Abstract
Among hematologic neoplasms, chronic myeloid leukemia (CML) is exquisitely sensitive to graft-versus-leukemia (GVL) because patients relapsing after allogeneic hematopoietic stem-cell transplantation (alloHSCT) can be cured by donor leukocyte infusion (DLI); however, the cellular mechanisms and strategies to separate GVL from GVHD are unclear. We used a BCR-ABL1 transduction/transplantation mouse model to study the mechanisms of DLI in MHC-matched, minor histocompatibility antigen–mismatched allogeneic chimeras with CML-like leukemia, in which DLI can be administered at the time of transplantation (early) or after recovery of hematopoiesis (delayed). After early DLI, CML-like leukemia cannot be transferred into immunocompetent secondary recipients as soon as 4 days after primary transplantation, demonstrating that cotransplantation of T lymphocytes blocks the engraftment of BCR-ABL1–transduced stem cells. In contrast, in allogeneic chimeras with established CML-like leukemia, combined treatment with delayed DLI and the kinase inhibitor imatinib eradicates leukemia with minimal GVHD. The GVL effect is directed against minor histocompatibility antigens shared by normal and leukemic stem cells, and is mediated predominantly by CD8+ T cells, with minor contributions from CD5− splenocytes, including natural killer cells. These results define a physiologic model of adoptive immunotherapy of CML that will be useful for investigating the cellular and molecular mechanisms of GVL.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm (MPN) caused by expression of a dysregulated tyrosine kinase, BCR-ABL1, in hematopoietic stem cells as a consequence of the t(9;22) Philadelphia (Ph) chromosome translocation.1 The only proven cure for CML is allogeneic hematopoietic stem cell transplantation (alloHSCT), which can eradicate the disease in up to 75% of recipients of stem cells from MHC-matched allogeneic donors.2 The curative potential of alloHSCT is mediated through a potent graft-versus-leukemia (GVL) effect of allogeneic immune effector cells, including T lymphocytes and possibly natural killer (NK) cells, directed against the malignant Ph+ CML stem/progenitor cells.3 The GVL effect in CML is most evident from data showing that in patients who are mixed allogeneic chimeras on relapse of leukemia or after transplantation with a nonmyeloablative conditioning regimen, infusions of peripheral blood leukocytes from the allogeneic donor can induce cytogenetic and molecular remission and long-term leukemia-free survival.4-6 However, donor leukocyte infusion (DLI) in allografted CML patients can be complicated by GVHD and graft failure.7,8 Efforts to preserve the GVL effect of DLI while decreasing the severity of GVHD, including modulating the number of total9 or CD8+ (see Giralt et al10 and Alyea et al11 ) T lymphocytes infused, have had only modest success.
Since their introduction into clinical practice in 2001, tyrosine kinase inhibitors such as imatinib mesylate have supplanted alloHSCT as the initial therapy for CML and can induce cytogenetic remissions in the majority of CML patients.12 However, complete molecular remission is not achieved in most imatinib-treated patients,13,14 and in those CML patients whose BCR-ABL1 transcripts become undetectable by PCR, the majority relapse quickly when imatinib is discontinued.15,16 This suggests that kinase inhibitor therapy is not curative in the majority of CML patients, possibly because of the intrinsic resistance of the most primitive Ph+ stem/progenitor cells to these drugs.17,18 This has rekindled interest in immunotherapy as a strategy to eradicate residual disease in CML patients and permanently cure the leukemia. In addition, alloHSCT is increasingly used for CML patients who are intolerant of or who relapse or exhibit disease progression on kinase inhibitor therapy.19,20 Improvements in adoptive immunotherapy for CML will require a better understanding of the basic immunologic mechanisms involved. For example, the specific cellular mechanisms of the GVL effect in CML are not understood, and it is not known whether GVL and GVHD are separate processes, if they are induced by different subsets of immune cells, or if GVL is directed against leukemia-specific antigens or minor histocompatibility antigens (miHAs).
To investigate these issues, we and others have used a strategy wherein BCR-ABL1 is expressed via retroviral transduction in murine hematopoietic stem cells to model adoptive immunotherapy in mice with CML-like MPN.21,22 Cotransplantation of T cell–depleted (TCD), BCR-ABL1–transduced BM together with allogeneic BM into lethally irradiated recipients yields mice with CML-like leukemia that are tolerant to infusions of allogeneic immune cells. When MHC-mismatched allogeneic donors were used, infusion of allogeneic lymphocytes could be given at the time of initial transplantation or delayed as much as 2 weeks after transplantation, which resulted in equivalent eradication of leukemia.21 In this setting, GVL was mediated by both CD4+ and CD8+ T lymphocytes, but was accompanied by a high incidence of fatal GVHD.21 However, when MHC-matched, miHA-mismatched allogeneic donors were used, a situation that more closely resembles alloHSCT in CML patients, delayed DLI was less effective and was unable to eradicate the leukemia in most recipients.21 In contrast, DLI administered at the time of transplantation (early DLI) efficiently eliminated CML-like leukemia and led to long-term survival, an effect mediated by both CD4+ and CD8+ T lymphocytes but independent of the Fas and TNF effector pathways.22 In the present study, we investigated the disparate effects of early or delayed DLI in MHC-matched, miHA-mismatched allogeneic chimeras with CML-like leukemia, and demonstrate that these distinct immunotherapy approaches mediate GVL through fundamentally different mechanisms.
Methods
Mice
Balb/cAnNTac mice (H-2d) were obtained from Taconic Farms and B10.D2 (H-2d), C3H.SW (H-2b), and C57Bl/6 (H-2b) mice were purchased from The Jackson Laboratory. B10.D2-Rag2−/− mice were generated by back-crossing the TCR-DO11.10/RAG2 knockout line (Taconic Farms) with wild-type B10.D2 mice.
BM transduction and transplantation
Retroviral transduction of BM from 5-fluorouracil–treated donors was carried out as described previously.21 In experiments in which immunotherapy was delivered at the time of initial transplantation (early DLI), transduced BM was TCD with biotinylated anti-CD5 Ab. When mixed allogeneic hematopoietic chimeras were generated for immunotherapy (delayed DLI), transduced BM was depleted of lymphoid and myeloerythroid lineage cells with biotinylated Abs against CD5, B220, Gr-1, and Ter-119 (BD Biosciences) and streptavidin-conjugated magnetic microbeads (Miltenyi Biotec). TCD (2 × 105) or lineage-depleted (Lin−) (1 × 104) BM was injected intravenously into Balb/c recipients irradiated with 700-900 cGy. To induce allogeneic chimerism, 1 × 107 allogeneic TCD BM cells from male B10.D2 donors were transplanted with the BCR-ABL1–transduced BM graft into irradiated recipients. In the C3H.SW→B6 strain pairing, TCD BM from 5-fluorouracil–treated B6 donors (CD45.1+) was transduced with BCR-ABL1 retrovirus and transplanted (1 × 104 cells) with 1 × 107 TCD C3H.SW BM (CD45.2+) into B6 recipients conditioned with 900 cGy γ irradiation. The clinical features and histopathology of BCR-ABL1–induced CML-like disease, B-lymphoid leukemia, and histiocytic sarcoma were described previously.23 All mouse experiments were approved by the Institutional Animal Care and Use Committee of Tufts Medical Center.
DLI
For early DLI treatment, allogeneic splenocytes from B10.D2 male mice were injected IV (3 × 107 cells per recipient) at the time of transplantation. For delayed DLI treatment, B10.D2 splenocytes were either injected directly into chimeras with CML-like disease or were subjected to cell depletion using biotinylated Abs against CD4, CD8, or CD5 (BD Biosciences) and streptavidin-conjugated magnetic microbeads (Miltenyi Biotec). DLI was administered weekly as described in the figure legends until eradication of leukemia as assessed by flow cytometry was reached. The dose of CD4-depleted (2.4 × 107, 80%), CD8-depleted (2.7 × 107, 90%), and CD5-depleted (2.1 × 107, 70%) splenocytes was adjusted to reflect the proportions in unfractionated DLI.
Flow cytometric analysis of chimerism
Peripheral blood chimerism was assessed by flow cytometry with an Ab specific to an allelic variant of β2-microglobulin (β2-Mb,c clone S19.8; BD Biosciences) that is expressed exclusively on allogeneic cells,21 whereas leukemia was monitored through GFP expression.
Southern blot analysis
Genomic DNA samples were collected from recipient mice at the time of death or morbidity, digested with Bgl II, and transferred to nylon membranes after electrophoresis on a 0.8% agarose slab gel. The blots were hybridized with a GFP probe to detect individual proviral clones, with an ABL1 probe to detect both endogenous c-Abl1 and BCR-ABL1 provirus or with an SRY probe to detect male sex. The proviral DNA copy number was calculated by the ratio of the BCR-ABL1 band to the c-Abl1 band, as described previously.23
PCR assay for Sry
Genomic DNA samples were collected from the secondary recipients at time of death or morbidity and assayed for Sry sequences as described previously.24
Results
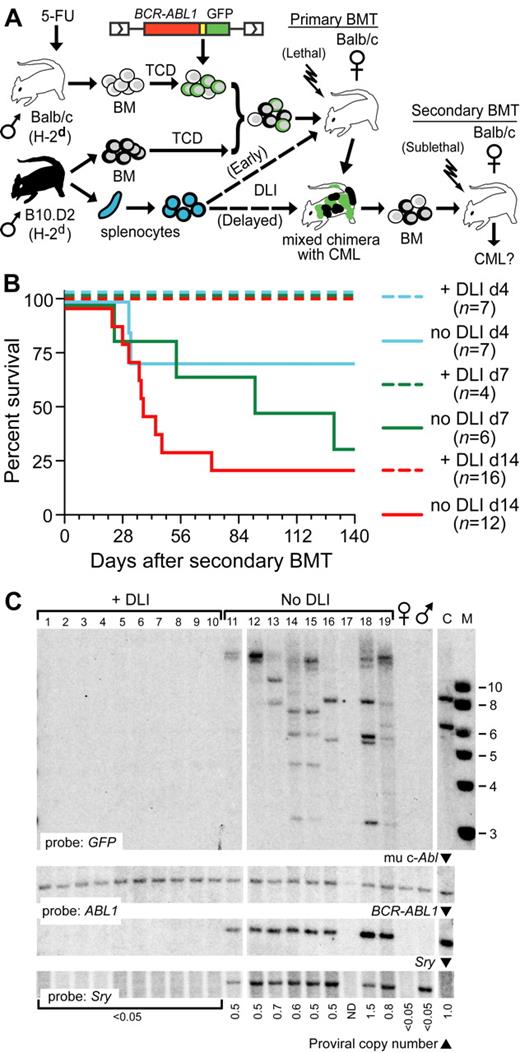
Cotransplantation of allogeneic lymphocytes with BCR-ABL1–transduced BM blocks engraftment of leukemic stem cells (LSCs).
We21 and others22 have reported a mouse immunotherapy model of CML-like MPN induced by BCR-ABL1 retroviral transduction of hematopoietic stem cells, followed by transplantation into irradiated syngeneic recipient mice. In this model, immune cells from an allogeneic donor (splenocytes or lymph node cells) can be delivered either early, at the time of initial transplantation of the mixture of BCR-ABL1–transduced BM and the allogeneic BM (Figure 1A), or later, after the establishment of mixed allogeneic chimerism. In both scenarios, circulating leukocytes expressing the retroviral reporter gene—either GFP (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) or truncated nerve growth factor receptor22 can be detected shortly after transplantation, but this likely reflects contributions from transduced committed progenitors25 rather than from hematopoietic stem cells. In the early DLI model, because the same transplantation procedure is used to both initiate the leukemia and deliver the immunotherapy, this may be quite different from the treatment of established leukemia. In addition, it has long been appreciated that the introduction of allogeneic lymphocytes at the time of transplantation tends to drive hematopoietic engraftment toward the allogeneic donor,26,27 raising the possibility that infusion of T cells at the time of transplantation might prevent leukemia by blocking the initial engraftment of the LSC.
Cotransplantation of allogeneic splenocytes with BCR-ABL1–transduced BM blocks the engraftment of LSCs. (A) Schematic diagram of secondary transplantation experiment. Primary Balb/c recipients (H-2d) were lethally irradiated (700-900 cGy) and received BCR-ABL1–transduced TCD syngeneic BM and TCD allogeneic BM from MHC-matched (H-2d), miHA-mismatched B10.D2 donors, with (dotted line) or without allogeneic splenocytes (DLI) administered at the time of transplantation (Early) or beginning 2 weeks after transplantation (Delayed). At different intervals after the primary transplantation, recipient BM was transplanted into sublethally (400-450 cGy) irradiated secondary Balb/c recipients, who were followed for development of CML-like leukemia. (B) Survival curve depicting the time to morbidity or death from CML-like disease of secondary recipients of BM from primary mice that received allogeneic splenocytes at the time of initial transplantation (+DLI; dashed lines) or did not receive allogeneic splenocytes (no DLI; solid lines). The secondary BMT was performed at 14 days (red curves), 7 days (green curves), or 4 days (blue curves) after the primary transplantation. Note that in this model, CML-like leukemia is not transplanted with 100% efficiency even from nonchimeric donors.29 (C) Southern blot analysis of genomic DNA from spleens of secondary recipients in panel C (d14 cohort) harvested at day 56 after BMT. The blot was hybridized with a GFP probe that detects individual LSC clones (top panel), an ABL1 probe that detects murine c-Abl1 and BCR-ABL1, allowing quantification of proviral DNA content23 (middle panels), and an SRY probe to detect male (donor) sex (bottom panel). Lanes 1-10 are from recipients of BM from DLI-treated primary mice, lanes 11-19 from recipients of primary mice who did not receive DLI, DNA from normal female and male Balb/c mice, and from a control cell line (C) containing a mixture of 2 proviral clones are on the right. Note that secondary recipients of BM from primary mice treated with early DLI did not engraft with any male BCR-ABL1+ stem cells. Similar results were obtained with analysis of the day 7 cohort (data not shown).
Cotransplantation of allogeneic splenocytes with BCR-ABL1–transduced BM blocks the engraftment of LSCs. (A) Schematic diagram of secondary transplantation experiment. Primary Balb/c recipients (H-2d) were lethally irradiated (700-900 cGy) and received BCR-ABL1–transduced TCD syngeneic BM and TCD allogeneic BM from MHC-matched (H-2d), miHA-mismatched B10.D2 donors, with (dotted line) or without allogeneic splenocytes (DLI) administered at the time of transplantation (Early) or beginning 2 weeks after transplantation (Delayed). At different intervals after the primary transplantation, recipient BM was transplanted into sublethally (400-450 cGy) irradiated secondary Balb/c recipients, who were followed for development of CML-like leukemia. (B) Survival curve depicting the time to morbidity or death from CML-like disease of secondary recipients of BM from primary mice that received allogeneic splenocytes at the time of initial transplantation (+DLI; dashed lines) or did not receive allogeneic splenocytes (no DLI; solid lines). The secondary BMT was performed at 14 days (red curves), 7 days (green curves), or 4 days (blue curves) after the primary transplantation. Note that in this model, CML-like leukemia is not transplanted with 100% efficiency even from nonchimeric donors.29 (C) Southern blot analysis of genomic DNA from spleens of secondary recipients in panel C (d14 cohort) harvested at day 56 after BMT. The blot was hybridized with a GFP probe that detects individual LSC clones (top panel), an ABL1 probe that detects murine c-Abl1 and BCR-ABL1, allowing quantification of proviral DNA content23 (middle panels), and an SRY probe to detect male (donor) sex (bottom panel). Lanes 1-10 are from recipients of BM from DLI-treated primary mice, lanes 11-19 from recipients of primary mice who did not receive DLI, DNA from normal female and male Balb/c mice, and from a control cell line (C) containing a mixture of 2 proviral clones are on the right. Note that secondary recipients of BM from primary mice treated with early DLI did not engraft with any male BCR-ABL1+ stem cells. Similar results were obtained with analysis of the day 7 cohort (data not shown).
We reasoned that if LSCs had engrafted and were present in these primary recipients, it should be possible to “rescue” them from the GVL effect by transplanting them into an immunocompetent syngeneic host, whose immune system would eliminate all allogeneic cells. To test this hypothesis, we performed secondary BM transplantations from primary mice that received BCR-ABL1–transduced BM and TCD allogeneic BM, with or without allogeneic splenocytes (B10.D2→Balb/c strain pairing), into sublethally irradiated (immunocompetent) syngeneic (Balb/c) recipients (Figure 1A). At day 14, day 7, or as early as day 4 after the initial transplantation, we could transfer CML-like leukemia into immunocompetent syngeneic secondary recipients from primary mice that did not receive splenocytes, representing bona fide evidence of LSCs in these primary recipients (Figure 1B and supplemental Table 1). These secondary recipients rapidly lost their allogeneic chimerism, which is consistent with a host-versus-graft effect (supplemental Figure 1B). In contrast, we were unable to transfer leukemia from any primary mice that received splenocytes at the time of the initial transplantation, indicating that LSCs were not present in these mice at these early time points and likely failed to engraft at all (Figure 1B and supplemental Table 1). Analysis of BM from secondary recipients revealed that, as expected, secondary recipients of BM from primary mice not treated with DLI engrafted with multiple LSCs of donor (male) origin, whereas secondary recipients from primary DLI-treated mice had no evidence of detectable provirus or cells of male origin, reconstituting hematopoiesis instead with host-derived (female) stem cells (Figure 1C).
To exclude the possibility that the allogeneic immune cells present in these primary donors might have specifically blocked leukemic stem-cell engraftment in secondary recipients, we generated mixed chimeras with CML-like disease, harvested their BM at day 14, and transplanted the cells into lethally or sublethally irradiated secondary Balb/c recipients with or without allogeneic splenocytes (1.5 × 107; supplemental Figure 2A). CML-like leukemia was efficiently transplanted from primary mixed chimeras to sublethally irradiated secondary recipients regardless of whether allogeneic splenocytes were added to the graft, whereas lethally irradiated recipients failed to develop CML when splenocytes were cotransplanted (supplemental Figure 2B-C), engrafting instead with allogeneic hematopoietic stem cells (HSCs). These observations indicate that cotransplantation of allogeneic immune cells can exert a potent antileukemia effect by preventing the engraftment of LSCs.
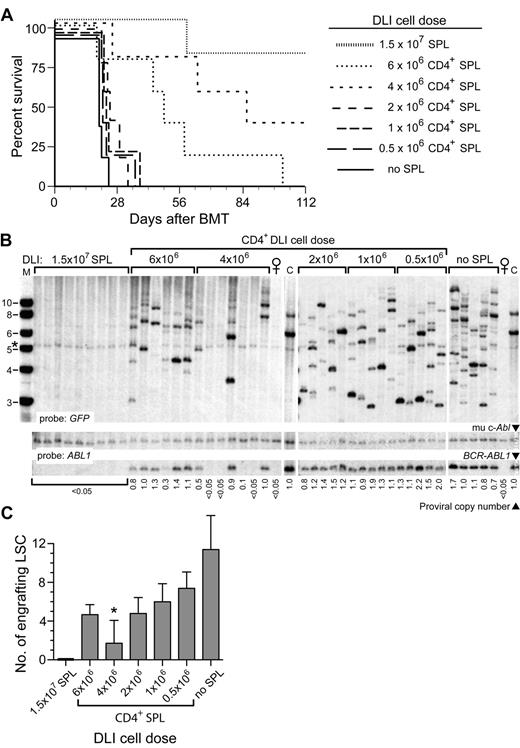
Previous studies have shown that both CD8+ and CD4+ T cells mediate the antileukemic effect of early DLI in the mouse CML model.22,28 When the dose of CD8+ or CD4+ T cells is decreased, the efficiency of the antileukemic effect is also reduced, with an increasing proportion of recipients succumbing to CML-like disease.22 To determine the effect of graded doses of T cells on LSC engraftment, we purified CD4+ allogeneic splenocytes and varied the number of CD4+ T cells added to the initial B10.D2→Balb/c graft. When the dose of CD4+ T cells was successively reduced from 6 × 106 to 0.5 × 106 CD4+ cells per recipient, there was a decrease in the antileukemic effect of early DLI, with an increasing proportion of recipients succumbing to CML-like MPN with successively shorter latency and survival (Figure 2A). However, analysis of the frequency of leukemia-initiating cells in these recipients demonstrated that reductions in the dose of CD4+ T cells were correlated with an increasing number of engrafting LSCs (Figure 2B), from 2-3 proviral clones to the polyclonal (> 10 clones) leukemia that is characteristic of the CML-like MPN induced in recipients who are not treated with DLI in this model23,29 (Figure 2C). These results demonstrate that the predominant antileukemic mechanism of early DLI is to prevent the engraftment of LSCs in this mouse CML model.
Reduction in CD4+ T-cell dose in early DLI is correlated with increased engraftment of LSCs. (A) Survival curve of B10.D2→Balb/c recipients of BCR-ABL1–transduced BM treated with early DLI with the indicated dose of purified CD4+ splenocytes (n = 5 for each cohort). As controls, recipients received either no DLI (no splenocytes, solid line) or DLI with unfractionated splenocytes at the standard dose (1.5 × 107 splenocytes). (B) Southern blot analysis of leukemia-initiating cell frequency in genomic DNA of BM from the cohorts in panel A. The blot was hybridized with a GFP probe (top panel) to quantify the number of engrafting LSCs or with an ABL1 probe (bottom panels) to quantify proviral DNA content, as in Figure 1C. Note that recipients of 4 × 106 T cells had only 1-2 clones per leukemic recipient. The band indicated by the asterisk is a background band. (C) Quantification of frequency of engrafting LSCs from the data shown in panel B. The difference in number of engrafting LSCs between untreated recipients and recipients of 4 × 106 CD4+ splenocytes was significant (P = .0002, unpaired t test).
Reduction in CD4+ T-cell dose in early DLI is correlated with increased engraftment of LSCs. (A) Survival curve of B10.D2→Balb/c recipients of BCR-ABL1–transduced BM treated with early DLI with the indicated dose of purified CD4+ splenocytes (n = 5 for each cohort). As controls, recipients received either no DLI (no splenocytes, solid line) or DLI with unfractionated splenocytes at the standard dose (1.5 × 107 splenocytes). (B) Southern blot analysis of leukemia-initiating cell frequency in genomic DNA of BM from the cohorts in panel A. The blot was hybridized with a GFP probe (top panel) to quantify the number of engrafting LSCs or with an ABL1 probe (bottom panels) to quantify proviral DNA content, as in Figure 1C. Note that recipients of 4 × 106 T cells had only 1-2 clones per leukemic recipient. The band indicated by the asterisk is a background band. (C) Quantification of frequency of engrafting LSCs from the data shown in panel B. The difference in number of engrafting LSCs between untreated recipients and recipients of 4 × 106 CD4+ splenocytes was significant (P = .0002, unpaired t test).
Attenuation of CML-like leukemia in mixed chimeras by reduction in LSC dose
To study the mechanism of immunotherapy against established CML-like leukemia in this mouse model, we returned our focus to the administration of delayed DLI to allogeneic mixed chimeras with CML-like disease, which resemble CML patients with cytogenetic relapse of disease after alloHSCT. In our previous study,21 the effectiveness of delayed DLI in the MHC-matched setting was limited primarily by the rapid pace of the chronic-phase CML-like disease in mice, which allowed only a short 2-week window between engraftment and death for DLI to be effective. However, studies in CML patients have suggested that GVL responses after DLI require weeks to months to be manifest.8 The aggressiveness of murine CML is primarily because of the polyclonal nature of the leukemia,23 and a previous study indicated that the CML-like disease could be converted into a more indolent oligoclonal or monoclonal disease by transplantation of limited numbers of Lin−BCR-ABL1–transduced BM cells.30 In this strategy, lineage depletion is essential to eliminate target cells for BCR-ABL1–induced B-cell acute lymphoblastic leukemia, which have the characteristics of early lymphoid progenitors and can cause death of recipients from lymphoid leukemia.23,31
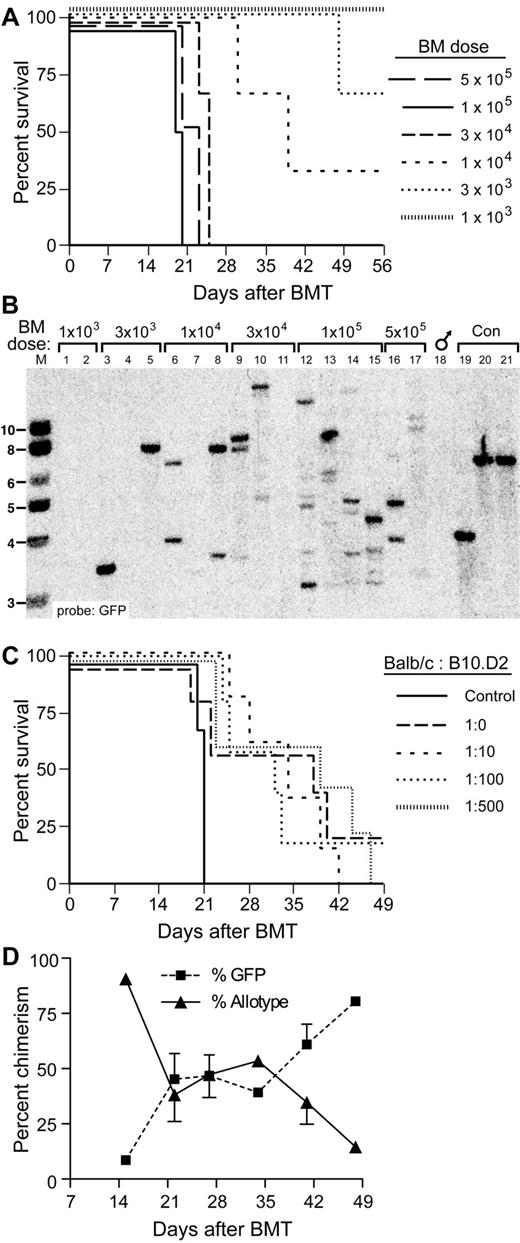
We tested the utility of this approach by performing limiting dilution repopulation of irradiated syngeneic Balb/c recipient mice with BCR-ABL1–transduced BM without allogeneic donor cells. We observed a gradual prolongation of survival associated with decreased transplanted cell dose that was optimal (median survival, 40 days) at a dose of approximately 1 × 104 cells per recipient, whereas recipients of lower cell doses failed to engraft efficiently with leukemia (Figure 3A). This was correlated with a gradual reduction in the clonality of the leukemia as assessed by Southern blotting, down to 1 or 2 clones per recipient (Figure 3B). Therefore, repopulation of mice with one or a few LSCs attenuates the severity of the CML-like leukemia and prolongs survival of recipients. We then tested the effect of cotransplanting different ratios of BCR-ABL1–transduced, Lin− syngeneic (Balb/c) cells and allogeneic (B10.D2) TCD BM cells on chimerism and survival. The results indicated that a mixture of 1 × 104 Lin−BCR-ABL1–transduced BM (containing 1-2 LSCs, Figure 3B) and a 100- to 500-fold excess of TCD allogeneic HSCs (based on an abundance of 1 HSC per 105 normal BM cells) was associated with a median survival of more than 40 days (Figure 3C) and yielded excellent allogeneic chimerism over a period of 14-50 days after transplantation (Figure 3D).
Prolonged survival of allogeneic chimeras engrafted with limiting numbers of LSCs. (A) Survival curve of Balb/c recipients of BCR-ABL1–transduced TCD BM from 5-fluorouracil–treated syngeneic donors injected with the indicated numbers of total BM cells. (B) Southern blot analysis of spleen genomic DNA from the cohorts shown in panel A. Lanes 1 and 2 were from recipients of 1 × 103BCR-ABL1–transduced BM cells, lanes 3-5 received 3 × 103 transduced cells, lanes 6-8 received 1 × 104 transduced cells, lanes 9-11 received 3 × 104 transduced cells, lanes 12-15 received 1 × 105 transduced cells, whereas lanes 16 and 17 received 5 × 105 transduced cells. Lanes 19-21 were from control cell lines each containing a single provirus. Note that reduction in BM cell dose to ≤ 1 × 104 cells results in repopulation with 1-2 LSCs. (C) Survival curve of mixed allogeneic chimeras repopulated with limiting numbers of TCD LSCs, together with increasing numbers of TCD allogeneic BM cells. The numbers represent the ratio of LSCs in 1 × 104BCR-ABL1–transduced TCD BM cells (approximately 2) to the number of allogeneic HSCs (assuming 1 HSC per 105 BM cells). Control mice received 2 × 105 syngeneic BCR-ABL1–transduced TCD BM cells only. (D) Percentage of circulating allogeneic cells and GFP+ cells versus time after BMT for the cohort that received the 1:500 mixture of BCR-ABL1–transduced and allogeneic BM from the experiment shown in panel C. Note the high level of allogeneic chimerism that persists until leukemic progression at 7 weeks. The GFP+ population between days 21 and 42 contained some cells that were allotype-positive because of phagocytosis of GFP+ leukemic cells by allogeneic macrophages.21
Prolonged survival of allogeneic chimeras engrafted with limiting numbers of LSCs. (A) Survival curve of Balb/c recipients of BCR-ABL1–transduced TCD BM from 5-fluorouracil–treated syngeneic donors injected with the indicated numbers of total BM cells. (B) Southern blot analysis of spleen genomic DNA from the cohorts shown in panel A. Lanes 1 and 2 were from recipients of 1 × 103BCR-ABL1–transduced BM cells, lanes 3-5 received 3 × 103 transduced cells, lanes 6-8 received 1 × 104 transduced cells, lanes 9-11 received 3 × 104 transduced cells, lanes 12-15 received 1 × 105 transduced cells, whereas lanes 16 and 17 received 5 × 105 transduced cells. Lanes 19-21 were from control cell lines each containing a single provirus. Note that reduction in BM cell dose to ≤ 1 × 104 cells results in repopulation with 1-2 LSCs. (C) Survival curve of mixed allogeneic chimeras repopulated with limiting numbers of TCD LSCs, together with increasing numbers of TCD allogeneic BM cells. The numbers represent the ratio of LSCs in 1 × 104BCR-ABL1–transduced TCD BM cells (approximately 2) to the number of allogeneic HSCs (assuming 1 HSC per 105 BM cells). Control mice received 2 × 105 syngeneic BCR-ABL1–transduced TCD BM cells only. (D) Percentage of circulating allogeneic cells and GFP+ cells versus time after BMT for the cohort that received the 1:500 mixture of BCR-ABL1–transduced and allogeneic BM from the experiment shown in panel C. Note the high level of allogeneic chimerism that persists until leukemic progression at 7 weeks. The GFP+ population between days 21 and 42 contained some cells that were allotype-positive because of phagocytosis of GFP+ leukemic cells by allogeneic macrophages.21
Combined therapy with delayed DLI and an ABL1 kinase inhibitor results in prolonged leukemia-free survival of mixed-allogeneic chimeras with BCR-ABL1–induced CML-like disease
A complementary strategy to extend the time period available for immunotherapy in the CML model is treatment of recipient chimeras with an ABL1 kinase inhibitor such as imatinib mesylate, which significantly prolongs the survival of mice reconstituted with larger numbers of BCR-ABL1–transduced cells32 and has been used in combination with DLI in CML patients relapsing after alloHSCT.33,34 To test this approach, we generated mice with mixed allogeneic chimerism (B10.D2→Balb/c) and CML-like MPN using the conditions defined in Figure 3C, and treated cohorts either with delayed DLI (allogeneic splenocytes) alone, imatinib at a low-intermediate dose (approximately 30 mg/kg once daily by oral gavage), or with both modalities (supplemental Figure 3). Treatment with imatinib alone prolonged survival but did not eradicate the leukemia, as the mice relapsed and succumbed to CML-like disease when the drug was discontinued (supplemental Figure 3 dotted line). In contrast, the addition of delayed DLI to imatinib treatment resulted in significantly prolonged survival relative to untreated mice and led to probable cure when kinase inhibitor therapy was discontinued. The surviving mice in this cohort had no evidence of circulating GFP+ leukemia cells (data not shown) and no overt evidence of GVHD.
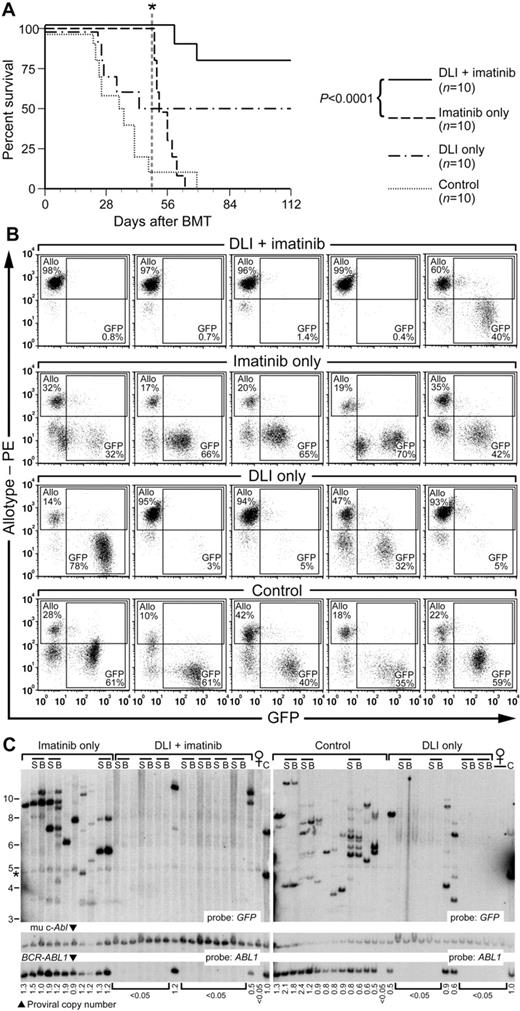
In subsequent experiments, we increased the dose of imatinib to 100-200 mg/kg/d (near the maximum tolerated dose in mice35 ) and increased the conditioning radiation dose from approximately 670 to approximately 750 cGy. Under these conditions, disease eradication and long-term survival was reproducibly achieved in 80%-100% of recipients treated with imatinib and delayed DLI (Figure 4A). Flow cytometric analysis of peripheral blood demonstrated elimination of circulating GFP+ leukemic cells in the majority of recipients of combined DLI plus imatinib therapy after 3 weeks of treatment, with conversion to full allogeneic chimerism (Figure 4B). Southern blot analysis confirmed the eradication of CML stem cells in these recipients, with replacement by male HSCs of allogeneic origin (Figure 4C). Interim analysis of the different cohorts of leukemic mixed allogeneic chimeras that were undergoing treatment demonstrated that therapy with imatinib alone was able to control but not eliminate the CML-like MPN, whereas combined treatment with imatinib and delayed DLI resulted in progressive elimination of the CML-like disease, restoration of normal organ histopathology, and conversion toward full allogeneic chimerism (supplemental Figure 4).
Delayed DLI and imatinib therapy leads to prolonged leukemia-free survival in MHC-matched/miHA-mismatched chimeras with BCR-ABL1–induced CML-like disease. (A) Survival curve for cohorts of Balb/c recipients of BCR-ABL1–transduced Lin− Balb/c BM (1 × 104 cells) mixed with 500-fold excess of MHC-matched allogeneic stem cells (B10.D2→Balb/c donors). All recipients developed mixed hematopoietic chimerism with CML-like leukemia (GFP+ myeloid cells) at day 14 after transplantation. Beginning at day 14, mice were treated with repeated weekly infusions of allogeneic (B10.D2) splenocytes (3 × 107 cells per treatment, total of 5 infusions per recipient), treated with high-dose imatinib (100 mg/kg once daily by oral gavage), or a combination of the 2 (DLI + imatinib). Imatinib treatment was discontinued at day 49 (indicated by the vertical dotted line and asterisk). The addition of delayed DLI to imatinib resulted in superior survival compared with imatinib alone (P < .0001, Mantel-Cox test), whereas the survival difference between cohorts receiving DLI + imatinib and DLI only was of borderline significance (P = .072). (B) Flow cytometric analysis of peripheral blood leukocytes from 5 representative mice each from the 4 cohorts in panel A, analyzed at 5 weeks after transplantation (after 3 DLI doses). Allogeneic chimerism (Y-axis) was detected by a polymorphism in β2-microglobulin as described in “Methods,” whereas GFP+ cells (X-axis) represent BCR-ABL1–expressing leukemic cells. The percentage of allotype-positive and GFP+ cells in each plot is indicated. Note the eradication of leukemia in the majority of recipients treated with DLI + imatinib compared with the persistent leukemia in control or imatinib-only mice. (C) Southern blot analysis of genomic DNA from the cohorts in panel A, harvested at day 120 after BMT. Paired samples from the spleen (S) and BM (B) of the same individual mouse are grouped by the bars; all other samples are from the spleen. Lanes with DNA from normal female mice are indicated, whereas C indicates DNA from cell lines containing 1 or 2 proviral copies. The blot was hybridized with probes for GFP and ABL1 as in Figure 1C. The band indicated by the asterisk is a background band.
Delayed DLI and imatinib therapy leads to prolonged leukemia-free survival in MHC-matched/miHA-mismatched chimeras with BCR-ABL1–induced CML-like disease. (A) Survival curve for cohorts of Balb/c recipients of BCR-ABL1–transduced Lin− Balb/c BM (1 × 104 cells) mixed with 500-fold excess of MHC-matched allogeneic stem cells (B10.D2→Balb/c donors). All recipients developed mixed hematopoietic chimerism with CML-like leukemia (GFP+ myeloid cells) at day 14 after transplantation. Beginning at day 14, mice were treated with repeated weekly infusions of allogeneic (B10.D2) splenocytes (3 × 107 cells per treatment, total of 5 infusions per recipient), treated with high-dose imatinib (100 mg/kg once daily by oral gavage), or a combination of the 2 (DLI + imatinib). Imatinib treatment was discontinued at day 49 (indicated by the vertical dotted line and asterisk). The addition of delayed DLI to imatinib resulted in superior survival compared with imatinib alone (P < .0001, Mantel-Cox test), whereas the survival difference between cohorts receiving DLI + imatinib and DLI only was of borderline significance (P = .072). (B) Flow cytometric analysis of peripheral blood leukocytes from 5 representative mice each from the 4 cohorts in panel A, analyzed at 5 weeks after transplantation (after 3 DLI doses). Allogeneic chimerism (Y-axis) was detected by a polymorphism in β2-microglobulin as described in “Methods,” whereas GFP+ cells (X-axis) represent BCR-ABL1–expressing leukemic cells. The percentage of allotype-positive and GFP+ cells in each plot is indicated. Note the eradication of leukemia in the majority of recipients treated with DLI + imatinib compared with the persistent leukemia in control or imatinib-only mice. (C) Southern blot analysis of genomic DNA from the cohorts in panel A, harvested at day 120 after BMT. Paired samples from the spleen (S) and BM (B) of the same individual mouse are grouped by the bars; all other samples are from the spleen. Lanes with DNA from normal female mice are indicated, whereas C indicates DNA from cell lines containing 1 or 2 proviral copies. The blot was hybridized with probes for GFP and ABL1 as in Figure 1C. The band indicated by the asterisk is a background band.
In human CML patients treated by allografting, DLI is effective across multiple HLA types.8 In our model, we also demonstrated the efficacy of allogeneic DLI in a second MHC-matched, miHA-mismatched strain pairing, C3H.SW→B6 (H-2b, supplemental Figure 5). These results demonstrate that combination treatment of leukemic mixed chimeras with delayed DLI and kinase inhibitor therapy can yield potent GVL effects across multiple MHC backgrounds in a scenario that is relevant to allografted CML patients.
GVL effect of delayed DLI is directed against miHAs shared by normal and leukemic HSCs, but not BCR-ABL1 or GFP
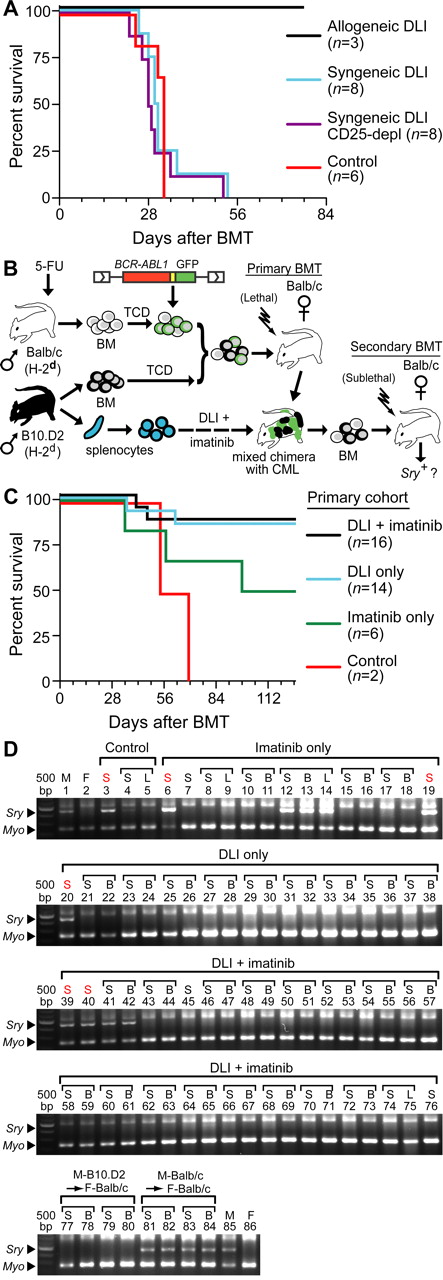
Whereas BCR-ABL1 (human) and GFP (jellyfish) are foreign proteins in this mouse model system, it is possible that epitopes derived from these potential antigens were responsible for the GVL effect of DLI that we observed. To test this possibility, we compared the GVL effect of syngeneic (Balb/c) and allogeneic (B10.D2) DLI in B10.D2→Balb/c chimeras with CML-like leukemia. In combination with imatinib treatment, allogeneic splenocytes were effective at clearing the leukemia, whereas recipients of syngeneic DLI succumbed to CML-like disease with similar latency as untreated chimeras regardless of whether inhibitory regulatory T cells were first depleted from the infused splenocytes (Figure 5A). Therefore, BCR-ABL1 and GFP alone are insufficient as target antigens, and miHA differences are absolutely required for GVL.
GVL is directed against miHAs shared by normal and leukemic stem cells. (A) Survival curve for B10.D2→Balb/c mixed allogeneic chimeras with CML who were untreated (red curve), or treated with weekly DLI (total of 3 infusions beginning at day 14 after BMT) of syngeneic (Balb/c) or allogeneic (B10.D2, black curve) DLI. For syngeneic DLI, splenocytes were given either unfractionated (blue curve) or after depletion of CD25+ regulatory T cells (purple curve). (B) Schematic diagram of secondary transplantation experiment. BM from primary chimeras (B10.D2→Balb/c) treated with allogeneic DLI + imatinib, DLI alone, imatinib alone, or no treatment was transplanted to sublethally (450 cGy) irradiated syngeneic female Balb/c recipients. The secondary transplantation was performed either at the time of morbidity or death of the primary chimera or at least 100 days after the primary transplantation. (C) Survival curve of secondary recipients of BM from primary leukemic chimeras that were untreated (Control) or treated with imatinib only, allogeneic DLI only, or the combination of DLI + imatinib. Note that in this experiment, imatinib treatment decreased the efficiency of secondary transplantation of CML-like leukemia to 50%. (D) PCR assay for male Sry sequences in hematopoietic tissues (S = spleen, B = BM, and L = lymph node) of the secondary recipients from panel C.
GVL is directed against miHAs shared by normal and leukemic stem cells. (A) Survival curve for B10.D2→Balb/c mixed allogeneic chimeras with CML who were untreated (red curve), or treated with weekly DLI (total of 3 infusions beginning at day 14 after BMT) of syngeneic (Balb/c) or allogeneic (B10.D2, black curve) DLI. For syngeneic DLI, splenocytes were given either unfractionated (blue curve) or after depletion of CD25+ regulatory T cells (purple curve). (B) Schematic diagram of secondary transplantation experiment. BM from primary chimeras (B10.D2→Balb/c) treated with allogeneic DLI + imatinib, DLI alone, imatinib alone, or no treatment was transplanted to sublethally (450 cGy) irradiated syngeneic female Balb/c recipients. The secondary transplantation was performed either at the time of morbidity or death of the primary chimera or at least 100 days after the primary transplantation. (C) Survival curve of secondary recipients of BM from primary leukemic chimeras that were untreated (Control) or treated with imatinib only, allogeneic DLI only, or the combination of DLI + imatinib. Note that in this experiment, imatinib treatment decreased the efficiency of secondary transplantation of CML-like leukemia to 50%. (D) PCR assay for male Sry sequences in hematopoietic tissues (S = spleen, B = BM, and L = lymph node) of the secondary recipients from panel C.
To investigate whether the GVL effect of allogeneic DLI is mediated by an immune response directed specifically at BCR-ABL1–expressing LSCs or against miHA shared by normal HSCs, we serially transplanted BM from leukemic B10.D2→Balb/c chimeras that were treated with allogeneic DLI to syngeneic immunocompetent female Balb/c recipients (Figure 5B). Because normal (untransduced) Balb/c HSCs are cotransplanted with BCR-ABL1–transduced HSCs to generate the primary chimeras,21 we reasoned that these normal (male) HSCs might persist in DLI-treated chimeras if the GVL effect were specific to LSCs. Whereas CML-like leukemia could be efficiently transplanted into secondary recipients from primary chimeras treated with syngeneic DLI, the majority of recipients of BM from primary chimeras treated with allogeneic DLI remained free of leukemia (Figure 5C) with no detectable circulating GFP+ cells (data not shown). To assess the presence of donor-derived male hematopoiesis in these secondary recipients, we used a PCR assay for the Sry gene in genomic DNA from hematopoietic tissues that was capable of detecting approximately 3% male DNA content (supplemental Figure 6). Except for a recipient of BM from a leukemic primary mouse who failed DLI treatment (Figure 5D lanes 41-42), Sry sequences were not detectable in any recipients of BM from primary chimeras successfully treated with allogeneic DLI plus imatinib or with DLI alone (Figure 5D). In contrast, Sry was readily detected in secondary recipients of BM from imatinib-treated primary mice that developed CML-like leukemia (Figure 5D lanes 12-14), or of BM from control male Balb/c donors (Figure 5D lanes 81-84). These results suggest that the anti-CML effect of DLI is directed against the entire population of miHA-mismatched HSCs regardless of whether they express BCR-ABL1.
The GVL effect of delayed DLI is mediated predominantly by CD8+ splenocytes
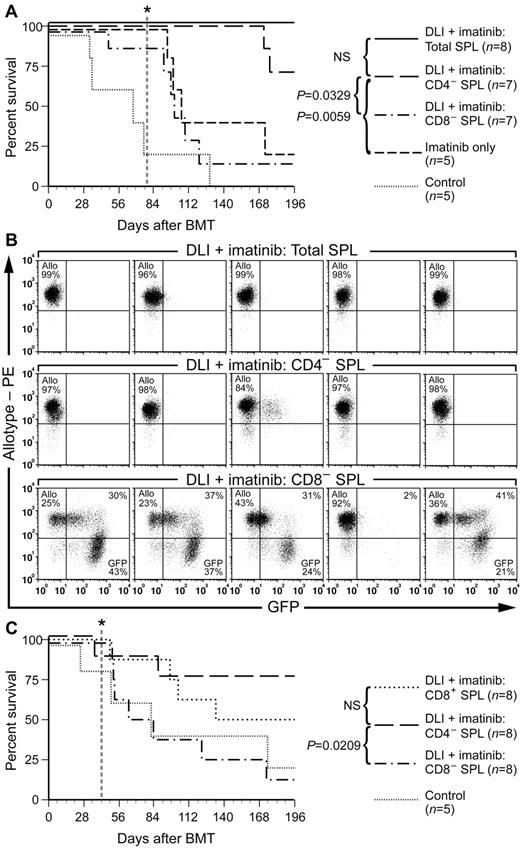
To determine the T-cell subsets responsible for the GVL effect of delayed DLI, we fractionated splenocytes by selectively depleting CD4+ or CD8+ cells. The resulting depleted splenocyte populations, when reanalyzed by FACS, had approximately 0.7% or 0.5% residual CD4+ or CD8+ T-cell content, respectively (supplemental Figure 7A). The GVL activity of the CD4- and CD8-depleted splenocytes was compared with that of the same relative dose of total unfractionated splenocytes using 4 DLI treatments of MHC-matched/miHA-mismatched leukemic chimeras generated using the transplantation conditions described in Figure 4. Under these conditions, the administration of DLI with unfractionated splenocytes in combination with imatinib at 100 mg/kg/d resulted in eradication of leukemia and cure in 100% of recipients (Figure 6A). Interestingly, depletion of CD4+ T cells had little impact on the GVL activity of delayed DLI, with the majority of recipients also clearing their GFP+ leukemia and converting to full allogeneic chimerism (Figure 6A-B). In contrast, depletion of CD8+ T cells profoundly impaired the antileukemic effect of delayed DLI (Figure 6A), with the majority of recipients failing to clear their leukemia (Figure 6B). In addition, most recipients of CD8-depleted DLI had evidence of clinical GVHD, with weight loss (supplemental Figure 7B) and skin changes (data not shown). The predominant role of CD8+ T cells in mediating GVL against CML-like leukemia was confirmed in an independent experiment in which purified CD8+ splenocytes were used as the source of DLI (Figure 6C). These results differed fundamentally from those after early DLI when T cells are administered at the time of initial transplantation of BCR-ABL–transduced stem cells, in which there are approximately equal antileukemic contributions from both CD4+ and CD8+ T cells.22 Therefore, our results demonstrate that allogeneic T lymphocytes use different cellular mechanisms to block the engraftment of LSCs or to eradicate established CML-like leukemia.
CD8+ T cells mediate GVL against CML-like leukemia. (A) Survival curve for B10.D2→Balb/c mixed allogeneic chimeras with CML that were untreated (Control) or treated with imatinib alone (100 mg/kg/d) or in combination with weekly DLI (total of 4 infusions beginning at day 14 after BMT) of unfractionated (Total splenocytes, solid line), CD4-depleted, or CD8-depleted splenocytes. Imatinib treatment was stopped at day 77 (vertical dotted line). The difference in survival between recipients of CD4-depleted DLI and recipients treated with imatinib only or with CD8-depleted DLI was significant (P = .0329 and P = .0059, respectively, by Mantel-Cox tests), whereas there was no significant difference in survival of recipients treated with total splenocyte DLI versus CD4-depleted DLI, or of recipients treated with CD8-depleted DLI versus imatinib only. (B) FACS analysis of leukemia burden (percentage of GFP+ leukocytes, X-axis) and allogeneic chimerism (β2-microglobulin b allele, Y-axis) at day 49 (2 weeks after the last DLI) in 5 representative recipients of DLI with total (top row), CD4-depleted (middle row), or CD8-depleted (bottom row) splenocytes. Note the eradication of GFP+ cells and full allogeneic chimerism in most recipients of CD4-depleted splenocytes, but persistent leukemia in most recipients treated with CD8-depleted splenocytes. (C) Survival curve for an independent transplantation cohort of B10.D2→Balb/c allogeneic chimeras with CML-like leukemia that were treated with imatinib and DLI consisting of CD4- or CD8-depleted splenocytes or purified CD8+ splenocytes, with imatinib treatment stopped at day 42 after transplantation (vertical dotted line). The survival of recipients treated with either CD4-depleted or CD8+ DLI was significantly improved compared with the CD8-depleted arm (P = .0209 and .0278, respectively, by Mantel-Cox tests), whereas there was no significant difference in survival of recipients of CD8-depeleted DLI versus control (P = .4259).
CD8+ T cells mediate GVL against CML-like leukemia. (A) Survival curve for B10.D2→Balb/c mixed allogeneic chimeras with CML that were untreated (Control) or treated with imatinib alone (100 mg/kg/d) or in combination with weekly DLI (total of 4 infusions beginning at day 14 after BMT) of unfractionated (Total splenocytes, solid line), CD4-depleted, or CD8-depleted splenocytes. Imatinib treatment was stopped at day 77 (vertical dotted line). The difference in survival between recipients of CD4-depleted DLI and recipients treated with imatinib only or with CD8-depleted DLI was significant (P = .0329 and P = .0059, respectively, by Mantel-Cox tests), whereas there was no significant difference in survival of recipients treated with total splenocyte DLI versus CD4-depleted DLI, or of recipients treated with CD8-depleted DLI versus imatinib only. (B) FACS analysis of leukemia burden (percentage of GFP+ leukocytes, X-axis) and allogeneic chimerism (β2-microglobulin b allele, Y-axis) at day 49 (2 weeks after the last DLI) in 5 representative recipients of DLI with total (top row), CD4-depleted (middle row), or CD8-depleted (bottom row) splenocytes. Note the eradication of GFP+ cells and full allogeneic chimerism in most recipients of CD4-depleted splenocytes, but persistent leukemia in most recipients treated with CD8-depleted splenocytes. (C) Survival curve for an independent transplantation cohort of B10.D2→Balb/c allogeneic chimeras with CML-like leukemia that were treated with imatinib and DLI consisting of CD4- or CD8-depleted splenocytes or purified CD8+ splenocytes, with imatinib treatment stopped at day 42 after transplantation (vertical dotted line). The survival of recipients treated with either CD4-depleted or CD8+ DLI was significantly improved compared with the CD8-depleted arm (P = .0209 and .0278, respectively, by Mantel-Cox tests), whereas there was no significant difference in survival of recipients of CD8-depeleted DLI versus control (P = .4259).
Non-T cells make a minor contribution to GVL against CML-like leukemia in MHC-matched/miHA-mismatched chimeras
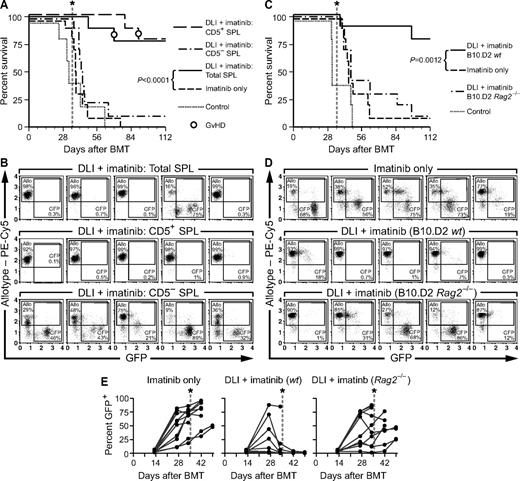
Our results demonstrate that delayed DLI with allogeneic splenocytes can cure mice with CML-like leukemia. The splenocyte population used for DLI is a heterogeneous mixture of CD4+ T cells (∼ 20%), CD8+ T cells (∼ 10%), B220+ B cells (∼ 30%), CD11b+ myeloid cells (∼ 20%), and NK1.1+ NK cells (∼ 2%-4%). To determine whether immune cells other than T lymphocytes can mediate GVL in this model, we depleted splenocytes of CD5+ T cells using anti-CD5 mAb (supplemental Figure 7A), and treated MHC-matched/miHA-mismatched leukemic chimeras with DLI and/or imatinib. In one experiment, we observed a modest but significant prolongation of the survival of a cohort of chimeric leukemic recipients after infusion of CD5-depleted splenocytes (supplemental Figure 8), with clearance of leukemia in 2 of 7 recipients. However, in a second experiment, CD5-depleted DLI was less effective, with a slight prolongation of survival that was not significant (Figure 7A) and a failure to clear circulating leukemia cells in the majority of recipients (Figure 7B). Whereas there was 1 long-term survivor in this cohort, this mouse had persistent leukemia in the BM at necropsy at the conclusion of the trial (data not shown). In contrast, DLI with either unfractionated splenocytes or with the equivalent number of purified CD5+ splenocytes mediated effective GVL against CML-like leukemia.
Contribution of non-T cells to GVL against CML-like leukemia. (A) Survival curve for leukemic allogeneic chimeras (B10.D2→Balb/c) treated with imatinib (100 mg/kg) alone or in combination with DLI consisting of unfractionated splenocytes (SPL; 3 × 107 total splenocytes per treatment, 4 infusions per recipient), CD5-depleted splenocytes (2.1 × 107 CD5-depleted splenocytes per treatment), or CD5+ splenocytes (0.9 × 107 CD5+ splenocytes per treatment). The numbers of CD5+ cells were adjusted to equal the number of T cells in the unfractionated DLI arm. Both imatinib and DLI treatments were discontinued at day 35 (vertical dotted line). The addition of delayed DLI to imatinib resulted in superior survival compared with imatinib alone (P < .0001 by Mantel-Cox test), whereas the survival differences between cohorts receiving CD5− splenocytes, imatinib only, and control were not statistically significant. (B) Flow cytometric analysis of allogeneic chimerism (β2-microglobulin allotype, y-axis) and leukemic burden (GFP+ cells, x-axis) in peripheral blood leukocytes from 5 representative mice each from the cohorts in panel A analyzed at day 43 after transplantation. The percentage of allotype-positive and GFP+ cells in each plot is indicated. Note the eradication of leukemia in the majority of recipients treated with total splenocytes + imatinib and CD5+ splenocytes + imatinib compared with persistent leukemia in other groups. (C) Survival curve of leukemic allogeneic chimeras (B10.D2→Balb/c) treated with imatinib and weekly infusions of allogeneic splenocytes from wild-type B10.D2 allogeneic donors (B10.D2-wt, 3 × 107 cells per treatment for a total of 4 infusions) or Rag2-deficient B10.D2 donors (B10.D2-Rag2−/−, 8-10 × 106 cells per treatment). Both treatments were discontinued at day 35 (vertical dotted line). The survival of recipients treated with imatinib + B10.D2-wt DLI was prolonged relative to the imatinib-only cohort (P = .012 by Mantel-Cox test), but there was no significant survival advantage imparted by B10.D2-Rag2−/− DLI. (D) Flow cytometric analysis of peripheral blood of 5 representative leukemic chimeras, analyzed at day 34 after transplantation (after 3 doses of DLI treatment). The percentage of allogeneic chimerism (y-axis) and GFP+ leukemic cells (x-axis) in indicated. Note the relative reduction of GFP+ cells and the high degree of allogeneic chimerism in some recipients of B10.D2 Rag2−/− DLI. (E) Percentage of GFP+ peripheral blood cells of individual recipients in each cohort over time. Note the relatively low burden of leukemic cells in some individuals receiving B10.D2-Rag2−/− DLI, with subsequent relapse after treatment cessation at day 35 (dotted lines).
Contribution of non-T cells to GVL against CML-like leukemia. (A) Survival curve for leukemic allogeneic chimeras (B10.D2→Balb/c) treated with imatinib (100 mg/kg) alone or in combination with DLI consisting of unfractionated splenocytes (SPL; 3 × 107 total splenocytes per treatment, 4 infusions per recipient), CD5-depleted splenocytes (2.1 × 107 CD5-depleted splenocytes per treatment), or CD5+ splenocytes (0.9 × 107 CD5+ splenocytes per treatment). The numbers of CD5+ cells were adjusted to equal the number of T cells in the unfractionated DLI arm. Both imatinib and DLI treatments were discontinued at day 35 (vertical dotted line). The addition of delayed DLI to imatinib resulted in superior survival compared with imatinib alone (P < .0001 by Mantel-Cox test), whereas the survival differences between cohorts receiving CD5− splenocytes, imatinib only, and control were not statistically significant. (B) Flow cytometric analysis of allogeneic chimerism (β2-microglobulin allotype, y-axis) and leukemic burden (GFP+ cells, x-axis) in peripheral blood leukocytes from 5 representative mice each from the cohorts in panel A analyzed at day 43 after transplantation. The percentage of allotype-positive and GFP+ cells in each plot is indicated. Note the eradication of leukemia in the majority of recipients treated with total splenocytes + imatinib and CD5+ splenocytes + imatinib compared with persistent leukemia in other groups. (C) Survival curve of leukemic allogeneic chimeras (B10.D2→Balb/c) treated with imatinib and weekly infusions of allogeneic splenocytes from wild-type B10.D2 allogeneic donors (B10.D2-wt, 3 × 107 cells per treatment for a total of 4 infusions) or Rag2-deficient B10.D2 donors (B10.D2-Rag2−/−, 8-10 × 106 cells per treatment). Both treatments were discontinued at day 35 (vertical dotted line). The survival of recipients treated with imatinib + B10.D2-wt DLI was prolonged relative to the imatinib-only cohort (P = .012 by Mantel-Cox test), but there was no significant survival advantage imparted by B10.D2-Rag2−/− DLI. (D) Flow cytometric analysis of peripheral blood of 5 representative leukemic chimeras, analyzed at day 34 after transplantation (after 3 doses of DLI treatment). The percentage of allogeneic chimerism (y-axis) and GFP+ leukemic cells (x-axis) in indicated. Note the relative reduction of GFP+ cells and the high degree of allogeneic chimerism in some recipients of B10.D2 Rag2−/− DLI. (E) Percentage of GFP+ peripheral blood cells of individual recipients in each cohort over time. Note the relatively low burden of leukemic cells in some individuals receiving B10.D2-Rag2−/− DLI, with subsequent relapse after treatment cessation at day 35 (dotted lines).
Several lines of evidence suggest that NK cells may contribute to the GVL effect against human CML, because the expression of particular NK-cell receptors or host ligands36-38 is correlated with decreased relapse in allografted CML patients. To address more directly the possibility that NK cells might contribute to GVL against murine CML-like leukemia, we used B10.D2 mice with homozygous null mutations in the Rag2 gene, which have spleens with no detectable CD3+ T cells and are enriched in NK1.1+ NK cells (supplemental Figure 9A), as the source of DLI. Leukemic mixed chimeras (B10.D2→Balb/c) were treated with imatinib alone or imatinib in combination with splenocyte DLI from B10.D2 wild-type or Rag2−/− donors. Despite the large number of NK cells present in the DLI population from B10.D2 Rag2−/− donors (∼ 4 × 106 NK cells per infusion for a total of 4 infusions), only the cohort treated with imatinib plus DLI from B10.D2 wild-type donors exhibited significantly prolonged survival (Figure 7C), with 90% of recipients clearing their leukemia (Figure 7D). In contrast, the majority of mice receiving DLI from B10.D2 Rag2−/− donors succumbed to leukemia shortly after cessation of imatinib treatment at day 35 after transplantation, although 40% of this cohort survived longer than 8 weeks (Figure 7C). Analysis of levels of allogeneic chimerism and circulating GFP+ leukemic cells (Figure 7D-E) showed that NK cell–enriched DLI was able to decrease the leukemic burden in some recipients in which > 4% circulating allogeneic NK cells were detected (supplemental Figure 9B), but the effect was transient and did not lead to elimination of LSCs. Consistent with this, there was no significant difference in the frequency of LSCs in the cohorts treated with imatinib or imatinib plus DLI from B10.D2 Rag2−/− donors as assessed by Southern blot (data not shown). These results suggest that NK cells make only a minor contribution to the GVL effect of allogeneic splenocyte infusions under the conditions used in this model system.
Discussion
Eradication of malignant disease and permanent cure of the patient is the ultimate goal of all cancer therapies. Whereas ABL1 kinase inhibitors have been an enormous clinical success and become the standard initial therapy for CML, it is now clear that the majority of CML patients are not cured by imatinib,15,16 and immunotherapy is a compelling avenue to explore as a complementary, potentially curative strategy. DLI from an allogeneic donor can induce remissions in the majority of CML patients who relapse after alloHSCT,4,5 but at the cost of significant GVHD and graft failure.6,7 Nonmyeloablative or reduced-intensity conditioning regimens can induce mixed hematopoietic chimerism in allografted CML patients,39,40 but DLI and conversion to full allogeneic chimerism is still complicated by frequent GVHD. In the imatinib era, exploiting the GVL effect in CML will require a better understanding of the basic immunologic mechanisms involved. For example, it is not known whether the GVL and GVHD are separate processes, if they are induced by different subsets of immune cells, if GVL actually represents GVHD that is restricted to the hematopoietic system, or if leukemia-specific antigens or miHAs are responsible for the immune responses.
In the present study, we used a physiologically relevant model of CML in laboratory mice to investigate the mechanisms of DLI in an MHC-matched, miHA-mismatched setting. When allogeneic immune cells are infused at the time of transplantation of BCR-ABL1–transduced BM (early DLI), they are effective at preventing fatal CML-like leukemia in both MHC-mismatched21 and MHC-matched22 chimeras. However, because the same transplantation procedure is used to both initiate the leukemia and deliver the immunotherapy, this may differ from immunotherapy of established CML-like leukemia. In addition, several lines of evidence suggest that the introduction of allogeneic lymphocytes or NK cells at the time of transplantation tends to drive hematopoietic engraftment toward the allogeneic donor.26,27,41 We demonstrate herein that in recipients of early DLI, CML-like leukemia cannot be adoptively transferred into sublethally irradiated syngeneic secondary recipients as early as 4 days after primary transplantation, indicating that cotransplantation of allogeneic lymphocytes at the time of transplantation prevents the engraftment of BCR-ABL1–transduced stem cells. Whereas male-derived hematopoiesis (originating from the syngeneic Balb/c donor) is not observed in these secondary recipients (Figure 1C), the effect of early DLI on HSC engraftment appears to be directed against both BCR-ABL1–transduced and normal stem cells. When the number of T lymphocytes delivered via early DLI is successively reduced, some recipients fail to clear their leukemia, but analysis of LSC frequency in these recipients demonstrates that this is because of the escape of limiting numbers of leukemia-initiating cells and their subsequent engraftment (Figure 2), causing sustained and fatal MPN. Our results demonstrate a potent anti-engraftment effect of allogeneic splenocytes that might be exploited in myeloid leukemia patients treated by autologous transplantation.42
To study the mechanism of GVL in a setting that models the cytogenetic relapse of CML after alloHSCT, in which GVL responses require weeks to months to be manifest,8 we developed methods to attenuate the severity of the CML-like disease by engrafting fewer LSCs and treating leukemia chimeras with imatinib (Figure 3). This procedure allowed the delivery of multiple rounds of delayed DLI that gradually resulted in clearance of circulating leukemia cells and long-term survival (Figure 4). The specific antigens involved in the GVL effect of delayed DLI are unknown, but it is likely that neither BCR-ABL1 nor GFP are major contributors, because infusion of splenocytes from syngeneic Balb/c donors was ineffective in mediating a GVL response (Figure 5). In addition, because residual normal syngeneic donor-derived HSCs do not persist in DLI-treated chimeras (Figure 5), the immune response appears to be directed equally against normal HSCs and LSCs. Therefore, it is likely that miHAs play a significant role in the GVL response to CML-like MPN, and this model system provides a platform for identifying these antigens.
Although CD4+ T cells play a significant role in blocking the engraftment of BCR-ABL1+ stem cells (Figure 2 and Matte et al22 ), there was little contribution by CD4+ splenocytes in the GVL effect of delayed DLI in allogeneic chimeras with established CML (Figure 6), emphasizing that different cellular mechanisms are involved. In the early DLI model, GVL mediated by CD4+ T cells is substantially but not completely reduced against leukemia target cells lacking MHC-II antigen expression,28,43 suggesting roles both for cytolytic CD4+ cells and for indirect, cytokine-mediated effects. It is plausible that some of these actions may be directed against processes involved in engagement of the HSC BM niche by the transplanted stem cells, such as adhesion and migration. In contrast, the GVL effect of delayed DLI was mediated predominantly by CD8+ T lymphocytes (Figure 6), which is consistent with the fact that most miHAs are presented in the context of MHC-I. Experiments to assess directly the role of MHC-I and MHC-II expression in the GVL response to delayed DLI are in progress.
We did not observe prominent signs or histopathological evidence of GVHD in allogeneic recipients of delayed splenocyte DLI when assessed at day 35 after transplantation (supplemental Figure 4), but did observe weight loss and skin changes consistent with GVHD in recipients of CD8-depleted DLI (supplemental Figure 7), which is consistent with previous studies showing GVHD in this strain pairing to be CD4 dependent and to predominantly involve the skin.44 When an empty MIG retrovirus was used for BM transduction, we observed only minimal GVHD in nonleukemic allogeneic chimeras treated with total or CD8-depleted splenocyte DLI (data not shown), suggesting that delayed DLI may be less potent for the induction of GVHD in this model than delivery of T cells at the time of acute radiation injury and BM transplantation. Our future immunotherapy studies will focus more on the C3H.SW→B6 strain pairing (supplemental Figure 5), in which GVHD responses are more robust.45
In addition to T lymphocytes, clinical studies suggest that NK cells may play a role in the GVL response in allografted CML patients. In such patients, donor-derived NK cells with cytolytic activity against CML cell lines and primary host CML progenitors can be identified,46,47 and retrospective analyses suggest that GVL effects of alloHSCT for CML are correlated with rapid recovery of donor-derived NK populations48 and with the expression of specific inhibitory49 and activating38 NK receptors. However, direct evidence for the participation of NK cells in adoptive immunotherapy for CML is lacking. The observation that immunotherapy with purified CD8+ T cells was somewhat less effective than DLI with CD4-depleted splenocytes (Figure 6C) suggests that non-T cells, possibly NK cells, might cooperate with cytotoxic T cells in the GVL response. However, when Rag2-deficient donors were tested as the source of DLI, the effects on leukemia burden and survival were modest and temporary. Interestingly, we have observed robust GVL effects of splenocyte DLI against CML-like leukemia when the leukemia targets lack expression of MHC class I molecules (Y.-F.L. and R.A.V., unpublished data), suggesting that NK cells can indeed mediate potent antileukemic effects when the influence of inhibitory NK receptor signaling triggered by self-MHC is negated. This model of adoptive immunotherapy will be useful in understanding the relative roles of T lymphocytes and NK cells in the GVL effect against CML, in defining the mechanisms of LSC killing, and in testing methods to optimize GVL by T and NK cells while minimizing GVHD. This should in turn lead to improvements in immunotherapy for CML and other leukemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Flow Cytometry and Small Animal Services Core Facilities of the Tufts Medical Center Cancer Center for invaluable assistance.
This work was supported by grants from the National Institutes of Health (CA090576 to R.A.V. and HL093981 to H.K. and R.A.V.).
National Institutes of Health
Authorship
Contribution: Y.-F.L., R.A.V., and H.K. designed the experiments; Y.-F.L., L.C.G, M.B., and K.L. performed the experiments, and Y.-F.L. and R.A.V.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard A. Van Etten, MD, PhD, Molecular Oncology Research Institute, Tufts Medical Ctr, 800 Washington St, no. 5609, Boston, MA 02111; e-mail: rvanetten@tuftsmedicalcenter.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal