Abstract
After stimulation of antigen-specific T cells, dendritic cell (DCs) are susceptible to killing by these activated T cells that involve perforin and Fas-dependent mechanisms. Fas-dependent DC apoptosis has been shown to limit DC accumulation and prevent the development of autoimmunity. However, a role for perforin in the maintenance of DC homeostasis for immune regulation remains to be determined. Here we show that perforin deficiency in mice, together with the deletion of Fas in DCs (perforin−/−DC-Fas−/−), led to DC accumulation, uncontrolled T-cell activation, and IFN-γ production by CD8+ T cells, resulting in the development of lethal hemophagocytic lymphohistiocytosis. Consistently, adoptive transfer of Fas−/− DCs induced over-activation and IFN-γ production in perforin−/− CD8+ T cells. Neutralization of IFN-γ prevented the spreading of inflammatory responses to different cell types and protected the survival of perforin−/−DC-Fas−/− mice. Our data suggest that perforin and Fas synergize in the maintenance of DC homeostasis to limit T cell activation, and prevent the initiation of an inflammatory cascade.
Introduction
Maintaining the homeostasis of different cell types in the immune system is critical for the prevention of immune dysregulation, such as uncontrolled inflammation. DCs are the most efficient antigen-presenting cells that process and present antigens to initiate the activation of antigen-specific lymphocytes.1-5 DCs represent a small population in the immune system and comprise approximately 1% of the total cell population in lymphoid organs.6 However, DC homeostasis has a major impact on the immune system. It has been shown that inhibition of Fas signaling in DCs induces DC accumulation and onset of autoimmune diseases.7,8 Although temporary deletion of DCs decreases immune responses,9 constitutive ablation of DCs by DC-specific expression of DTA in CD11c-DTA transgenic mice has been shown to induce fatal autoimmune and inflammatory responses.10 This suggests that homeostasis of DCs plays a major role in maintaining a balanced immune system.
Activated antigen-specific T cells may promote the clearance of antigen-bearing DCs in vivo.11 Interestingly, it has been shown that activated antigen-specific T cells are capable of killing exogenously administrated antigen-bearing DCs through perforin in vivo, thereby limiting antigen-specific T-cell responses.12-17 Inhibition of Fas signaling or deletion of Fas in DCs leads to DC accumulation and the development of autoimmunity.7,8 However, a role for perforin in controlling DC homeostasis in the absence of infections remains to be established.
Haemophagocytic lymphohistiocytosis (HLH) is an inflammatory disorder that displays hypercytokinaemia and uncontrolled activation of T cells and macrophages.18-21 In humans, mutations in perforin or other genes that affect perforin-dependent cytotoxicity were first identified in patients with familial HLH.22-26 Perforin is a cytolytic molecule secreted by cytotoxic T cells and NK cells.27-30 Knockout of perforin in mice has been shown to impair the cytolytic activity of T cells toward targets cells.31,32 Although perforin−/− mice do not spontaneously develop inflammation, infection of these mice with LCMV induced uncontrolled T-cell activation, especially in CD8+ T cells,33-37 leading to the development of inflammatory symptoms resembling familial HLH.38 Perforin-deficient mice mount CD8+ T–cell mediated and IFN- γ–dependent lethal inflammation in response to infection by LCMV.38 Increased T-cell activation has been linked to elevated antigen presentation in perforin-deficient mice during LCMV infection.39 This suggests that deficiency in perforin synergizes with other factors during viral infections to trigger the onset of lethal inflammation. It remains to be determined whether defective killing of antigen presenting cells causes HLH in humans or mice deficient in perforin.
Deficiencies of both perforin and Fas/Fas ligand cause severe autoimmune and inflammatory responses in mice.40-42 This suggests a compound effect for perforin and Fas/Fas ligand in controlling autoimmunity and inflammation. We have observed that perforin and Fas are both involved in T cell–mediated killing of DCs in vitro.7 However, whether perforin might synergize with Fas in the maintenance of DC homeostasis for immune regulation is yet to be determined. We examined the compound effects of perforin deficiency and DC-specific Fas deletion by crossing perforin−/− mice with DC-Fas−/− mice. Perforin−/−DC-Fas−/− mice showed accelerated accumulation of DCs and overactivation of CD8+ T cells that produced high levels of IFN-γ. Uncontrolled production of IFN-γ contributed to secondary activation of other cell types to produce different inflammatory cytokines, resulting in the development of lethal inflammation. Our data suggest perforin and Fas-dependent killing of DCs protects against the development of systemic inflammation.
Methods
Mice
Wild-type, perforin−/−, lpr, CD11c-cre transgenic, OT1 and OT2 transgenic mice, and Fasflox mice were obtained from The Jackson Laboratory and maintained on the C57BL/6 background. Perforin−/− mice were crossed with OT1 or OT2 transgenic mice to generate perforin−/−OT1 or perforin−/−OT2 mice. Perforin−/− mice were also crossed with lpr mice to obtain perforin−/−lpr mice. CD11c-cre transgenic mice were crossed with Fasflox mice to obtain CD11c-cre/Fasflox/flox mice with DC-specific deletion of Fas (DC-Fas−/−). Perforin−/− mice were crossed with DC-Fas−/− mice to obtain perforin−/−DC-Fas−/− mice. Granzyme A−/−granzyme B−/− mice and wild-type controls on the 129 × 1/SvJ background were also obtained from The Jackson Laboratory. FoxP3GFP knock-in mice were obtained from Dr Alexander Rudensky43 and crossed with perforin−/− mice to generate perforin−/−FoxP3GFP mice. The mice were maintained in a specific–pathogen-free facility at Baylor College of Medicine and used with the approval of the Institutional Animal Care and Use Committee.
In vitro killing of DCs
DCs were derived from bone marrow monocytes by culturing in GM-CSF and IL-4, followed by purification with CD11c+ MACS beads (Miltenyi Biotec) as described.44 CD4+ OT2 T cells (106/mL) were stimulated with DCs (105/mL) pulsed with OVA323-339 for 4 days. Wild-type or Fas−/− DCs were pulsed with OVA323-339 and labeled with 5μM CFSE at 37°C for 10 minutes. CFSE-labeled DCs (2 × 104/well) were mixed with activated OT2 T cells at different ratios and incubated at 37°C for 5 hours, followed by staining with 5 ng/mL 7-amino-actinomycin D (7-AAD; BD Biosciences) and analysis with flow cytometry. Induction of cell death in DCs was quantified essentially as described45 with the following formula: percentages of killing of DCs by T cells = 100% × (DCcontrol-DCT)/DCcontrol, with DCcontrol and DCT representing CFSE+7-AAD− DCs in the absence or presence of T cells, respectively. To determine killing of DCs by CD8+ T cells, wild-type or perforin−/− CD8+ OT1 T cells (106/mL) were stimulated with DCs (105/mL) pulsed with OVASIINFEKL for 4 days. Wild-type or Fas−/− DCs were pulsed with OVASIINFEKL and labeled with CFSE. CFSE-labeled DCs (2 × 104/well) were incubated with activated OT1 T cells at 37°C for 5 hours, and the killing of DCs was quantitated as previously stated.
CD4+FoxP3+ Treg cells were sorted from the spleen and lymph node cells of FoxP3GFP and perforin−/−FoxP3GFP mice. CD4+CD25high Treg cells were also sorted from granzyme A−/−granzyme B−/− mice and wild-type controls. The sorted Treg cells were expanded in vitro with anti–CD3-coated and anti-CD28–coated Dynabeads (Invitrogen; 10 mL beads/106 cells) in the presence of 1000 U/mL IL-2 for 3 days. CFSE-labeled DCs (2 × 104/well) were mixed with expanded Treg cells at different ratios and the killing of DCs was calculated as previously stated.
NK cells were purified from wild-type or perforin−/− mouse splenocytes with anti[ne]DX5α-MACS beads (Miltenyi Biotec) and cultured in RPMI complete medium containing 1000 U/mL IL-2 for 6 days. NK cells were incubated with CFSE-labeled wild-type or Fas−/− DCs at different ratios for 4 hours. Percentages of DC killing by NK cells were determined as previously stated.
Flow cytometry
Single cell suspensions from mouse spleens were first blocked with 1 μg/mL of anti-CD16/CD32, rat IgG, and hamster IgG. The cells were then stained with various antibodies conjugated to FITC, PE, or PE-Cy5, and analyzed on a BD LSR II flow cytometer (BD Biosciences). The data were analyzed using FlowJo Version 6.4.7 software (TreeStar). The conjugated antibodies to CD11c, CD11b, CD40, B7.1, B7.2, I-Ab, ICAM-1, CD4, CD8, and CD69 were obtained from BD Biosciences. PE-conjugated anti–PDCA-1 was from Miltenyi Biotec.
To detect intracellular IFN-γ, cells were stained with PE-conjugated antibodies to CD8, DX5α, or CD4, followed by fixation and permeabilization with Cytofix/Cytoperm solution (BD Biosciences), and staining with APC-conjugated anti–IFN-γ (BD Biosciences) or APC-conjugated rat IgG1 as isotype control. The cells were then analyzed by flow cytometry.
CFSE-labeled DCs in the draining lymph nodes
Measurement of CFSE-labeled DCs after interaction with antigen-specific T cells was performed as described.44
DC-induced T-cell activation in vivo
CD8+ T cells from wild-type or perforin−/− OT1 mice were labeled with 5μM CFSE and injected into congenic CD45.1 mice retro-orbitally (2 × 106/mouse). WT or Fas−/− DCs were pulsed with OVASIINFEKL peptide and injected at the footpad 24 hours later. Three days later, draining popliteal lymph node cells were harvested and stained with PE-Cy5–anti-CD8 and PE–anti-CD45.2 (BD Biosciences) and analyzed by flow cytometry. CD8+CD45.2+ T cells were gated to analyze CFSE dilution because of cell division. Similarly, CD4+ T cells from wild-type or perforin−/− OT2 mice were purified, labeled with CFSE and injected into CD45.1 mice retro-orbitally. Wild-type or Fas−/− DCs were pulsed with OVA323-339 peptide (106/mouse) and injected at footpad 24 hours later. Three days later, popliteal lymph nodes were collected and CD4+CD45.2+ T cells were analyzed by flow cytometry. In parallel experiments, OT1 or OT2 T cells without CFSE labeling were injected into recipients with peptide-pulsed DCs as described in “In vitro killing of DCs.” Three days later, draining popliteal lymph node cells were harvested and stained with anti-CD45.2 and anti-CD8, and (for OT1) or anti-CD4 (for OT2), followed by intracellular staining with anti–IFN-γ, and analysis by flow cytometry. Similarly, DX5α+ NK cells (5 × 105/mouse) were injected into congenic CD45.1 mice retro-orbitally, followed by injection of DCs at the footpad 24 hours later. Three days later, NK cells in the draining lymph nodes were stained with anti-DX5α and anti-CD45.2, followed by intracellular staining with anti–IFN-γ as previously stated.
Histochemistry
Immunohiostochemistry and H&E staining were performed as described.7 The sections were examined under an Olympus BX51 microscope with 10× or 40× objective amplification. Images were captured with an Olympus DP70 camera using DP Controller Version 1.2 software (Olympus).
Measurement of cytokines by multiplex and whole blood analyses
Sera from 4-week-old or 7-week-old perforin−/−DC-Fas−/− mice or controls were measured by the multiplex method using the Milliplex Map mouse cytokine/chemokine kit (Millipore) and quantitated in a Bio-Plex System (Bio-Rad). Whole blood collected from 7-week-old perforin−/−DC-Fas−/− mice and controls were analyzed in a Scil Vet Abc counter (Scil Diagnostics).
Neutralization of IFN-γ or cell depletion in perforin−/−DC-Fas−/− mice
Three-week-old perforin−/−DC-Fas−/− mice (5 mice/group) were injected with anti–IFN-γ (200 μg/mouse; Biolegend) or rat IgG control intraperitoneally every 2 days until 10-week old or when mice died. Percentages of survival of the mice were plotted. In parallel experiments, mice injected with anti–IFN-γ or rat IgG control until 7 weeks old. Measurement of cytokine in the serum by multiplex, whole blood analyses and histochemistry were performed as previously stated. In some experiments, perforin−/−DC-Fas−/− mice (5 mice/group) were injected with antibodies (Biolegend) to CD8 (53-6.7), CD4 (GK1.5), or NK1.1 (PK136), or with control IgG intraperitoneally (100 μg/mouse) every 3 days until 10 weeks old or when mice died. Successful deletion of CD8+ T cells, CD4+ T cells or NK cells were confirmed by flow cytometry analyses of peripheral blood cells. IFN-γ in the sera at 6 weeks of age was measured as previously stated. The percentages of survival of the mice were also shown.
Statistical Analysis
Statistical significance was determined by 2-tailed unpaired Student t test.
Results
Killing of DCs by antigen-specific T cells through perforin and Fas
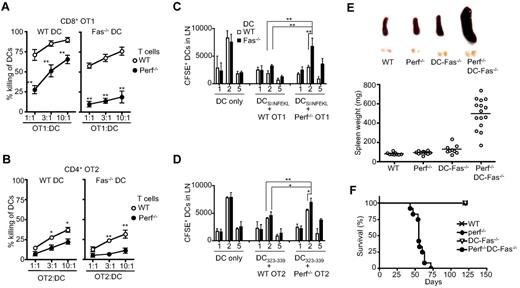
We have previously found that DCs are susceptible to killing by allo-specific T cells.7 We also determined the involvement of perforin and Fas in the killing of DCs by antigen-specific CD4+ or CD8+ T cells. Although OVA-specific CD8+ OT1 T cells were capable of killing antigen-pulsed DCs, perforin−/− OT1 T cells were significantly weaker in such killing (Figure 1A). This indicates that perforin plays an important role for antigen-specific CD8+ T cells in the killing of DCs. We also determined the roles of Fas in CD8+ OT1 T cell–mediated killing of DCs. Wild-type DCs were lysed more efficiently than Fas-deficient DCs by CD8+ T cells (Figure 1A), indicating that Fas is also involved in CD8+ T cell–mediated killing of DCs. Fas-deficient DCs showed most resistance to killing by perforin−/− OT1 T cells (Figure 1). Therefore, perforin and Fas are major effectors and can work synergistically in the killing of DCs by antigen-specific CD8+ T cells. We also detected the killing of OVA-pulsed DCs by wild-type CD4+ OT2 T cells. However, CD4+ T cells were significantly weaker than CD8+ T cells in the killing of DCs (Figure 1B). Nevertheless, the lysis of antigen-pulsed DCs by CD4+ T cells was similarly dependent on perforin and Fas (Figure 1B).
Perforin and Fas in the interactions between T cells and DCs. (A) Activated wild-type (WT) or perforin−/− CD8+ OVA-specific OT1 T cells were incubated with CFSE-labeled WT or Fas-deficient lpr DCs pulsed with OVASIINFEKL peptide. Killing of DCs were quantified with WT versus perforin−/− (mean ± SD): **P < .01 (n = 3). (B) WT or Fas-deficient lpr DCs were pulsed with OVA223-239 peptide and labeled with CFSE. Activated WT or perforin−/− CD4+ OVA-specific OT2 T cells were incubated with DCs. Killing of DCs was quantified (mean ± SD). WT versus perforin−/−: *P < .05, **P < .01 (n = 3). (C) WT or perforin−/− OT1 T cells were injected into recipient mice, followed by injection of CFSE-labeled WT of Fas−/− DCs pulsed with OVASIINFEKL peptide. Draining lymph nodes were harvested. Total cell numbers were counted and percentages of CFSE+ DCs were quantified by flow cytometry. Total CFSE+ DCs were determined (mean ± SD). **P < .01 (n = 5). (D) WT or perforin−/− OT2 T cells were injected into recipient mice, followed by injection of CFSE-labeled WT of Fas−/− DCs pulsed with OVA223-239 peptide. Draining lymph nodes were harvested and total CFSE+ DCs were determined as in (C). *P < .05, **P < .01 (n = 5). (E) Spleen and inguinal lymph nodes were harvested from 6-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. The weight of spleens of 6-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice was also determined. WT versus perforin−/−DC-Fas−/−: P < .01. (F) Percentages of survival of WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice at different ages (n = 20).
Perforin and Fas in the interactions between T cells and DCs. (A) Activated wild-type (WT) or perforin−/− CD8+ OVA-specific OT1 T cells were incubated with CFSE-labeled WT or Fas-deficient lpr DCs pulsed with OVASIINFEKL peptide. Killing of DCs were quantified with WT versus perforin−/− (mean ± SD): **P < .01 (n = 3). (B) WT or Fas-deficient lpr DCs were pulsed with OVA223-239 peptide and labeled with CFSE. Activated WT or perforin−/− CD4+ OVA-specific OT2 T cells were incubated with DCs. Killing of DCs was quantified (mean ± SD). WT versus perforin−/−: *P < .05, **P < .01 (n = 3). (C) WT or perforin−/− OT1 T cells were injected into recipient mice, followed by injection of CFSE-labeled WT of Fas−/− DCs pulsed with OVASIINFEKL peptide. Draining lymph nodes were harvested. Total cell numbers were counted and percentages of CFSE+ DCs were quantified by flow cytometry. Total CFSE+ DCs were determined (mean ± SD). **P < .01 (n = 5). (D) WT or perforin−/− OT2 T cells were injected into recipient mice, followed by injection of CFSE-labeled WT of Fas−/− DCs pulsed with OVA223-239 peptide. Draining lymph nodes were harvested and total CFSE+ DCs were determined as in (C). *P < .05, **P < .01 (n = 5). (E) Spleen and inguinal lymph nodes were harvested from 6-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. The weight of spleens of 6-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice was also determined. WT versus perforin−/−DC-Fas−/−: P < .01. (F) Percentages of survival of WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice at different ages (n = 20).
We also measured DCs in the draining nodes after adoptive transfer. Similar numbers of wild-type and Fas-deficient DCs were detected in the draining lymph nodes 2 days after adoptive transfer (Figure 1C). Cotransfer of wild-type OT1 T cells with DCs led to a reduction of DCs in the draining lymph nodes (Figure 1C). This is consistent with the possibility of killing of DCs by antigen-specific T cells in vivo. When either Fas on DCs or perforin in OT1 T cells was deficient, increased DCs were found in the draining lymph nodes (Figure 1C). When Fas−/− DCs and perforin−/− OT1 T cells were co-injected, significantly more DCs were detected in the draining lymph nodes (Figure 1C). Similar results were obtained when CD4+ OVA-specific OT2 T cells were cotransferred with DCs (Figure 1D). This indicates that compound deficiencies of perforin in T cells and Fas in DCs lead to defective clearance of DCs by antigen-specific T cells in vivo.
Enlargement of lymphoid organs and early lethality of perforin−/−DC-Fas−/− mice
It has been shown that DC-Fas−/− mice develop systemic autoimmunity.8 However, perforin−/− mice do not display salient defects unless challenged by infections.31,32,38 To examine whether Fas and perforin might synergize to regulate DC survival in vivo, we crossed perforin−/− mice with DC-Fas−/− mice to generate perforin−/−DC-Fas−/− mice. Successful deletion of Fas on DCs was confirmed in DC-Fas−/− and perforin−/−DC-Fas−/− mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, T cells from DC-Fas−/− or perforin−/−DC-Fas−/− mice did not show deletion of the Fas on the cell surface (supplemental Figure 1). By 6 weeks of age, significant enlargement of spleen and lymph nodes was found in perforin−/−DC-Fas−/− mice compared with age-matched wild-type controls, perforin−/− or DC-Fas−/− mice (Figure 1E). Strikingly, perforin−/−DC-Fas−/− mice developed hunched posture, reduced mobility and reduced weight, and died between 7 and 10 weeks of age (Figure 1F). In contrast, no such phenotypes and lethality was found in wild-type, perforin−/− or DC-Fas−/− mice during a 20-week observing period (Figure 1F).
DC expansion and spontaneous T cell activation in perforin−/−DC-Fas−/− mice
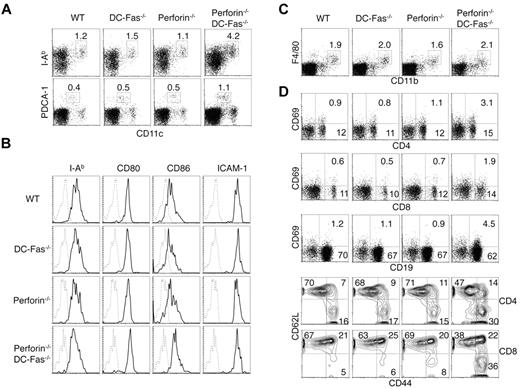
We examined DCs and lymphocytes in 4-week-old perforin−/−DC-Fas−/− mice before they display significant enlargement of lymphoid organs and clinical symptoms. We found that CD11chighMHC-II+ conventional DCs and CD11clowPDCA-1+ plasmacytoid DCs were expanded in perforin−/−DC-Fas−/− mice compared with wild-type, perforin−/− or DC-Fas−/− mice (Figure 2A). CD80 and CD86 were increased on DCs from perforin−/−DC-Fas−/− mice (Figure 2B), suggesting that DCs in perforin−/−DC-Fas−/− mice display a more activated phenotype. In contrast to DCs, macrophages were not expanded in 4-week-old perforin−/−DC-Fas−/− mice (Figure 2C). Increased CD69 expression was detected on T and B cells from perforin−/−DC-Fas−/− mice (Figure 2D). Expansion of the CD44hiCD62L− activated/memory cells was observed in both CD4+ and CD8+ T cells in perforin−/−DC-Fas−/− mice (Figure 2D). These data suggest that perforin−/−DC-Fas−/− mice develop DC accumulation and spontaneous T-cell activation at an early age.
DC accumulation and spontaneous activation of T cells in perforin−/−DC-Fas−/− mice. (A) Flow cytometry of CD11chighI-Ab+ DCs and CD11clowPDCA-1+ pDCs in 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (B) Staining of I-Ab, CD80, CD86, and ICAM-1 on CD11chighCD11b+ DCs from 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (C) Flow cytometry of F4/80+CD11b+ macrophages in 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (D) CD69 staining on CD4+, CD8+, and CD19+ cells from the spleen of 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice were analyzed by flow cytometry. Splenocytes were also stained with APC–anti-CD4 or APC–anti-CD8, and PE–anti-CD44 and cychrome–anti-CD62L. The cells were analyzed by flow cytometry. CD44 and CD62L staining of CD4+ or CD8+ cells were plotted. Data are representative of 5 independent experiments.
DC accumulation and spontaneous activation of T cells in perforin−/−DC-Fas−/− mice. (A) Flow cytometry of CD11chighI-Ab+ DCs and CD11clowPDCA-1+ pDCs in 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (B) Staining of I-Ab, CD80, CD86, and ICAM-1 on CD11chighCD11b+ DCs from 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (C) Flow cytometry of F4/80+CD11b+ macrophages in 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice. (D) CD69 staining on CD4+, CD8+, and CD19+ cells from the spleen of 4-week-old WT, perforin−/−, DC-Fas−/−, and perforin−/−DC-Fas−/− mice were analyzed by flow cytometry. Splenocytes were also stained with APC–anti-CD4 or APC–anti-CD8, and PE–anti-CD44 and cychrome–anti-CD62L. The cells were analyzed by flow cytometry. CD44 and CD62L staining of CD4+ or CD8+ cells were plotted. Data are representative of 5 independent experiments.
Uncontrolled production of IFN-γ in perforin−/−DC-Fas−/− mice at early ages
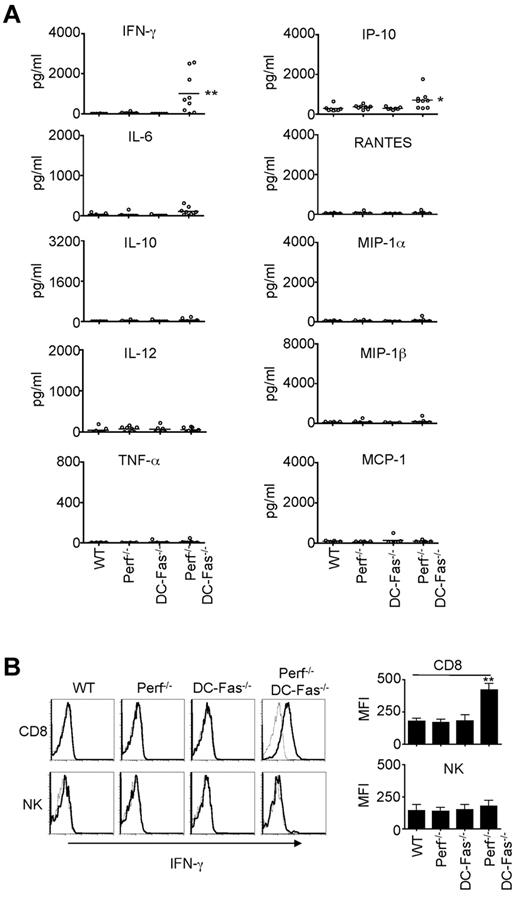
We next determined whether perforin−/−DC-Fas−/− mice demonstrated uncontrolled production of inflammatory cytokines. We did not find significant increases in the production of IL-6, IL-10, IL-12, TNF-α, RANTES, MIP-1α, MIP-1β, or MCP-1 in the sera of 4-week-old perforin−/−DC-Fas−/− mice (Figure 3A). However, we detected high levels of IFN-γ in 4-week-old perforin−/−DC-Fas−/− mice, but not in wild-type, DC-Fas−/−, or perforin−/− mice (Figure 3A). A small but significant increase in IP-10, an IFN-γ-inducible chemokine,46 was also detected (Figure 3A). We next performed intracellular staining to determine what cell types produced IFN-γ in perforin−/−DC-Fas−/− mice. Interestingly, CD8+ T cells, but not CD4+ cells, DCs, macrophages, or NK cells from perforin−/−DC-Fas−/− mice showed significant intracellular staining for IFN-γ (Figure 3B, supplemental Figure 2A, and data not shown). This suggests that CD8+ T cells, but not other cell types, are the major sources of IFN-γ production in perforin−/−DC-Fas−/− mice. On stimulation with anti-CD3 and anti-CD28, CD4+ T cells from perforin−/−DC-Fas−/− mice had similar induction of IFN-γ–producing cells (supplemental Figure 2B). More CD8+ T cells from perforin−/−DC-Fas−/− mice were IFN-γ–positive after stimulation with anti-CD3 and anti-CD28 (supplemental Figure 2B). However, this could be because of increased spontaneous IFN-γ production in CD8+ T cells before CD3 stimulation in these mice (supplemental Figure 2A). Increased IFN-γ production by CD8+ T cells in perforin−/−DC-Fas−/− mice is potentially because of interactions with external factors.
Uncontrolled IFN-γ production in perforin−/−DC-Fas−/− mice. (A) Sera from 4-week-old WT, perforin−/− (Perf−/−), DC-Fas−/−, and Perf−/−DC-Fas−/− mice were used for the measurement of different cytokines by multiplex. WT versus Perf−/−DC-Fas−/−: IFN-γ, ** P < .01; IP-10, *P < .05; other cytokines, not significant. (B) Intracellular staining for IFN-γ in CD8+ T cells and DX5α+ NK cells. Mean fluorescence intensity (MFI) for IFN-γ staining: **P < .01.
Uncontrolled IFN-γ production in perforin−/−DC-Fas−/− mice. (A) Sera from 4-week-old WT, perforin−/− (Perf−/−), DC-Fas−/−, and Perf−/−DC-Fas−/− mice were used for the measurement of different cytokines by multiplex. WT versus Perf−/−DC-Fas−/−: IFN-γ, ** P < .01; IP-10, *P < .05; other cytokines, not significant. (B) Intracellular staining for IFN-γ in CD8+ T cells and DX5α+ NK cells. Mean fluorescence intensity (MFI) for IFN-γ staining: **P < .01.
Adoptive transfer of Fas−/− DCs led to overproduction of IFN-γ in perforin−/− T cells
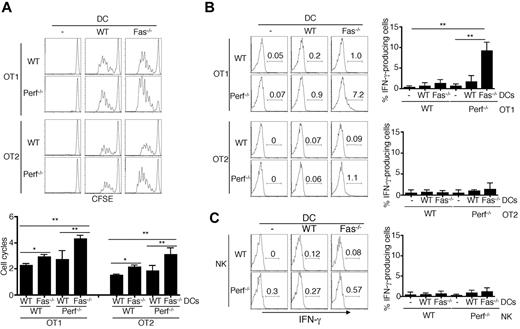
To further determine whether Fas deficiency in DCs in combination with perforin deficiency in T cells lead to uncontrolled T-cell activation, we performed adoptive transfer studies. Although deficiency of either Fas in DCs or perforin in T cells led to better T cell proliferation assayed by CFSE dilution, cotransfer of Fas−/− DCs together with perforin−/− OT1 T cells resulted in highest T cell proliferation (Figure 4A left panels). Similar results were observed with DC-dependent stimulation of CD4+ OT2 T cells (Figure 4A right panels). These data suggest that compound deficiencies of Fas on DCs and perforin in T cells lead to more robust activation of T cells in vivo.
Over-activation and IFN-γ production of perforin−/− T cells induced by Fas−/− DCs after adoptive transfer. (A) CD45.1 mice were injected with CFSE-labeled WT or perforin−/− OT1 or OT2 cells, followed by injections of WT or Fas−/− DCs pulsed with corresponding OVA peptides at the footpad. Three days later, draining lymph node cells were analyzed by flow cytometry. CD8+CD45.2+ OT1 cells or CD4+CD45.2+ OT2 cells were gated to determine CFSE dilution. Average numbers of cell division (mean ± SD) were plotted. **P < .01, *P < .05, n = 5. (B) CD45.1 mice were injected with WT or perforin−/− OT1 or OT2 cells, followed by injections of WT or Fas−/− DCs pulsed with corresponding OVA peptides as in panel A at the footpad. Three days later, draining LN cells were stained with PE–anti-CD4 or anti-CD8 and FITC-CD45.2, followed by intracellular staining with APC-anti–IFN-γ and flow cytometry. **P < .01 (n = 5). (C) CD45.1 mice were injected with WT or perforin−/− NK cells, followed by injections of WT or Fas−/− DCs at the footpad. Three days later, IFN-γ staining in CD45.2+DX5α+ NK cells from draining LN was analyzed (n = 5).
Over-activation and IFN-γ production of perforin−/− T cells induced by Fas−/− DCs after adoptive transfer. (A) CD45.1 mice were injected with CFSE-labeled WT or perforin−/− OT1 or OT2 cells, followed by injections of WT or Fas−/− DCs pulsed with corresponding OVA peptides at the footpad. Three days later, draining lymph node cells were analyzed by flow cytometry. CD8+CD45.2+ OT1 cells or CD4+CD45.2+ OT2 cells were gated to determine CFSE dilution. Average numbers of cell division (mean ± SD) were plotted. **P < .01, *P < .05, n = 5. (B) CD45.1 mice were injected with WT or perforin−/− OT1 or OT2 cells, followed by injections of WT or Fas−/− DCs pulsed with corresponding OVA peptides as in panel A at the footpad. Three days later, draining LN cells were stained with PE–anti-CD4 or anti-CD8 and FITC-CD45.2, followed by intracellular staining with APC-anti–IFN-γ and flow cytometry. **P < .01 (n = 5). (C) CD45.1 mice were injected with WT or perforin−/− NK cells, followed by injections of WT or Fas−/− DCs at the footpad. Three days later, IFN-γ staining in CD45.2+DX5α+ NK cells from draining LN was analyzed (n = 5).
We next performed adoptive transfer experiments to determine whether Fas−/− DCs induced overproduction of IFN-γ by perforin−/− CD8+ T cells. No significant intracellular staining of IFN-γ in T cells from the draining lymph nodes was detected in these T cells after stimulation with wild-type DCs (Figure 4B). However, compound deficiencies of perforin in T cells and Fas on DCs led to significant IFN-γ production by CD8+ OT1 T cells (Figure 4B top panels). This provides direct evidence to suggest that Fas−/− DCs can trigger IFN-γ production in perforin−/− CD8+ T cells, and induce an IFN-γ–dependent inflammation cascade.
In comparison, CD4+ OT2 T cells were much weaker in producing IFN-γ, even when perforin−/− T cells and Fas−/− DCs were cotransferred (Figure 4B bottom panels). No significant IFN-γ production was detected in wild-type or perforin−/− NK cells when cotransferred with wild-type or Fas−/− DCs (Figure 4C). These data support the finding in perforin−/−DC-Fas−/− mice that compound perforin deficiency in combination with deletion of Fas in DCs induce uncontrolled IFN-γ production in CD8+ T cells.
Tissue inflammation and anemia in perforin−/−DC-Fas−/− mice
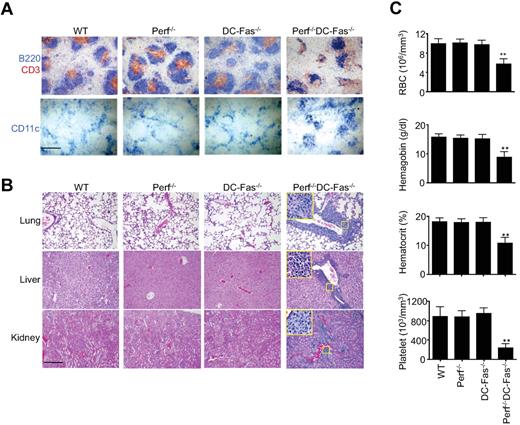
We next examined different tissues for signs of inflammatory responses in 7-week-old perforin−/−DC-Fas−/− mice before they succumbed to inflammation. The B cell and T cell areas were disorganized in the spleen of perforin−/−DC-Fas−/− mice (Figure 5A). More CD11c+ DCs were detected in the spleen of perforin−/−DC-Fas−/− mice (Figure 5A). All perforin−/−DC-Fas−/− mice displayed severe perivascular lymphocyte infiltration in the lung, liver, and kidney at 7 weeks of age (Figure 5B). Those mice also showed loss of red blood cells, hemoglobin, and platelets in the peripheral blood (Figure 5C) resembling HLH. Perforin−/−DC-Fas−/− mice died within a week when they started to show hutched back, slow movement, and weight loss. Despite systemic inflammation, we did not detect autoantibodies in 7 week-old perforin−/−DC-Fas−/− mice, or in DC-Fas−/− and perforin−/− mice at these ages. It has been shown that DC-Fas−/− mice develop autoantibodies at 3 months of age.8 However, most perforin−/−DC-Fas−/− mice died before they reach 2 and one-half months of age (Figure 1C). Therefore, lethal inflammation may preclude the development of autoimmune response in perforin−/−DC-Fas−/− mice.
Histochemistry and blood analyses of perforin−/−DC-Fas−/− mice. (A) Spleen sections of 7-week-old perforin−/−DC-Fas−/− and control mice were stained with anti-CD3 and anti-B220, or anti-CD11c. Scale bar: 50 μm. (B) Section of lung, liver, and kidney from 7-week-old perforin−/−DC-Fas−/− and control mice were used for H&E staining. Scale bar: 50 μm. (C) Blood analyses of 7-week-old perforin−/−DC-Fas−/− and control mice. WT versus perforin−/−DC-Fas−/−: **P < .01 (n = 5).
Histochemistry and blood analyses of perforin−/−DC-Fas−/− mice. (A) Spleen sections of 7-week-old perforin−/−DC-Fas−/− and control mice were stained with anti-CD3 and anti-B220, or anti-CD11c. Scale bar: 50 μm. (B) Section of lung, liver, and kidney from 7-week-old perforin−/−DC-Fas−/− and control mice were used for H&E staining. Scale bar: 50 μm. (C) Blood analyses of 7-week-old perforin−/−DC-Fas−/− and control mice. WT versus perforin−/−DC-Fas−/−: **P < .01 (n = 5).
Elevated cytokine production in perforin−/−DC-Fas−/− mice
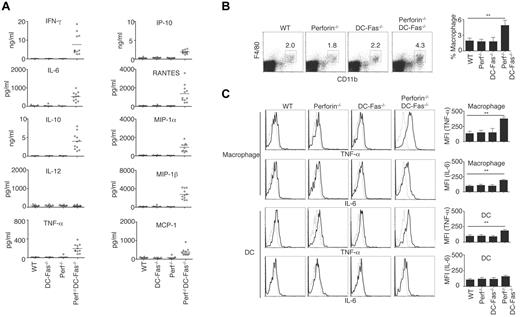
Most perforin−/−DC-Fas−/− mice died between 7 and 10 weeks of age (Figure 1F). We therefore measured inflammatory cytokines in 7-week-old perforin−/−DC-Fas−/− mice. We detected higher levels of IFN-γ in the sera of 7-week-old perforin−/−DC-Fas−/− mice (Figure 6A). Different from 4-week-old perforin−/−DC-Fas−/− mice, these older mice also produced increased levels of TNF-α, IL-6, IL-10, IP-10, RANTES, MIP-1α, MIP-1β, and MCP-1 (Figure 6A). Although macrophages were not increased in 4-week-old perforin−/−DC-Fas−/− mice (Figure 2C), they were expanded by 7 weeks of age (Figure 6B). We also found the production of TNF-α and IL-6 by macrophages in 7-week-old perforin−/−DC-Fas−/− mice (Figure 6C), although they were undetectable at 4 weeks of age. This suggests that secretion of IFN-γ by CD8+ T cells precedes increased inflammatory cytokine production by macrophages.
Uncontrolled production of inflammatory cytokines and chemokines in 7-week-old perforin−/−DC-Fas−/− mice. (A) Sera from 7-week-old WT, perforin−/− (Perf−/−), DC-Fas−/−, and Perf−/−DC-Fas−/− mice were used for measurement of different cytokines by multiplex. Statistic comparison between WT and Perf−/−DC-Fas−/−: for all cytokine except IL-12, P < .001; IL-12, not significant. (B) Percentages of F4/80+CD11b+ macrophages in mice as in panel A. P < .01 (n = 5). (C) Intracellular staining of TNF-α and IL-6 in F4/80+CD11b+ macrophages or CD11c+CD1b+ DCs from mice as in (A). Mean fluorescent intensity (MFI) for intracellular cytokine staining was plotted. **P < .01 (n = 5).
Uncontrolled production of inflammatory cytokines and chemokines in 7-week-old perforin−/−DC-Fas−/− mice. (A) Sera from 7-week-old WT, perforin−/− (Perf−/−), DC-Fas−/−, and Perf−/−DC-Fas−/− mice were used for measurement of different cytokines by multiplex. Statistic comparison between WT and Perf−/−DC-Fas−/−: for all cytokine except IL-12, P < .001; IL-12, not significant. (B) Percentages of F4/80+CD11b+ macrophages in mice as in panel A. P < .01 (n = 5). (C) Intracellular staining of TNF-α and IL-6 in F4/80+CD11b+ macrophages or CD11c+CD1b+ DCs from mice as in (A). Mean fluorescent intensity (MFI) for intracellular cytokine staining was plotted. **P < .01 (n = 5).
Neutralization of IFN-γ suggests a cascade of systemic inflammation in perforin−/−DC-Fas−/− mice
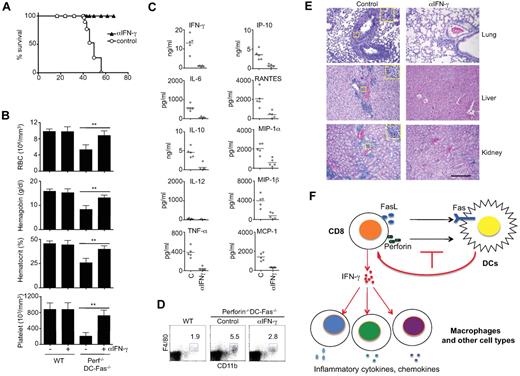
Because IFN-γ was detected in young perforin−/−DC-Fas−/− mice earlier than other cytokines (Figure 3), we determined whether IFN-γ is a critical inflammatory cytokine that initiates lethal inflammation in these mice. We therefore started injection of perforin−/−DC-Fas−/− mice from 3 weeks of age with a neutralizing anti–IFN-γ antibody before these mice develop systemic inflammatory symptoms. All perforin−/−DC-Fas−/− mice injected with anti–IFN-γ appeared healthy and survived throughout the duration of the experiments (Figure 7A). In contrast, perforin−/−DC-Fas−/− mice injected with control IgG died around 2 months of age (Figure 7A).
Rescue of perforin−/−DC-Fas−/− mice from lethal inflammation by neutralization of IFN-γ. (A) Perforin−/−DC-Fas−/− mice (5 mice/group) were injected with anti–IFN-γ (200 μg/mouse) or rat IgG control intraperitoneally once every 2 days starting from 3 weeks of age. The survival of the mice was plotted. (B-E) Seven-week-old perforin−/−DC-Fas−/− mice or controls with or without anti–IFN-γ treatment as in panel A were used for blood analyses (B), measurement of cytokines by multiplex (C), flow cytometry analyses (D), and H&E staining for tissue sections (E). **P < .01. Statistic comparison between control and anti–IFN-γ treatment: for all cytokines except IL-12, P < .01; for IL-12, not significant. Scale bar in panel E: 50 μm. (F) A diagram for T cell–mediate killing of DCs in the prevention of inflammation. When perforin and Fas-dependent killing of DCs is inhibited, overactivation of T cells leads to IFN-γ production. IFN-γ then triggers a cascade of inflammation by activating macrophages and other cell types to produce different inflammatory cytokines and chemokines.
Rescue of perforin−/−DC-Fas−/− mice from lethal inflammation by neutralization of IFN-γ. (A) Perforin−/−DC-Fas−/− mice (5 mice/group) were injected with anti–IFN-γ (200 μg/mouse) or rat IgG control intraperitoneally once every 2 days starting from 3 weeks of age. The survival of the mice was plotted. (B-E) Seven-week-old perforin−/−DC-Fas−/− mice or controls with or without anti–IFN-γ treatment as in panel A were used for blood analyses (B), measurement of cytokines by multiplex (C), flow cytometry analyses (D), and H&E staining for tissue sections (E). **P < .01. Statistic comparison between control and anti–IFN-γ treatment: for all cytokines except IL-12, P < .01; for IL-12, not significant. Scale bar in panel E: 50 μm. (F) A diagram for T cell–mediate killing of DCs in the prevention of inflammation. When perforin and Fas-dependent killing of DCs is inhibited, overactivation of T cells leads to IFN-γ production. IFN-γ then triggers a cascade of inflammation by activating macrophages and other cell types to produce different inflammatory cytokines and chemokines.
Treatment with anti–IFN-γ prevented the decreases in hemoglobin and hematocrit, and the loss of red blood cells and platelets in perforin−/−DC-Fas−/− mice (Figure 7B). This is consistent with the critical roles for IFN-γ in causing acute cytopenias and hemophagocytosis.39,47 Anti–IFN-γ treatment inhibited the production of not only IFN-γ, but also IL-6, IL-10, TNF-α, IP-10, RANTES, MIP-1α, MIP-1β, and MCP-1 in these mice (Figure 7C). Anti–IFN-γ treatments also reduced macrophage expansion in the spleen, and attenuated lymphocyte infiltration in the lung, liver, and kidney of perforin−/−DC-Fas−/− mice (Figure 7D-E). However, neutralization of IFN-γ did not prevent splenomegaly or spontaneous T-cell activation in perforin−/−DC-Fas−/− mice (supplemental Figure 3), indicating that abnormal T-cell activation occurs upstream of IFN-γ production. Together, these data suggest that IFN-γ produced by overactivated CD8+ T cells is critical for initiating the spreading of uncontrolled inflammatory responses to macrophages and other cell types (Figure 7F).
CD8+ T cells are the main effectors for inflammation in perforin−/−DC-Fas−/− mice
Consistent with the finding that CD8+ T cells are the major producers of IFN-γ (Figure 3B, supplemental Figure 2A), deletion of CD8+ T cells protected the survival and significantly lowered the levels of IFN-γ in the sera of perforin−/−DC-Fas−/− mice (supplemental Figure 4A). In contrast, deletion of NK or CD4+ T cells in perforin−/−DC-Fas−/− mice did not have such protective effects (supplemental Figure 4A), suggesting that NK or CD4+ T cells are dispensable for inducing the lethal inflammation in the presence of perforin-deficient CD8+ T cells.
It has been shown that NK cells can kill DCs in a perforin-dependent manner.48 We also observed that NK cells activated by IL-2 killed DCs through Fas and perforin (supplemental Figure 4B), suggesting that NK cells may regulate CD8+ T-cell activation and expansion through modulating survival of DCs and T cells. Interestingly, wild-type, but not perforin−/− NK cells, suppressed the production of IFN-γ in perforin-deficient CD8+ T cells induced by Fas-deficient DCs during adoptive transfer (supplemental Figure 5A). Similar results were observed with CD4+ T cells (supplemental Figure 5A). Therefore, perforin-sufficient NK cells or CD4+ T cells may partially protect against the production of inflammatory IFN-γ by perforin−/− CD8+ T cells.
We observed that the killing of DCs by T regulatory cells was independent of Fas, perforin or granzymes A and B (supplemental Figure 5B-C). This indicates that deficiencies in Fas or perforin do not affect the function of T regulatory cells in the killing of DCs. It has been reported that FoxP3+ T regulatory cells are capable of killing DCs through perforin in tumor environmen.17 Such discrepancy might be because that some factors present in the tumor environment regulates perforin pathway. Our data indicate that lethal inflammation in perforin−/−DC-Fas−/− mice is mainly caused by overactivation of perforin−/− CD8+ T cells by Fas−/− DCs (Figure 7F). Perforin-deficient NK cells or CD4+ cells are not sufficient to induce hyperinflammation in the absence of CD8+ T cells.
Discussion
We observed that antigen-specific T cells killed DCs through both perforin and Fas. Although CD8+ T cells were potent in inducing cell death in DCs, CD4+ T cells showed less cytotoxicity. Activated antigen-specific CD8+ T cells are potentially the predominant cell type for restricting the lifespan of antigen-presenting DCs. Combined deficiencies of perforin in T cells and Fas on antigen-bearing DCs led to prolonged survival of DCs and abnormal activation of CD8+ T cells. At 4 weeks of age, perforin−/−DC-Fas−/− mice started to show DC accumulation, abnormal T-cell activation and production of IFN-γ by CD8+ T cells. At older ages, these mice produced additional inflammatory cytokines and chemokines by macrophages and other cell types. They also manifested elevated levels of macrophages, and reduced red blood cells, hemoglobin, and platelets resembling HLH. Most perforin−/−DC-Fas−/− mice succumbed to inflammation between 7 and 10 weeks of age. Adoptive transfer studies showed that compound deficiencies of perforin in T cells and Fas on DCs led to uncontrolled T-cell activation and IFN-γ production. Depletion studies indicate that perforin deficient NK or CD4+ T cells are not critical for inducing the lethal inflammation in perforin−/−DC-Fas−/− mice. These results suggest that perforin and Fas synergize in the killing of DCs to maintain DC homeostasis and prevent overactivation of CD8+ T cells that initiate a lethal inflammatory cascade.
After presenting antigen for the activation of antigen-specific T cells, DCs are subject to killing by activated T cells. This may serve as a negative feedback mechanism to limit the duration and scope of T-cell activation by antigen-presenting DCs. Although perforin is involved in the killing of DCs by T cells,12-17 Fas also plays an important role. It has been reported that deficiency in perforin promotes lymphoproliferation, infiltration of myeloid cells, pancreatitis and systemic autoimmunity in Fas-deficient lpr or Fas ligand-deficient gld mice.40-42 In addition, perforin deficiency may exacerbate the clinical symptoms in the human autoimmune lymphoproliferative syndrome harboring Fas mutations.49 However, whether perforin synergizes with Fas in regulating survival and function of DCs has not been investigated in those studies. The mechanism for Fas and perforin in the induction of cell death are distinct. Fas signaling triggers an apoptosis pathway involving the activation of caspase-8, whereas perforin directly forms pores on targets to mediate the delivery of cytolytic granules for target lysis. Our data indicate that perforin-dependent killing of DCs may synergize with Fas-dependent death in DCs to ensure the clearance of DCs after antigen-specific T cells are activated.
We found that neutralization of IFN-γ suppressed the production of TNF-α, IL-6 and other cytokines in perforin−/−DC-Fas−/− mice. Production of these inflammatory cytokine by different cell types potentially contributes to the lethality initiated by IFN-γ. Chemokines probably serve as attractants for lymphocyte infiltration in various tissues. Although DCs may function as initiators of inflammation, macrophages and lymphocytes are potentially the key effector cell types for lethal inflammation in perforin−/−DC-Fas−/− mice. Interestingly, it has been shown that TNF exacerbates the immunopathology caused by perforin and Fas ligand double deficiency.42 TNF-α may contribute to the manifestation of severe inflammation in perforin−/−DC-Fas−/− mice. IFN-γ has been shown to induce the hemophagocytic activities of macrophages and cause acute cytopenia and hemophagocytosis.39,47 Elevated IFN-γ probably contributes to anemia in perforin−/−DC-Fas−/− mice. Induction of IFN-γ–dependent inflammation has been reported in LCMV-infected perforin−/− mice, SOCS-1−/− mice, CD70 transgenic mice, and acute graft-versus-host reactions.38,50-52 This suggests the convergence of downstream inflammation events induced by different initiating mechanisms.
Perforin−/−DC-Fas−/− mice showed significant overactivation of CD8+ T cells, whereas CD4+ T cells were affected to a lesser extent. CD8+ T cells were far more efficient than CD4+ T cells in the killing of antigen-bearing DCs. This is potentially because of the ability of CD8+ T cells to produce more perforin than CD4+ T cells.53 Therefore, CD8+ T cells may be more critical for the down-regulation of DCs during T-DC interactions. It has been shown that infection of perforin−/− mice with LCMV also leads to IFN-γ–dependent inflammatory responses driven by persistent presentation of antigens to stimulate CD8+ T cells.38,39 The killing of antigen presenting cells by CD8+ T cells may be defective in this infection model, resulting in increased antigen presentation and T-cell activation.
Although preactivated NK cells were capable of killing DCs through Fas and perforin, deletion of NK cells did not prevent lethal inflammation in perforin−/−DC-Fas−/− mice (supplemental Figure 4). Why NK cells are not required for the induction of inflammation in perforin−/−DC-Fas−/− mice is unclear. One possibility is that DCs alone are not sufficient for the activation of NK cells. Consequently, abnormal accumulation of DCs may not have a salient effect on NK cells to induce inflammation. In this regard, we did not detect significantly increased production of IFN-γ by NK cells in perforin−/−DC-Fas−/− mice (Figure 3B). Alternatively, the effect of CD8+ T cells on DC homeostasis may be dominant and mask the effect of NK cells.
Perforin-deficient mice have the potential to develop hemophagocytic lymphohistiocytosis, but an additional trigger, such as LCMV infection,38 is required. Although defective perforin-dependent cytotoxic function is probably to be responsible for the disease manifestation in this model, it is possible that the disease progression is caused by the inability of cytotoxic T cells to remove virally infected cells, leading to persistent presence of antigen and antigen-driven activation of macrophages. Alternatively, HLH may be caused by the inability of cytotoxic T cells to clear antigen-presenting cells, leading to persistent antigen presentation, prolonged T-cell activation and uncontrolled cytokine production. In our mouse model, Fas deficiency in DCs serves as an additional trigger for HLH. Perforin−/−DC-Fas−/− mice therefore provide an in vivo model to support the hypothesis that defective killing of DCs plays a prominent role in the onset of HLH-like diseases.
Our results suggest a hierarchy model for the induction of inflammation. Defects in perforin and Fas-dependent killing of DCs may lead to DC accumulation and chronic activation of CD8+ T cells. The production of an excessive amount of IFN-γ by CD8+ T cells may then trigger the secretion of different cytokines by macrophages and other cell types. Neutralization of the initiating inflammatory cytokine may prevent the production of other inflammatory cytokines and widespread inflammatory responses. It is probably important to identify the initiating cell type or initiating cytokines in inflammatory disorders. Targeting such inflammatory cytokines and initiating cell type may be most effective in preventing the onset of uncontrolled inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Y. Zhang, S. Bai, and Y. Shi in their laboratory for technical assistance.
This work was supported by National Institutes of Health grants R01AI074949, R01 GM087710 (J.W.), and R01DK083164 (M.C.).
National Institutes of Health
Authorship
Contribution: M.C. and J.W. designed and performed experiments and wrote the paper; and K.F. performed tissue sectioning and H&E staining.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jin Wang, Department of Pathology and Immunology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030; e-mail: jinwang@bcm.edu; and Min Chen, Department of Pathology and Immunology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030; e-mail: minc@bcm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal