Abstract
Peroxisome proliferator–activated receptor-γ (PPARγ) is an anti-inflammatory molecule. To study its biologic function in myeloid cells, dominant-negative PPARγ (dnPPARγ) was overexpressed in a myeloid-specific bitransgenic mouse model. In this bitransgenic system, overexpression of the dnPPARγ-Flag fusion protein in myeloid-lineage cells abnormally elevated frequencies and total numbers of IL-7Rα−Lin−c-Kit+Sca-1−, Lin−/Scal+/c-Kit+, common myeloid, and granulocyte-monocyte progenitor populations in the BM. dnPPARγ overexpression led to up-regulation of IL-1β, IL-6, and TNFα in the blood plasma. As a result, CD11b+Ly6G+ cells were systemically increased in association with activation of Stat3, NF-κB, Erk1/2, and p38 molecules. Myeloid-derived suppressor cells (MDSCs) inhibited the proliferation and lymphokine production of wild-type CD4+ T cells in vitro. CD4+ T cells from doxycycline-treated bitransgenic mice displayed reduced proliferation and lymphokine release. Both CD4+ and CD8+ T-cell populations were decreased in doxycycline-treated bitransgenic mice. Multiple forms of carcinoma and sarcoma in the lung, liver, spleen, and lymph nodes were observed in doxycycline-treated bitransgenic mice. BM transplantation revealed that a myeloid-autonomous defect was responsible for MDSC expansion, immunosuppression, and tumorigenesis in these mice. These studies suggest that anti-inflammatory PPARγ in myeloid-lineage cells plays a key role in controlling pro-inflammatory cytokine synthesis, MDSC expansion, immunosuppression, and the development of cancer.
Introduction
Inflammatory responses are initiated in response to pathogen infection and tissue injury, contributing to protective host immunity. However, failure to clear excessive amounts of inflammatory cells or to control exuberant expression of pro-inflammatory molecules at the inflammatory sites can result in organ damage and cancer.1,2 The identification of molecules and pathways that modulate inflammation and eliminate excessive inflammatory cells is therefore important in preventing chronic, inflammation-induced cancer.
Recently, we reported that neutral lipid metabolism controlled by lysosomal acid lipase (LAL) plays a critical role in controlling inflammation. LAL hydrolyzes cholesteryl ester and triglycerides in the lysosome of cells to generate free cholesterol and free fatty acids.3 Blocking cholesterol ester and triglyceride metabolism in LAL-knockout mice (lal−/−) resulted in abnormal myeloid development, differentiation, and homeostasis within the BM.4 LAL loss led to severe inflammation and pathogenic phenotypes in multiple organs.5-9 One of manifestations of inflammation in lal−/− mice was expansion of myeloid-derived suppressor cells (MDSCs).4 MDSCs are a heterogeneous population of myeloid cells composed of myeloid progenitor cells and immature myeloid cells, which are commonly defined by the markers CD11b (αM-integrin) and Gr-1 (Ly6-C/G) in the mouse.10-12 MDSCs from lal−/− mice displayed strong suppression of T-cell proliferation and function in vitro.4 Infiltration of MDSCs in lal−/− mice impaired T-cell development in the thymus and T-cell maturation in the spleen.4,13 Myeloid-specific expression of LAL in lal−/− mice ameliorated inflammation and tissue pathogenesis.4,9,14 Therefore, LAL and downstream lipid metabolites in myeloid-lineage cells are critical in controlling inflammation, immune response, and tissue pathogenesis.
Free cholesterol and the free fatty acid–derivative compounds hydroxyeicosatetraenoic acid, hydroxyoctadecanoic acid, and 15-deoxy−Δ12,14-prostaglandin J2 serve as ligands for peroxisome proliferator-activated receptor-γ (PPARγ). It is likely that phenotypes exhibited in lal−/− mice are, at least in part, due to inactivation of PPARγ as a result of blocking ligand synthesis. PPARγ belongs to the nuclear receptor superfamily. After binding to ligands, PPARγ interacts with the retinoid X receptor to form a dimer that subsequently binds to specific PPAR-responsive elements on target genes. PPARγ is well known for its anti-inflammatory function because it reduces pro-inflammatory gene expression in macrophages.8,15-18 Overexpression of pro-inflammatory molecules negatively regulated by PPARγ induces chronic inflammation and spontaneous tumor formation, as we reported recently.17-21
To determine the functional role of PPARγ in myeloid-lineage cells, a doxycycline-controlled c-fms-rtTA/(tetO)7-CMV-dnPPARγ bitransgenic mouse model was generated. In this model, a dominant negative PPARγ (dnPPARγ) was overexpressed in myeloid-lineage cells, resulting in up-regulated pro-inflammatory cytokines/chemokines and MDSC expansion. These dnPPARγ-induced MDSCs suppressed T-cell function. Carcinomas and sarcomas developed in both immune and nonimmune organs of doxycycline-treated c-fms-rtTA/(tetO)7-CMV-dnPPARγ bitransgenic mice. Therefore, the LAL/hormonal ligand/PPARγ axis and its downstream gene products are essential for controlling chronic inflammation (especially MDSC homeostasis) and tumorigenesis.
Methods
Animal care
All scientific protocols involving the use of animals were approved by the Institutional Animal Care and Usage Committee of Indiana University School of Medicine, and followed the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. Protocols involving the use of recombinant DNA or biohazard materials have been approved by the Institutional Biosafety Committee and followed the guidelines established by the National Institutes of Health. Animals were housed under Institutional Animal Care and Usage Committee–approved conditions in a secured animal facility at Indiana University School of Medicine. Animals were regularly screened for common pathogens and were specific pathogen-free). CO2 narcosis was used to minimize animal discomfort for experiments requiring that they be killed.
Generation of doxycycline-controlled dnPPARγ-transgenic mice
FACS analysis
BM, blood, spleen, and lung single-cell suspensions were prepared as described previously.17 Approximately 1-2 × 106 cells from various organs in FACS buffer were stained with isotype control or primary Abs. The procedure used for analysis of hematopoietic progenitor and intracellular molecules was described previously.20
Isolation of CD4+ T cells and MDSCs
To purify CD4+ T cells, erythrocyte-depleted splenocytes were incubated with anti-CD4 microbeads and then passed over a MS column following the manufacturer's instructions (Miltenyi Biotec). To purify Ly-6G+ MDSCs, erythrocyte-depleted splenocytes were incubated with anti–Ly-6G-biotin Abs, followed by positive magnetic selection using anti-biotin microbeads, following the manufacturer's instructions (Miltenyi Biotec). The purity of the MDSC population was typically higher than 90%.
ChIP assay
Ly-6G MDSCs were isolated from the BM of doxycycline-treated or untreated c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. Proteins were cross-linked to DNA by adding formaldehyde directly to the RPMI 1640 culture medium to a final concentration of 1%, and incubated for 10 minutes at room temperature. The cells were washed with 1× PBS 3 times and lysed with SDS lysis buffer (0.1% SDS, 3mM EDTA, 25mM Tris, pH 8.1, and 1× protease inhibitor cocktail). Cells were sonicated for 10 seconds 3 times with 30-second intervals. A fraction of 1% sonicated chromatin was saved from each sample for input normalization of real-time PCR. The rest of chromatin samples were incubated with 20 μg of anti-Flag polyclone Ab (Sigma-Aldrich) overnight at 4°C. Protein A agarose beads were added to adsorb the immunocomplexes and were rotated at 4°C for 2 hours. DNA-protein immunocomplexes were eluted off from the beads with ChIP elution buffer (1% SDS and 0.1M NaHCO3) and de-cross-linked at 65°C for 4 hours. DNA was eluted by chloroform-phenol extraction. For real-time PCR, 2 μL of DNA isolated was amplified by a pair of sequence-specific DNA oligonucleotide primers for each gene promoter listed below in 20 μL of reaction mixture containing SYBR Green PCR Master Mix (Applied Biosystems). The reactions were analyzed using the Relative Quantification Assay and StepOnePlus System (both Applied Biosystems). All PCR reactions were normalized by input DNA fraction according to the formula: ΔCt = Ct(testing) − (Ct(input) − Log2 (input dilution factor), where CT is the cycle threshold, as described by the manufacturer in the ChampionChIP qPCR Primers User Manual (SABiosciences).
Primers for ChIP-real-time PCR were as follows: for mApi-6, 5′- GGAAGCTGGTTGAAGGTAGGAA-3′ (upstream), 5′-GTAAGTCACGATTGTGGCATCTATCT-3′ (downstream); for mIL-1β, 5′-CACTATCTGCCACCCCTTGAC-3′ (upstream), 5′-GAAGAGGCTATTGCTACCCTGAAA-3′ (downstream); for mIL-6, 5′-TGGGATCAGCACTAACAGATAAGG-3′ (upstream), 5′-TGGTCTCTTGGCTATCTTCTTAGTTAAG-3′ (downstream); for mMMP-12, 5′-GCAGAAAAATTGAAATGGGTAAAGA-3′ (upstream), 5′-TGGGTTGCTTTGGGAGGTATT-3′ (downstream); and for mTNF-α, 5′-CCCAGATTGCCACAGAATCC-3′ (upstream), 5′-CCTACACCTCTGTCTCGGTTTCTT-3′ (downstream).
Real-time RT-PCR
The procedure used for reverse transcription reactions was described previously.19 Relative expression was determined by 2(ΔΔCt), in which ΔΔCt = ΔCt (+Dox) − ΔCt (−Dox), where ΔCt represents the PCR Ct of a testing molecule normalized by the Ct of GAPDH. Therefore, ΔCt = Ct (testing molecule) − Ct (GAPDH). Primers for real-time PCR were as follows: for mApi6, 5′-CCCATGGCGAGGACACA-3′ (upstream), 5′-CCCACCAGCTTCAGCTCAAA-3′ (downstream); for mIL-1β, 5′-TTGACGGACCCCAAAAGATG-3′ (upstream), 5′-CAGGACAGCCCAGGTCAAA-3′ (downstream); for mIL-6, 5′-GAGGCTTAATTACACATGTTC-3′ (upstream), 5′-TGCCATTGCACAACTCTTTTCT-3′ (downstream); for mMMP-12, 5′-TGGTATTCAAGGAGATGCACATTT-3′ (upstream), 5′-GGTTTGTGCCTTGAAAACTTTTAGT-3′ (downstream); for mTNF-α, 5′-CCCCAAAGGGATGAGAAGTTC-3′ (upstream), 5′-TGAGGGTCTGGGCCATAGAA-3′ (downstream); and for GAPDH, 5′-GGCCATCAAGCCAGAGCTT-3′ (upstream), 5′-CCAAACCATCACTGACACTCAGA-3′ (downstream).
Neutralization study
Anti–mouse IL-6, IL-1β, and TNF-α (30 μg/mice) Abs (R&D Systems) were injected IP into bitransgenic mice every 3 days, and 2 weeks later, the spleen, lungs, and blood were harvested. Single cells were stained with anti-mouse CD11b and Ly6G cell-surface markers for flow cytometry analysis.
In vivo T-cell proliferation assay
The sorted CD4+ T cells from spleens were cultured in 96-well plates coated with anti-CD3 mAb (2 μg/mL) and anti-CD28 mAb (5 μg/mL). After 48 hours, cells were harvested and stained with allophycocyanin (APC)–labeled anti-CD4 mAb and PE-labeled anti-CD69 mAb (eBiosciences) and analyzed using a flow cytometer (BD Biosciences). Data were analyzed using BD CellQuest Pro Version 5.21 software (BD Biosciences).
MDSC suppression on T-cell proliferation
The sorted CD4+ T cells from wild-type spleens were stained in PBS containing 1μM CFSE (Molecular Probes) at 37°C for 10 minutes, and then pelleted and resuspended in complete medium for 40 minutes. CD4+ T cells were washed twice in PBS and then cultured in 96-well flat-bottom plates coated with anti-CD3 mAb (2 μg/mL) and anti-CD28 mAb (5 μg/mL). Purified MDSCs (1 × 105) from the spleens of wild-type, doxycycline-treated, or untreated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice were added into 5 × 105 CD4 T lymphocytes. MDSCs and splenocytes were cocultured for 4 days. Cells were harvested, stained with APC-labeled anti-CD4 mAb (eBiosciences), and analyzed using flow cytometry. Transwell experiments were performed in 96-well plates (Corning). The Ly-6G+ MDSCs (1 × 105) from the spleens of doxycycline-treated or untreated bitransgenic mice were plated to the upper wells and the CFSE-labeled CD4+ T cells from the wild-type spleens were plated in the lower wells (5 × 105) in the presence or absence of anti-CD3 mAb (2 μg/mL) and anti-CD28 mAb (5 μg/mL). After 4 days, cells were harvested and stained with APC-labeled anti-CD4 mAb (eBiosciences) for flow cytometry analysis.
For the CD69-expression assay, MDSCs from the spleens of doxycycline-treated or untreated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice and splenocytes from wild-type mice were cocultured in 96-well plates coated with anti-CD3 mAb (2 μg/mL) and anti-CD28 mAb (5 μg/mL) for 48 hours. Cells were harvested, stained with anti-CD4–APC mAb and anti-CD69–PE mAb (eBiosciences), and analyzed by flow cytometry.
Cytokine measurement by ELISA
The expression levels of IL-2, IL-4, and IFN-γ in the supernatants of the culture medium were measured using ELISA kits according to the manufacturer's instructions (BD Biosciences). Levels of IL-1β, IL-6, and TNF-α in the blood serum were measured using ELISA kits according to the manufacturer's instructions (BD Biosciences).
BM transplantation
Whole BM cells were harvested from 8- to 10-week-old male wild-type (CD45.2 congenic strain) or c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice (CD45.1 congenic strain). RBCs were lysed in ACK red blood cell lysis buffer and the remaining mononuclear cells were resuspended in sterile PBS. Nucleated BM cells (5 × 106) in 200 μL of PBS were injected IV into isoflurane-anesthetized 8-week-old wild-type or c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic recipient mice that had been lethally irradiated with 1000 rad 4 hours earlier. Nontransplanted control mice died within 2 weeks of irradiation. Twelve weeks after transplantation, BM, peripheral blood, spleens, and lungs from the chimeras were collected and stained with CD45.1 or CD45.2 (eBiosciences) Abs to verify donor-derived reconstitution with other cell-lineage markers.
Histology and immunohistochemical staining
The lungs, livers, spleens, and lymph nodes from doxycycline-treated (with tumor) or untreated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice were dissected out and fixed with a fixative solution (4% paraformaldehyde and 1 × PBS) at 4°C for 24 hours. After fixation and embedding in paraffin, tissue sections were cut to 5 μm thick. H&E staining and immunohistochemical staining were performed by the Histological Core Facility, Department of Pathology and Laboratory Medicine, Indiana University. The following Abs were tested: CD3, B220, MAC2, F4/80, lysozyme, myeloperoxidase, CD117, cytokeratin, Ki67, and caspase 3.23
Statistical analysis
The data shown are mean values of at least 3 independent experiments and are expressed as means ± SD. A paired Student t test or ANOVA was used to evaluate the significance of the differences. Statistical significance was set at a level of P < .05.
Results
Overexpression of dnPPARγ alters hematopoietic progenitor populations in the BM
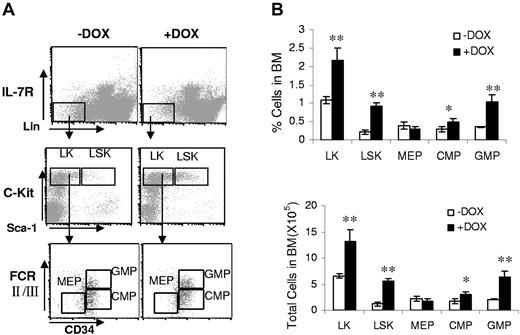
A doxycycline-controlled bitransgenic mouse model was generated to specifically direct dnPPARγ expression in myeloid cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In this system, a previously established c-fms-rtTA–transgenic mouse line9 and a (TetO)7-CMV-dnPPARγ–transgenic mouse line22 were crossbred. To assess the effect of overexpression of dnPPARγ on progenitor populations, the BM cells from c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice treated with doxycycline for 3 months were analyzed for the percentage and total cell numbers of IL-7Rα−Lin−Sca-1+c-Kit+ (LSK), IL-7Rα−Lin−Sca-1−c-Kit+ (LK), IL-7Rα−Lin−Sca-1−c-Kit+CD34+FcRII/IIIlow common myeloid progenitor (CMP), IL-7Rα−Lin−Sca-1−c-Kit+CD34+FcRII/III+ granulocyte-monocyte progenitor (GMP), and IL-7Rα−Lin−Sca-1−c-Kit+CD34−FcRII/III− megakaryocyte-erythroid progenitor (MEP) populations.24 Overexpression of dnPPARγ resulted in significant increase in the percentages of LSK, LK, CMP, and GMP progenitors from doxycycline-treated bitransgenic mice compared with untreated mice (Figure 1). However, the frequency (percentage) of MEPs decreased and the total number of MEPs remained relatively unchanged in doxycycline-treated bitransgenic mice. These data suggest that overexpression of dnPPARγ selectively increased myeloid-lineage progenitors, similar to what was observed in lal−/− mice.4 No alteration in hematopoietic progenitor populations was observed in c-fms-rtTA single-transgenic mice and (TetO)7-CMVdnPPARγ single-transgenic mice (data not shown) regardless of doxycycline treatment.
dnPPARγ overexpression alters hematopoietic progenitor development. (A) FACS staining profiles of the BM progenitor populations, including LKs, LSKs, CMPs, GMPs, and MEPs from the BM of 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (B) Frequencies and total numbers of LK, LSK, CMP, MEP and GMP populations in the BM of 3-month doxycycline-treated (+DOX) or untreated (−DOX) bitransgenic mice. Values were derived from 4 mice in each group (n = 4). **P < .01.
dnPPARγ overexpression alters hematopoietic progenitor development. (A) FACS staining profiles of the BM progenitor populations, including LKs, LSKs, CMPs, GMPs, and MEPs from the BM of 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (B) Frequencies and total numbers of LK, LSK, CMP, MEP and GMP populations in the BM of 3-month doxycycline-treated (+DOX) or untreated (−DOX) bitransgenic mice. Values were derived from 4 mice in each group (n = 4). **P < .01.
Overexpression of dnPPARγ increases myeloid-derived suppressive cells
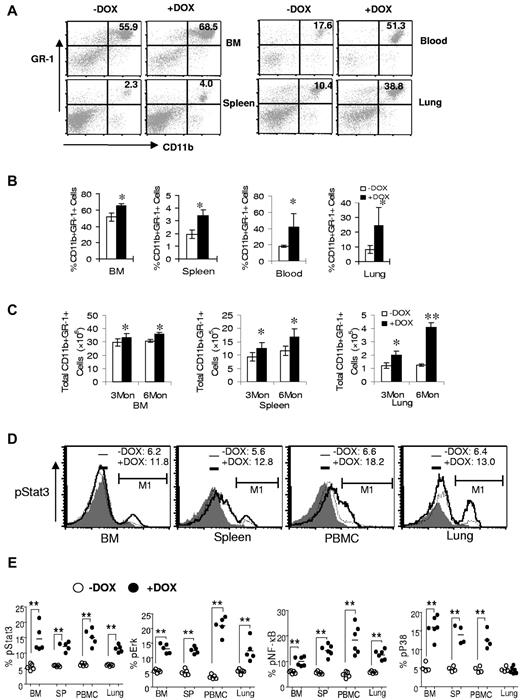
To determine whether abnormal myelopoiesis in BM progenitor cells changes myeloid homeostasis, bitransgenic mice were treated with or without doxycycline for 3 months. Single cells from the BM, blood, spleens, and lungs of doxycycline-treated or untreated bitransgenic mice were isolated and stained with CD11b+ and GR-1(Ly6G)+ Abs for flow cytometry analysis. Compared with doxycycline-untreated mice, both the percentages and the total numbers of CD11b+GR-1+ cells were systemically increased in the BM, blood, spleens, and lungs of doxycycline-treated bitransgenic mice (Figure 2A-C). In c-fms-rtTA single-transgenic mice and (TetO)7-CMVdnPPARγ single-transgenic mice (data not shown), no change was observed regardless of doxycycline treatment. When single cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were stained with CD11b+, GR-1+ surface-specific Abs and fluorochrome-conjugated anti–phosphor-Stat3, anti–phosphor-Erk1/2, anti–phosphor-NF-κB, or anti–phosphor-p38 Abs, the percentages of phosphor-Stat3, phosphor-Erk1/2, anti–phosphor-NF-κB, and phosphor-p38–positive cells were increased in the gated CD11b+GR-1+ cell population of doxycycline-treated bitransgenic mice (Figure 2D-E). These results suggest an intrinsic defect in myeloid cell autonomy in bitransgenic mice.
dnPPARγ overexpression causes expansion and abnormality of myeloid cells. (A) Flow cytometry analysis of the BM, blood, spleens, and lungs from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. A representative analysis of the percentage of CD11b+GR-1+ cells is presented. (B) Percentage of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice. (C) Total numbers of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice. (D) Representative flow cytometry analysis of pStat3 in CD11b+GR-1+ cells from BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice. (E) Percentages of pStat3, pErk, pNFκB, and pP38 in 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. Values are means ± SD (n > 5). * P < .05; ** P < .01.
dnPPARγ overexpression causes expansion and abnormality of myeloid cells. (A) Flow cytometry analysis of the BM, blood, spleens, and lungs from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. A representative analysis of the percentage of CD11b+GR-1+ cells is presented. (B) Percentage of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice. (C) Total numbers of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice. (D) Representative flow cytometry analysis of pStat3 in CD11b+GR-1+ cells from BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice. (E) Percentages of pStat3, pErk, pNFκB, and pP38 in 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. Values are means ± SD (n > 5). * P < .05; ** P < .01.
dnPPARγ has a dominant-negative effect on pro-inflammatory genes in MDSCs of bitransgenic mice
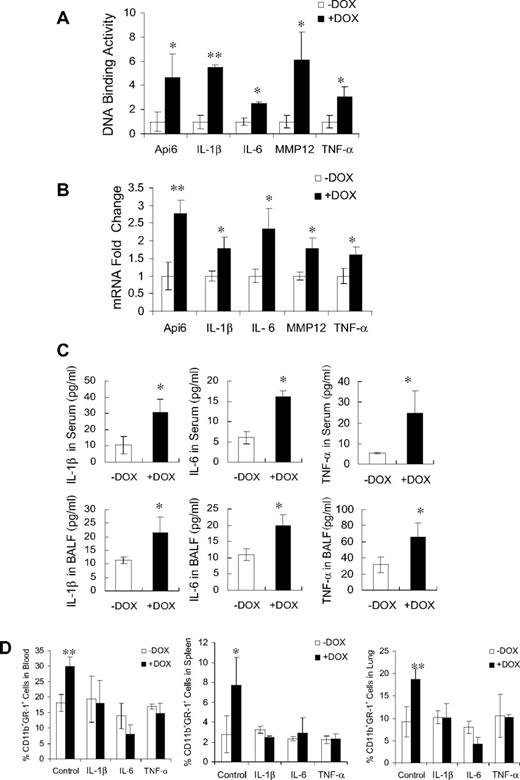
Because PPARγ is an anti-inflammatory molecule, it was important to evaluate whether pro-inflammatory molecules were up-regulated in c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. These molecules are potentially important for initiating inflammation. PPARγ, which binds to specific DNA sites (PPARE) on the promoters after ligand binding, prevents transcriptional events of pro-inflammatory molecules. Because Api6, IL-1β, IL-6, MMP12, and TNF-α are downstream genes of PPARγ,8,15,25 it is possible that overexpression of dnPPARγ in doxycycline-treated c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice interferes with endogenous PPARγ function at the transcriptional level by binding PPARE sites within the gene's promoters. To test this assumption, ChIP assays were performed to investigate the association between dnPPARγ and PPARE sites within the Api6, IL-1β, IL-6, MMP12, and TNF-α promoters using BM MDSCs from 3-month doxycycline-treated or untreated c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. The putative PPARE sites within the promoters of Api6, IL-1β, IL-6, MMP12, and TNF-α were identified as described previously.22 An anti-Flag polyclonal Ab was used to immunoprecipitate the dnPPARγ-Flag/DNA complex. In doxycycline-treated mice, Flag-tagged dnPPARγ was detected bound to the promoters of the Api6, IL-1β, IL-6, MMP12, and TNF-α genes by quantitative real-time PCR analysis (Figure 3A). dnPPARγ binding to these sites was not observed in c-fms-rtTA and (TetO)7-CMVdnPPARγ single-transgenic mice (data not shown). These results showed that the dnPPARγ-Flag fusion protein can bind the promoters of pro-inflammatory genes with measurable affinity and may compete to block endogenous PPARγ regulation.
DN effects of dnPPARγ-Flag fusion protein on inflammatory molecule genes. (A) DNA binding of dnPPARγ-Flag fusion protein on promoter regions of Api6, IL-1β, IL-6, MMP12, and TNF-α genes using ChIP assay followed by real-time PCR (n = 4). Relative DNA-binding levels were determined by 2(−ΔΔCt), in which ΔΔCt = ΔCt (+DOX) − ΔCt (−DOX). Testing samples Ct value was normalized by each input DNA fraction Ct value. Therefore, ΔCt = Ct (testing) − (Ct (input) − Log2 (input dilution factor). Values are means ± SD. *P < .05; **P < .01. (B) Real-time PCR analysis of mRNA expression levels of inflammatory cytokines in BM MDSCs from c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice that were treated or untreated with doxycycline for 3 months (n = 5). The mRNA expression level of GAPDH was used for normalization. ΔCt = Ct(testing) − Ct(GAPDH). Relative expression levels were determined by 2(−ΔΔCt), in which ΔΔCt = ΔCt (+DOX) − ΔCt (−DOX). Values are means ± SD. **P < .01. (C) Protein expression levels of IL-1β, IL-6, and TNF-α in serum and bronchoalveolar lavage fluid from the lungs of 3-month doxycycline-treated or untreated bitransgenic mice as shown by ELISA (n = 5). Values are means ± SD. *P < .05. (D) Percentage of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice after injection of anti–IL-1β, anti–IL-6, and anti–TNF-α Abs in neutralization studies. −DOX indicates doxycycline-untreated bitransgenic mice; +DOX, doxycycline-treated bitransgenic mice. *P < .05, **P < .01.
DN effects of dnPPARγ-Flag fusion protein on inflammatory molecule genes. (A) DNA binding of dnPPARγ-Flag fusion protein on promoter regions of Api6, IL-1β, IL-6, MMP12, and TNF-α genes using ChIP assay followed by real-time PCR (n = 4). Relative DNA-binding levels were determined by 2(−ΔΔCt), in which ΔΔCt = ΔCt (+DOX) − ΔCt (−DOX). Testing samples Ct value was normalized by each input DNA fraction Ct value. Therefore, ΔCt = Ct (testing) − (Ct (input) − Log2 (input dilution factor). Values are means ± SD. *P < .05; **P < .01. (B) Real-time PCR analysis of mRNA expression levels of inflammatory cytokines in BM MDSCs from c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice that were treated or untreated with doxycycline for 3 months (n = 5). The mRNA expression level of GAPDH was used for normalization. ΔCt = Ct(testing) − Ct(GAPDH). Relative expression levels were determined by 2(−ΔΔCt), in which ΔΔCt = ΔCt (+DOX) − ΔCt (−DOX). Values are means ± SD. **P < .01. (C) Protein expression levels of IL-1β, IL-6, and TNF-α in serum and bronchoalveolar lavage fluid from the lungs of 3-month doxycycline-treated or untreated bitransgenic mice as shown by ELISA (n = 5). Values are means ± SD. *P < .05. (D) Percentage of CD11b+GR-1+ cells in 3-month doxycycline-treated or untreated bitransgenic mice after injection of anti–IL-1β, anti–IL-6, and anti–TNF-α Abs in neutralization studies. −DOX indicates doxycycline-untreated bitransgenic mice; +DOX, doxycycline-treated bitransgenic mice. *P < .05, **P < .01.
To determine whether dnPPARγ overexpression up-regulates proinflammatory cytokines in vivo, total RNA was purified from BM MDSCs of 3-month doxycycline-treated or untreated c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. The expression levels of multiple cytokines were quantitatively determined by real-time PCR. Among these, mRNA expression levels of Api6, IL-1β, IL-6, MMP12, and TNF-α in doxycycline-treated mice were increased compared with those in untreated mice (Figure 3B). In the blood and bronchoalveolar lavage fluid, protein concentrations of secreted IL-1β, IL-6, and TNF-α were steadily increased (as measured by ELISA) compared with those in untreated mice (Figure 3C). It is known that these cytokines stimulate MDSC expansion.10,11 No change was observed in c-fms-rtTA and (TetO)7-CMVdnPPARγ single-transgenic mice (data not shown). When anti–IL-1β, IL-6, and TNF-α Abs were injected into bitransgenic mice to neutralize cytokines, MDSC expansion in multiple organs was inhibited in doxycycline-treated bitransgenic mice (Figure 3D).
Overexpression of dnPPARγ decreases the T-lymphocyte population
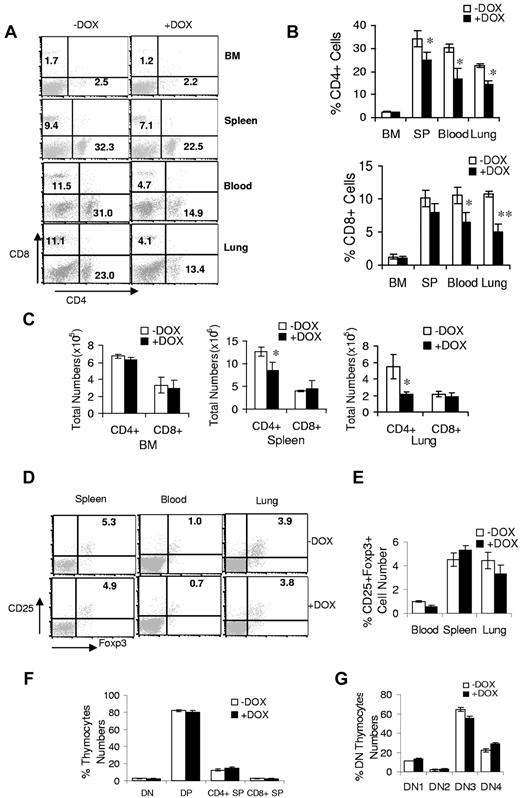
To assess T-cell proliferation in bitransgenic mice, BM, blood, spleen, and lung mononuclear cells were isolated from 3-month doxycycline-treated or untreated bitransgenic mice and stained with fluorochrome-conjugated anti–mouse CD4 and CD8 Abs for flow cytometric analysis. In contrast to myeloid cells, the percentage and total numbers of CD4+ and CD8+ cells in the blood, spleens, and lungs were reduced significantly in doxycycline-treated bitransgenic mice (Figure 4A-C). There were no changed T-cell levels in the BM for doxycycline-treated and untreated bitransgenic mice. In c-fms-rtTA single-transgenic mice and (TetO)7-CMVdnPPARγ single-transgenic mice (data not shown), no change in T-cell levels was observed regardless of doxycycline treatment. There was no change in the natural killer–cell population, whereas pathways (granzyme B and IFN-γ) indicating natural killer–cell activation were increased in doxycycline-treated bitransgenic mice (supplemental Table 1). T-regulatory (Treg) cells remained relatively unchanged in the blood, spleens, and lungs (Figure 4D-E). The decline in T cells in bitransgenic mice could be a result of impairment in T-cell development. The thymus is among the most important organs for T-cell development, and T-cell development in the thymus can be divided into various stages, including CD4−CD8− double-negative, CD4+CD8+ double-positive, and CD4+CD8−/CD4−CD8+ single-positive stages. To determine possible alterations at the earliest stages of intrathymic T-cell development, T cells at different developmental stages of thymocyte subsets were analyzed as defined by CD4 and CD8 markers. There was no significant change in various thymocyte subsets from 1-month doxycycline-treated or untreated mice (Figure 4F). When further evaluated, various DN developmental stages were found by CD44 and CD25 markers: DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) thymocytes showed no change (Figure 4G). These results suggest that, unlike in lal−/− mice, T-cell development does not contribute to the decrease in T-cell numbers in bitransgenic mice.
dnPPARγ overexpression causes a decrease in the T-cell population. (A) A representative flow cytometric analysis of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (B) Percentage of CD4+ and CD8+ T cells in the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (C) Total numbers of CD4+ and CD8+ T cells in the BM, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (D) Representative flow cytometric analysis of CD25+Foxp3+ CD4+ Treg cells from the blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice. (E) Percentages of CD25+Foxp3+ CD4+ Treg cells from the blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (F) Frequencies of CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), CD4+ single-positive (SP), and CD8+ SP thymocytes in 1-month doxycycline-treated or untreated bitransgenic mice. (G) Frequencies of DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) thymocytes in the same groups of mice. In all analyses, values were derived from 5 mice in each groups (n = 5). *P < .05; **P < .01.
dnPPARγ overexpression causes a decrease in the T-cell population. (A) A representative flow cytometric analysis of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (B) Percentage of CD4+ and CD8+ T cells in the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (C) Total numbers of CD4+ and CD8+ T cells in the BM, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (D) Representative flow cytometric analysis of CD25+Foxp3+ CD4+ Treg cells from the blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice. (E) Percentages of CD25+Foxp3+ CD4+ Treg cells from the blood, spleens, and lungs of 3-month doxycycline-treated or untreated bitransgenic mice were statistically analyzed. (F) Frequencies of CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), CD4+ single-positive (SP), and CD8+ SP thymocytes in 1-month doxycycline-treated or untreated bitransgenic mice. (G) Frequencies of DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) thymocytes in the same groups of mice. In all analyses, values were derived from 5 mice in each groups (n = 5). *P < .05; **P < .01.
Overexpression of dnPPARγ decreases proliferation and function of CD4+ T lymphocytes in vitro
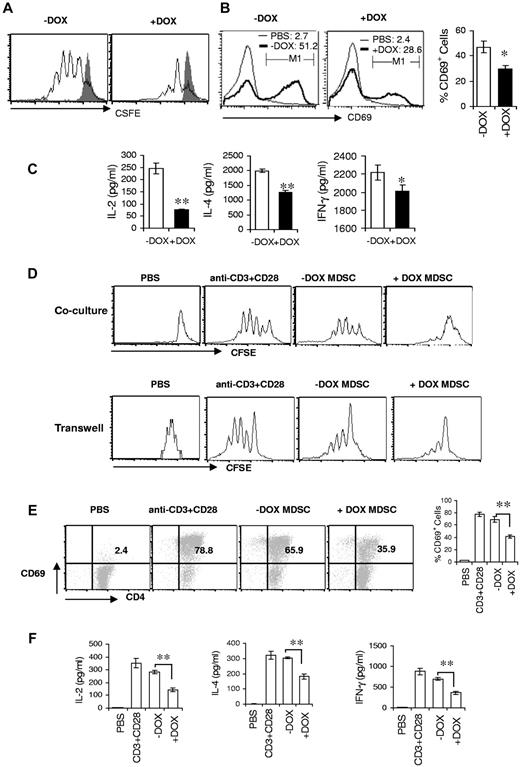
Because T-cell development was not impaired and Treg cells remained relatively unchanged in bitransgenic mice, the reduced numbers and function of T cells might be linked to the increased expansion of MDSCs. To assess how dnPPARγ overexpression in myeloid cells influences T-cell proliferation, TCR stimulation of splenic CD4+ T cells from 3-month doxycycline-treated or untreated bitransgenic mice was measured with the CFSE-labeling assay. Compared with doxycycline-untreated mice, the CD4+ T-cell proliferation was significantly decreased in doxycycline-treated bitransgenic mice when cultured in vitro for 4 days after anti-CD3 mAb plus anti-CD28 mAb stimulation (Figure 5A). In addition, the expression of CD4+ T-cell surface–proliferating marker CD69 was significantly reduced in doxycycline-treated mice after anti-CD3 mAb plus anti-CD28 mAb stimulation when cultured in vitro for 2 days (Figure 5B). Furthermore, secretion of lymphokines (IL-2, IL-4, and IFN-γ) by splenic CD4+ T cells after TCR activation in cultured medium was much less in doxycycline-treated mice compared with untreated mice, as assessed by ELISA (Figure 5C), suggesting functional impairment of T cells in bitransgenic mice.
MDSCs from bitransgenic mice suppress T-cell proliferation and function. (A) CFSE-labeled splenic CD4+ T cells from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice were stimulated with anti-CD3 mAb plus anti-CD28 mAb for 4 days. Proliferation of labeled CD4+ T cells was analyzed by flow cytometry. Peaks represent cell division cycles. PBS stimulation control is shown as the shaded areas. (B) CD4+ T cells from the spleens of 3-month doxycycline-treated or untreated bitransgenic mice were cultured and stimulated with anti-CD3 mAb plus anti-CD28 mAb. After 48 hours, CD4+ T cells were stained with anti-CD69 and CD4 Abs for the flow cytometry analysis. Percentages of CD69+ CD4+ T cells were statistically analyzed. (C) Lymphokine production of splenic CD4+ T cells in the medium measured by ELISA analysis; (D) CFSE-labeled wild-type CD4+ T cells were stimulated with anti-CD3 mAb plus anti-CD28 mAb for 4 days in the presence or absence of CD11b+Gr-1+ cells from the spleens of wild-type, doxycycline-untreated, or 3-month doxycycline-treated bitransgenic mice. The ratio of MDSCs to CD4+ T cells was 1:5. The proliferation of labeled CD4+ T cells was analyzed by flow cytometry. Peaks represent cell division cycles. Top panel, MDSCs and T cells in direct contact coculture study. Bottom panel, MDSCs and T cells in Transwell coculture study. (E) Similar to panel B, CD69+ expression on CD4+ T cells was analyzed by flow cytometry 48 hours after anti-CD3 mAb plus anti-CD28 mAb stimulation. (F) Lymphokine production of CD4+ T cells in the culture medium was measured by ELISA analysis. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01.
MDSCs from bitransgenic mice suppress T-cell proliferation and function. (A) CFSE-labeled splenic CD4+ T cells from 3-month doxycycline-treated (+DOX) or untreated (−DOX) c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice were stimulated with anti-CD3 mAb plus anti-CD28 mAb for 4 days. Proliferation of labeled CD4+ T cells was analyzed by flow cytometry. Peaks represent cell division cycles. PBS stimulation control is shown as the shaded areas. (B) CD4+ T cells from the spleens of 3-month doxycycline-treated or untreated bitransgenic mice were cultured and stimulated with anti-CD3 mAb plus anti-CD28 mAb. After 48 hours, CD4+ T cells were stained with anti-CD69 and CD4 Abs for the flow cytometry analysis. Percentages of CD69+ CD4+ T cells were statistically analyzed. (C) Lymphokine production of splenic CD4+ T cells in the medium measured by ELISA analysis; (D) CFSE-labeled wild-type CD4+ T cells were stimulated with anti-CD3 mAb plus anti-CD28 mAb for 4 days in the presence or absence of CD11b+Gr-1+ cells from the spleens of wild-type, doxycycline-untreated, or 3-month doxycycline-treated bitransgenic mice. The ratio of MDSCs to CD4+ T cells was 1:5. The proliferation of labeled CD4+ T cells was analyzed by flow cytometry. Peaks represent cell division cycles. Top panel, MDSCs and T cells in direct contact coculture study. Bottom panel, MDSCs and T cells in Transwell coculture study. (E) Similar to panel B, CD69+ expression on CD4+ T cells was analyzed by flow cytometry 48 hours after anti-CD3 mAb plus anti-CD28 mAb stimulation. (F) Lymphokine production of CD4+ T cells in the culture medium was measured by ELISA analysis. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01.
MDSCs are known to suppress T-cell proliferation and function. It is possible that the decreased numbers and function of T cells resulted from defects in these myeloid cells in bitransgenic mice. To test this assumption, CD4+ splenic T cells from wild-type mice were cocultured in vitro with MDSCs from 3-month doxycycline-treated or untreated mice and stimulated with anti-CD3 mAb plus anti-CD28 mAb for 4 days. T-cell proliferation was measured with the CFSE-labeling assay. As demonstrated in Figure 5D, TCR-stimulated proliferation of wild-type CD4+ T cells was dramatically suppressed by MDSCs from doxycycline-treated bitransgenic mice compared with those from untreated mice. Interestingly, this was also observed in the Transwell study (Figure 5D), suggesting that a paracrine mechanism is used by MDSCs to suppress T-cell proliferation. Expression of CD69 was reduced to 35.9% by MDSCs from doxycycline-treated bitransgenic mice compared with those from untreated bitransgenic mice (65.9%; Figure 5E). MDSCs from doxycycline-untreated mice induced a modest inhibition of T-cell proliferation compared with control T-cell cultures in the absence of MDSC coculturing (78.8%; Figure 5D-E). In the PBS control group, no proliferation was observed. MDSCs from doxycycline-treated mice also markedly inhibited the secretion of CD4+ T lymphokines (IL-2, IL-4, and IFN-γ), as shown by ELISA (Figure 5F). Therefore, abnormal MDSCs from dnPPARγ-bitransgenic mice had an inhibitory effect on T-cell proliferation and function.
BM transplantation on myeloid cells
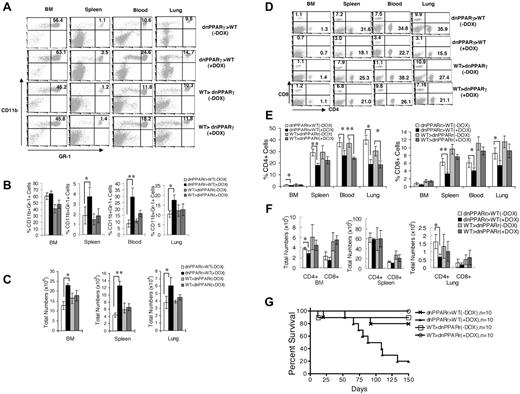
BM transplantation was performed to determine whether the immune defects in c-fins-rtTA/(TetO)-CMV-dnPPARγ mice were due to dnPPARγ-induced myeloid cell–autonomous or tissue microenvironment changes. BM cells from c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice (CD45.1) and wild-type mice (CD45.2) were reciprocally transplanted in recipient mice that were lethally irradiated to generate BM chimeric mice. Donor cells were analyzed by gating with CD45.1 or CD45.2 Ag derived from donor BM cells by flow cytometry. In the BM, blood, spleens, and lungs from c-fms-rtTA/(TetO)7-CMV-dnPPARγ BM-transplanted recipient wild-type mice, the CD11b+GR-1+ MDSC population was increased (Figure 6A-C), whereas the CD4+ and CD8+ T-cell populations were decreased (Figure 6D-F) after doxycycline treatment compared with those in doxycycline-untreated recipient wild-type mice. This suggests that a myeloid-autonomous defect was responsible for the MDSC expansion and T-cell decrease. In the BM, blood, spleens, and lungs from wild-type BM-transplanted c-fms-rtTA/(TetO)7-CMV-dnPPARγ recipient mice, no MDSC expansion was observed regardless of doxycycline treatment, suggesting that the loss of PPARγ function in the tissue microenvironment had no effect on myeloid cell development. CD4+ and CD8+ T-cell numbers were significantly decreased in the same group of mice after doxycycline treatment (Figure 6D-F), suggesting that factors beyond the myeloid cell defect also contributed to the defects in T cells. Bitransgenic BM-transplanted wild-type recipient mice were more prone to death than wild-type BM-transplanted bitransgenic recipient mice after doxycycline treatment (Figure 6G).
Characterization of MDSCs and T cells in BM-transplanted chimeric mice. (A) Representative flow cytometric analysis of donor CD11b+GR-1+ cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated (+DOX) or untreated (−DOX) wild-type or bitransgenic BM-transplanted recipient mice. (B) Percentages of CD11b+GR-1+ cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. (C) Total numbers of CD11b+GR-1+ cells from the BM, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01. (D) Representative flow cytometry analysis of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs from 3-month doxycycline-treated or untreated wild-type and bitransgenic BM-transplanted recipient mice. (E) Percentages of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. (F) Total numbers of CD4+ and CD8+ T cells from the BM, spleens, and lungs from 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01. (G) Death curve of doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice (n = 10). dnPPARγ>WT indicates bitransgenic to wild-type transplantation; WT>dnPPARγ, wild-type to bitransgenic transplantation.
Characterization of MDSCs and T cells in BM-transplanted chimeric mice. (A) Representative flow cytometric analysis of donor CD11b+GR-1+ cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated (+DOX) or untreated (−DOX) wild-type or bitransgenic BM-transplanted recipient mice. (B) Percentages of CD11b+GR-1+ cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. (C) Total numbers of CD11b+GR-1+ cells from the BM, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01. (D) Representative flow cytometry analysis of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs from 3-month doxycycline-treated or untreated wild-type and bitransgenic BM-transplanted recipient mice. (E) Percentages of CD4+ and CD8+ T cells from the BM, blood, spleens, and lungs of 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. (F) Total numbers of CD4+ and CD8+ T cells from the BM, spleens, and lungs from 3-month doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice were statistically analyzed. In all analyses, values were derived from 4 mice in each group (n = 4). *P < .05; **P < .01. (G) Death curve of doxycycline-treated or untreated wild-type or bitransgenic BM-transplanted recipient mice (n = 10). dnPPARγ>WT indicates bitransgenic to wild-type transplantation; WT>dnPPARγ, wild-type to bitransgenic transplantation.
Overexpression of dnPPARγ in myeloid cells induces carcinoma and myeloid sarcoma in multiple organs of bitransgenic mice
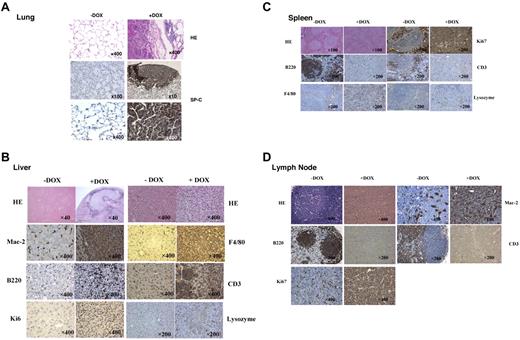
Because dnPPARγ overexpression caused severe inflammation, especially MDSC expansion–modulated T cells, pathogenesis in c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice was analyzed by histology. After 9 to 12 months of induced dnPPARγ in myeloid cells, adenocarcinomas were observed in the lungs. Various other forms of tumors were also observed in the livers, intestines, spleens, and lymph nodes of bitransgenic mice (supplemental Figure 2A). In the lung, adenocarcinomas were positively stained with the lung epithelial marker SP-C Ab (Figure 7A). In the liver, neoplastic cells were arranged in sheets and infiltrated adjacent parenchyma, often surrounded by areas of necrosis. Neoplastic cells were uniformly round to polygonal with round to oval nuclei and scant vesiculated cytoplasm exhibiting prominent increases in the nuclear to cytoplasmic ratio (Figure 7B). Many cells in the tumor areas showed positive staining with the monocyte/macrophage markers Mac2, F4/80, or lysozyme based on immunohistochemical staining. Some cells in the tumor areas were B220+ or CD3+. The proliferation marker Ki67 was positive in select areas of the tumor and caspase 3 was largely negative. The morphologic features, together with immunoreactivity for monocyte/macrophage markers, were consistent with histiocytic sarcoma. In the spleen, the red pulp and white pulp structures were completely destroyed, and both B220 and CD3 Abs showed negative staining in the tumor areas (Figure 7C); instead, F4/80 staining was positive. A few cells were lysozyme positive. Tumor cells were highly proliferative, as evidenced by Ki67 Ab staining and caspase 3–highlighted apoptotic bodies (data not shown). Strikingly, some bitransgenic mice developed enlarged lymph nodes (lymphadenopathy) after doxycycline treatment (supplemental Figure 2A). Lymph nodes of doxycycline-treated bitransgenic mice were diffusely enlarged and showed effacement of the lymphoid follicles, which were replaced by dense sheets of round cells with oval to irregular nuclei, dispersed chromatin, visible nucleoli, and scant cytoplasm (Figure 7D). Numerous cell mitosis and frequent apoptotic bodies were seen. Whereas B220+ and CD3+ cells had vanished, Mac2+ cells were observed throughout the areas with tumors. Similar to what was observed in the spleen, Ki67 staining was positive and caspase 3 highlighted apoptotic bodies. The expression of myeloid-lineage markers strongly suggests that these tumors were myeloid/monocytic sarcomas. In the BM transplantation study, some bitransgenic BM-transplanted wild-type recipient mice developed lung adenocarcinomas and lymph node sarcomas with doxycycline treatment (supplemental Figure 2B), similar to those observed in supplemental Figure 2A. This suggests that tumorigenesis depends on the myeloid compartment in c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice.
Tumorigenesis in c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (A) H&E and immunohistochemical staining of lung tissue sections with anti–SP-C Ab in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. (B) H&E and immunohistochemical staining of liver tissue sections with anti-Mac2, F4/80, B220, CD3, Ki67, and lysozyme Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were 40×, 200×, and 400×. (C) H&E and immunohistochemical staining of spleen tissue sections with anti-Ki67, B220, CD3, F4/80, and lysozyme Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were ×100 and ×200. (D) H&E and immunohistochemical staining of lymph node tissue sections with anti-Mac2, B220, CD3, and Ki67 Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were ×200 and ×400.
Tumorigenesis in c-fms-rtTA/(tetO)7-CMV-dnPPARγ–bitransgenic mice. (A) H&E and immunohistochemical staining of lung tissue sections with anti–SP-C Ab in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. (B) H&E and immunohistochemical staining of liver tissue sections with anti-Mac2, F4/80, B220, CD3, Ki67, and lysozyme Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were 40×, 200×, and 400×. (C) H&E and immunohistochemical staining of spleen tissue sections with anti-Ki67, B220, CD3, F4/80, and lysozyme Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were ×100 and ×200. (D) H&E and immunohistochemical staining of lymph node tissue sections with anti-Mac2, B220, CD3, and Ki67 Abs in the tumor areas of doxycycline-treated bitransgenic mice. Untreated mice served as controls. Original magnifications were ×200 and ×400.
Discussion
Because inflammation is a major factor contributing to tumor formation, it is essential to identify the mechanisms that control inflammation to block tumor formation. Based on our previous studies, LAL and its downstream neutral lipid metabolites in myeloid cells play a critical role in suppressing inflammation. To further elucidate the molecular mechanism contributing to this anti-inflammatory pathway and the role of myeloid cells, we investigated whether PPARγ in myeloid cells is required for preventing inflammation-induced tumors. PPARγ is a known downstream effector of LAL-derived hormones and suppresses pro-inflammatory molecules. In the present study, overexpression of dnPPARγ in myeloid cells was used to investigate the role of this cell lineage in inflammation and tumor formation. It has been reported previously that the expression and activity of PPARγ show a diverse pattern among various macrophage and dendritic cell subtypes with differential ability to respond to certain lipid signals.26
In the c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mouse model, in which a DN form of PPARγ is overexpressed by doxycycline under the control of the 7.2-kb c-fms promoter/intron 2 fragment, dnPPARγ overexpression altered progenitor cell development skewing toward the myeloid lineage by increasing the frequencies and numbers of LSK, LK, CMP, and GMP progenitors in doxycycline-treated bitransgenic mice (Figure 1). As a consequence, the immature CD11b+Gr-1+ cell population was dramatically increased in multiple organs of these mice, including the BM, blood, spleens, and lungs (Figure 2A-C). Compared with the normal immature CD11b+Gr-1+ cell population, this CD11b+Gr-1+ cell population showed much stronger activation of oncogenic intracellular molecules, including pStat3, pErk, pP38, and pNF-κB (Figure 2D-E). This observation is strikingly similar to phenotypes of myeloproliferative neoplasms previously reported in LAL-knockout mice.4 PPARγ negatively regulates Api6, IL-1β, IL-6, MMP12, and TNF-α genes. In c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice, overexpression of dnPPARγ antagonized the endogenous PPARγ function by binding to the promoter regions (Figure 3A). Although there is no direct evidence for dnPPARγ preventing endogenous PPARγ from binding to the promoter regions in vivo, increased expression of inflammatory genes (Figure 3B) suggests dnPPARγ interference of negative gene regulation by endogenous PPARγ. The production of IL-1β, IL-6, and TNF-α was up-regulated in the blood serum and bronchoalveolar lavage fluid (Figure 3C). These molecules are known to stimulate MDSC expansion.10 Injection of anti–IL-1β, anti–IL-6, and anti–TNF-α Abs reduced MDSC expansion in doxycycline-treated bitransgenic mice (Figure 3D). These observations provide a mechanistic linkage between dnPPARγ and MDSC expansion.
In addition to their enhanced expansion, the CD11b+Gr-1+ cell population in doxycycline-treated mice demonstrated strong immunosuppressive activity. These MDSCs suppressed TCR-stimulated wild-type T-cell proliferation and function, as judged by CFSE and CD69 expression assays (Figure 5D-E) and cytokine release (Figure 5F) in vitro. Soluble factors released by MDSCs were able to suppress T-cell proliferation in the Transwell study (Figure 5D). These observations were further confirmed in doxycycline-treated bitransgenic mice in vivo, with decreases in overall CD4+ and CD8+ T-cell populations (Figure 4A-C), reductions in T-cell proliferation (Figure 5A-B), and loss of T-cell cytokine secretory function (Figure 5C). Because myeloid cell expansion in doxycycline-treated bitransgenic mice did not alter T-cell progenitors at various stages in the thymus, it is unlikely that the T-cell defects were linked to alterations in T-cell development (Figure 4F-G). Likewise, Treg cells were not responsible for T-cell suppression and loss of function (Figure 4D-E). In lal−/− mice, T-cell development in the thymus and Treg cells were altered.13 It is unlikely that these alterations were mediated through PPARγ activity in myeloid cells. The BM transplantation study confirmed that the enhanced production and malfunction of myeloid cells were the result of a myeloid-autonomous defect (Figure 6A-C). However, the malformation and malfunction of T cells were not strictly linked to changes in monocytic cells (Figure 6D-F) in doxycycline-treated bitransgenic mice. These observations support the notion that abnormal hematopoietic progenitor development in the BM contributes to MDSC expansion and immunosuppression in the absence of PPARγ function.
Defects in the myeloid cells and T cells of doxycycline-treated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice may be responsible for the development of multiple tumors in these mice (Figure 7 and supplemental Figure 2A). In the lung, adenocarcinoma was identified (Figure 7A and supplemental Figure 2A). This is consistent with our previous observations, in which up-regulation of the PPARγ downstream gene MMP12 and Api6 in mouse models caused adenocarcinoma.17,18,20,21 Activation of pStat3, pErk, pP38, and pNF-κB is a common feature in the lungs of all of these mouse tumor models (Figure 2D-E).17,18,20,21 We reported previously that persistent activation of the Stat3 pathway in the lung alone was sufficient to cause spontaneous, inflammation-induced adenocarcinoma.19 It has been well documented that PPARγ inhibits the proliferation of lung carcinoma cells by suppressing the Erk pathway.27 NF-κB is frequently expressed in lung cancer and preneoplastic lesions.28
In the liver and intestine (Figure 7B and supplemental Figure 2A), myeloid sarcomas were identified in doxycycline-treated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice based on myeloid marker expression. Immunohistochemical staining showed massive infiltration of immune cells in tumor lesion areas (Figure 7B). Interestingly, myeloid sarcomas were also found in T- and B-cell developing organs, including the spleen (Figure 7C) and lymph nodes (Figure 7D). In these organs, cells expressing lymphoid lineages disappeared, whereas myeloid cells accumulated along with tumors. Destruction of the normal architecture of the spleen and lymph nodes may partially account for the decrease in T-cell populations in doxycycline-treated c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice.
In conclusion, the LAL/hormonal ligand/PPARγ axis is critical to controlling inflammation and the induction of various tumors. Disruption of this pathway in myeloid cells, either by blocking ligand synthesis, as in lal−/− mice, or by inhibition of PPARγ, as in c-fms-rtTA/(TetO)7-CMV-dnPPARγ–bitransgenic mice, can initiate up-regulation of inflammatory molecules such as Api6, MMP12, IL-1β, IL-6, and TNF-α, which cause hematopoietic progenitors skewing toward myeloid-lineage expansion to form MDSCs. MDSCs hijack the immune surveillance system by suppressing T-cell proliferation and function to create an environment for tumor growth. The present study and our previous findings show the importance of neutral lipids in anticancer applications, thus providing new options for cancer therapy. We reported previously that overexpression of dnPPARγ in lung epithelial cells also promotes regional MDSC expansion, immune suppression, and emphysema in the lung.22 Pathogenesis of LAL is a complex process involving multiple pathways and biologic events. In addition to the PPARγ pathway, other molecular pathways and mechanisms may also be involved in LAL function and remained to be identified in future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health (grants HL087001 to H.D.; CA138759 and CA152099 to C.Y.; HL061803 and HL067862 to C.Y. and H.D.; and AI079065 to J.B.).
National Institutes of Health
Authorship
Contribution: L.W. performed the research, collected, analyzed, and interpreted the data, and performed the statistical analysis; C.Y. and H.D. designed the research, collected, analyzed, and interpreted the data, and wrote the manuscript; M.C. and O.F. analyzed and interpreted the data and contributed ideas; and J.S.B. and R.K. contributed ideas and proofread the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cong Yan, Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Walter Hall C418, 980 W Walnut St, Indianapolis, IN, 46202- 5188; e-mail: coyan@iupui.edu or Hong Du, Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, VanNuys Medical Science Bldg, Rm A132, 635 Barnhill Dr, Indianapolis, IN 48202; e-mail: hongdu@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal