yi et al showed enhanced Th1 differentiation of donor T cells by increased production of IL-12 from dendritic cells in the absence of IL-17.33 By contrast, Kappel et al showed reduced numbers of IFN-γ–positive CD4+ T cells and IFN-γ secretion in culture in the absence of IL-17.34 These results together with our results suggest that IL-17 may induce IFN-γ, although such a hierarchy of Th1/Th17 pathways may be context or model dependent or both and will need to be studied in the future. Nonetheless, it should be noted that cGVHD still developed in the absence of donor IL-17 cells in our study. Taken together, it is probable that Th17 is not an absolute requirement for cGVHD, and either Th1 or Th17 is sufficient to cause cGVHD.
We demonstrated that IFN-γ−/− donor mice and injecting anti–IFN-γ mAb ameliorated cGVHD. Thus, Th1 and Th17 responses play a pathogenic role in cGVHD in this model. These results were consistent with a recent study reporting that cGVHD is mediated by Th1 and Th17 responses because of the progressive loss of CD4+CD25+Foxp3+ T cells during acute GVHD in mice.39 These results were also consistent with clinical studies showing that Th1 cells and Th17 cells increased in patients with active cGVHD.40-43 Increased transcription of IFN-γ has also been detected in the affected skin and oral mucosa of patients with cGVHD.41,44 In this study, we found no differences in Th17 cells between IFN-γ−/− and WT recipients, although significantly greater numbers of Treg cells were detected in IFN-γ−/− recipients. CD25-depleted T cells from IFN-γ−/− mice induced more severe skin cGVHD compared with CD25-replete IFN-γ−/− T cells, but still less severe cGVHD compared with CD25-depleted T cells from WT mice (Figure 3E), suggesting that IFN-γ contributes to the pathogenesis of cGVHD by both Treg-independent and -dependent pathways. Neutralization of IFN-γ ameliorated cGVHD in the absence of donor IL-17 (Figure 3H), suggesting again that both Th1 and Th17 responses contribute to the pathogenesis of cGVHD.
We found that donor-derived Th17 cells were generated in recipients of syngeneic transplantation in addition to allogeneic transplantation. However, the kinetics of Th17 development differed between the syngeneic and allogeneic settings; Th17 cells developed in the early phase after syngeneic transplantation. Kappel et al speculated that Th17 development may be the result of increased immune reconstitution of syngeneic hosts compared with allogeneic hosts with GVHD.34 We additionally identified a population of donor-derived IFN-γ+IL-17+ cells after allogeneic BMT. It has been shown that a subset of IL-17–producing cells can also produce IFN-γ in vivo.34,45 Such CD4+IFN-γ+IL-17+ T cells have been postulated to play a causative role in the pathogenesis of experimental autoimmune encephalomyelitis (EAE).46 IFN-γ+IL-17+ cells were only detected after allogeneic BMT, but not after syngeneic BMT, suggesting that this population is generated by allogeneic stimulation, but not because of lymphopenia-induced proliferation. Further investigations are required to clarify the difference in function between IL-17 single-positive and IFN-γ/IL-17 double-positive cells.
ATRA suppresses Th17 differentiation and effector function by RARα signaling,18 but ATRA can also bind to RARβ and RARγ, which can form a variety of homodimers and heterodimers with 3 retinoid X receptors.15 Nonselective receptor binding is thought to be a main cause of the side effects associated with the administration of ATRA and other pan-RAR agonists. Am80 is a synthetic RAR agonist that shows high affinity to RARα/β. In addition to a greater specificity for RARα, Am80 offers several other advantages over ATRA as a therapeutic agent, including less toxicity, greater stability, fewer potential side effects, and superior bioavailability. Am80 is effective in autoimmune disease models of collagen-induced arthritis,20,47 EAE,21,29 2,4-dinitrofluorobenzene–induced contact dermatitis,22 and atherosclerosis.23 Because retinoids can down-regulate Th1 and Th17 cells and can ameliorate autoimmune diseases, we hypothesized that these retinoids would attenuate cGVHD. We demonstrated that Am80 down-regulated Th1 and Th17 differentiation of donor T cells in BALB/c recipients of B10.D2 donors, resulting in reduced cGVHD. Our results suggest that combined blockade of Th1 and Th17 responses may represent a promising strategy to prevent or treat cGVHD, as has been suggested for acute and chronic GVHD.32,39,48 Most recently, Yu et al used mice deficient for both T-bet and RORγt as T-cell donors and clearly showed that blockade of both Th1 and Th17 differentiation is required to prevent acute GVHD.14 In addition, TGF-β mRNA expression in the skin decreased in the Am80 recipients of WT and IL-17−/− but not IFN-γ−/− donors. These results suggest that Am80 down-regulates TGF-β and that this effect is more dependent on IFN-γ than on IL-17. Unexpectedly, those recipients administered Am80 had a significantly lower frequency of Foxp3+ cells. These results differ from those of in vitro studies performed by Mucida et al,28 in which retinoic acids were shown to be capable of inhibiting the IL-6–driven induction of Th17 cells and to promote Treg cell differentiation. Thus, retinoic acids enhance Treg differentiation and inhibit both Th17 and Th1 in vitro; however, the effects of retinoids may be more complex in vivo, because retinoids can affect not only T cells but also other immunoregulatory cells. For example, previous in vivo studies reported that Am80 suppressed Treg cells in experimental models of EAE29 and collagen-induced arthritis,47 similar to our study. In our study, Am80 suppressed TGF-β expression, a key cytokine in Treg development, which may have resulted in the suppression of Treg.
In conclusion, both Th1 and Th17 contribute to the development of cGVHD. Am80 down-regulates TGF-β and also regulates both Th1 and Th17 cells in vitro and in vivo, resulting in attenuation of cGVHD. Thus, administration of Am80, which is currently available as medication for acute promyelocytic leukemia in Japan,49 may represent effective strategy for prevention and treatment of cGVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Misako Shibakura, Dr Terumasa Toraya, and Dr Akiko Uenaka for their technical assistance and Mai Henmi, Chikara Takahashi, Shinsaku Matsumoto, Hiroki Kobayashi, and Dan Liu for their help in the experiments.
This work was supported by research funds from the Ministry of Education, Culture, Sports, Science, and Technology (no. 21591244), and research grants from the Health and Labor Science.
Authorship
Contribution: H.N. conducted the experiments, analyzed the data, and wrote the manuscript; Y.M. designed the experiments, supervised the research, and wrote the manuscript; H.S., K.K., Y.Y., S.K., and H.U. performed the research; K.T., T. Tanaka, and T.Y. performed histopathologic analyses of the organs; Y.I. provided vital new reagents for the study; and T. Teshima and M.T. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshinobu Maeda, Department of Hematology and Oncology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Shikata-cho 2-5-1, Kita-ku, Okayama City, Okayama, 700-8558 Japan; e-mail: yosmaeda@md.okayama-u.ac.jp.