Abstract
Although well recognized that expression of E-selectin on marrow microvessels mediates osteotropism of hematopoietic stem/progenitor cells (HSPCs), our knowledge regarding the cognate E-selectin ligand(s) on HSPCs is incomplete. Flow cytometry using E-selectin-Ig chimera (E-Ig) shows that human marrow cells enriched for HSPCs (CD34+ cells) display greater E-selectin binding than those obtained from mouse (lin−/Sca-1+/c-kit+ [LSK] cells). To define the relevant glycoprotein E-selectin ligands, lysates from human CD34+ and KG1a cells and from mouse LSK cells were immunoprecipitated using E-Ig and resolved by Western blot using E-Ig. In both human and mouse cells, E-selectin ligand reactivity was observed at ∼ 120- to 130-kDa region, which contained two E-selectin ligands, the P-selectin glycoprotein ligand-1 glycoform “CLA,” and CD43. Human, but not mouse, cells displayed a prominent ∼ 100-kDa band, exclusively comprising the CD44 glycoform “HCELL.” E-Ig reactivity was most prominent on CLA in mouse cells and on HCELL in human cells. To further assess HCELL's contribution to E-selectin adherence, complementary studies were performed to silence (via CD44 siRNA) or enforce its expression (via exoglycosylation). Under physiologic shear conditions, CD44/HCELL-silenced human cells showed striking decreases (> 50%) in E-selectin binding. Conversely, enforced HCELL expression of LSK cells profoundly increased E-selectin adherence, yielding > 3-fold more marrow homing in vivo. These data define the key glycoprotein E-selectin ligands of human and mouse HSPCs, unveiling critical species-intrinsic differences in both the identity and activity of these structures.
Introduction
A greater understanding of the molecular effector(s) directing trafficking and engraftment of hematopoietic stem/progenitor cells (HSPCs) into bone marrow is critical for optimizing therapeutic outcomes of hematopoietic stem cell transplantation. It is well established that homing of HSPCs into marrow is a conspicuously nonrandom process regulated by discrete adhesive interactions between HSPCs in blood flow and target bone marrow endothelium.1-4 Studies in both humans and mice indicate that E-selectin is constitutively expressed on marrow endothelial cells,5,6 and intravital studies have revealed that HSPC migration to marrow occurs at specialized microvascular beds expressing E-selectin.2 These and several other independent lines of evidence3,4,7-9 have highlighted a central role for E-selectin–dependent interactions in HSPC recruitment to marrow. However, our knowledge of the cognate E-selectin coreceptors expressed on human and mouse HSPCs remains incomplete.
E-selectin, together with L- and P-selectin, comprise the selectin family of adhesion molecules, which are cell membrane calcium-dependent lectins that function as principal regulators of Step 1 rolling interactions that are requisite for tissue-specific cell migration.10 All 3 selectins bind to specialized carbohydrate determinants, composed of sialofucosylations containing an α2,3-linked sialic acid substitution on galactose, and an α1,3-linked fucose modification on N-acetylglucosamine, prototypically displayed as the terminal tetrasaccharide sialyl Lewis X (sLex; also known as CD15s).10 This structure is recognized by monoclonal antibodies (mAbs), such as HECA-452 and CSLEX-1.7,11,12 Although each of the 3 selectins binds to sLex, additional structural modifications, principally involving sulfation, increase binding affinity of P- and L-selectin to sLex, whereas no such modifications are needed for optimal binding of E-selectin.13,14 Importantly, enforced expression of sLex on cell surface CD44 by ex vivo glycan engineering licenses homing of human mesenchymal stem cells to marrow,9 providing direct evidence of the importance of sLex-dependent E-selectin receptor/ligand interactions in mediating osteotropism. Although sLex can be displayed on cell surfaces via a protein scaffold (ie, a glycoprotein) or a lipid scaffold (ie, a glycolipid), several studies have shown that membrane glycoproteins serve more effectively as E-selectin ligands under physiologically relevant flow conditions because of the greater extension of glycoprotein-based sLex beyond the glycocalyx.9,15-17
In contrast to limited information regarding the E-selectin ligands of HSPCs, the glycoprotein E-selectin ligands expressed on mouse and human leukocytes have been extensively characterized. These studies have revealed a rather bewildering array of E-selectin ligands, many of which have not been studied on HSPCs. For example, L-selectin has been reported to serve as an E-selectin ligand on human neutrophils but not lymphocytes, yet it does not appear to serve as an E-selectin ligand on mouse leukocytes,18 and its contribution(s) to E-selectin ligand activity of mouse or human HSPCs has not been determined. E-selectin ligand-1 (ESL-1) has been described to be an E-selectin ligand on mouse neutrophils,15 but it is unknown whether it plays such a role on HSPCs. The leukocyte β2 integrins LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) carry sLex,19 and biochemical assays have revealed that they can serve as E-selectin ligands on human leukocytes.20 CD43 has also been implicated to be an E-selectin ligand on mouse neutrophils and activated T cells,21,22 and we have recently reported that CD43 from cutaneous lymphocyte antigen-positive (CLA+) human T cells bears E-selectin, but not P-selectin, ligand activity.23 However, it is unknown whether CD43 is an E-selectin ligand on either human or mouse HSPCs.
To date, two well-described glycoprotein E-selectin ligands are known to be expressed among human hematopoietic progenitors: a HECA-452–reactive glycoform of P-selectin glycoprotein ligand-1 (PSGL-1) known as CLA12 and a specialized HECA-452–reactive glycoform of CD44 termed HCELL.7,24,25 CLA is also expressed on leukocytes (including dermatotrophic lymphocytes) in mice and humans, but expression of HCELL is restricted to hematopoietic progenitors.1 All human cells that express HCELL, including native HSPCs,7,24,25 de novo acute myeloid leukemia (AML) cells,7,24,25 the AML-derived primitive human hematopoietic progenitor cell line KG1a,7,24,25 and G-CSF–mobilized peripheral blood leukocytes,26 display higher binding to E-selectin than cells that lack HCELL7,24-26 ; however, it is unclear whether this property is the result of HCELL per se or other presently uncharacterized E-selectin ligands that are coexpressed with HCELL. In mouse HSPCs, the only E-selectin ligand described at present is the CLA-type glycoform of PSGL-1. Studies in mice genetically deficient in PSGL-1 expression have shown that CLA contributes approximately 60% of the E-selectin–mediated homing activity to marrow,27 indicating that, although CLA serves as the dominant E-selectin ligand, other functional E-selectin ligands exist on mouse HSPCs. Notably, no studies to date have examined the operational contribution(s) of non-CLA glycoprotein E-selectin ligand(s) on murine HSPCs, and our knowledge of the relative contribution(s) of the human HSPC E-selectin ligands to E-selectin adherence is similarly incomplete. Accordingly, we sought to fully characterize the glycoprotein E-selectin ligands expressed on both mouse and human HSPCs. Collectively, the results of our biochemical studies and functional assays reveal a previously unsuspected role for CD43 as an E-selectin ligand on marrow cells from both human and mouse enriched for HSPCs, and highlight the unique and prominent role of HCELL in mediating E-selectin binding on human cells. Our findings thus offer novel perspectives on the functional and structural diversity of the molecular effectors mediating osteotropism of human and mouse HSPCs.
Methods
Cells, antibodies, and reagents
All human cells were obtained and used in accordance with the procedures approved by the Human Experimentation and Ethics Committees of Partners Cancer Care Institutions (Massachusetts General Hospital, Brigham and Women's Hospital, and Dana-Farber Cancer Institute). Human peripheral blood mononuclear cells (PBMCs) were prepared from whole blood using established methods.24,25 Bone marrow (BM) cells were isolated from filter sets of BM harvests performed at the Massachusetts General Hospital and Brigham and Women's Hospital. BM mononuclear cells (BM-MNCs) were isolated by Ficoll-Hypaque density separation, and human cells enriched for HSPCs (CD34+ cells) were collected by positive selection using anti-CD34 immunomagnetic beads (Miltenyi Biotec).
For isolation of mouse marrow cells enriched for HSPCs (lin−sca-1+c-kit+ cells, [LSK]), 6- to 8-week-old C57BL/6 (The Jackson Laboratory) mice were killed (in accordance with the National Institutes of Health guidelines for the care and use of animals and under approval of the Institutional Animal Care and Use Committees of Harvard Medical School), and the femurs and tibia were harvested. The bone marrow was flushed, and single-cell suspensions were made. Red blood cells were cleared by use of red blood cell lysing buffer (Sigma-Aldrich). Cells were then washed and filtered through a 100-μm cell strainer (BD Falcon) before lineage depletion using the Lineage Cell Depletion Kit from Miltenyi Biotec, and magnetic separation using the autoMACS Separator (Miltenyi Biotec). After depletion, the cells were then positively selected for c-kit using CD117 MicroBeads (Miltenyi Biotec), and selected cells were then selected for Sca-1 expression using Anti-Sca-1(FITC) MicroBead Kit (Miltenyi Biotec). Confirmation of doubly positive c-Kit (phycoerythrin [PE]) and Sca-1 (FITC) mouse cells consistently showed > 98% positive cells by flow cytometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
KG1a, a human AML-derived HSPC-like CD34+ cell line (ATCC), and Chinese hamster ovary cells transfected with full-length E-selectin (CHO-E) or mock-transfected (CHO-M) were maintained as described previously.7,9,25
The following antibodies were purchased from BD Biosciences PharMingen: function blocking mouse anti–human E-selectin (68–5H11; IgG1), rat anti–human sLex (HECA-452; IgM), rat anti–mouse PSGL-1 (2PH1 and 4RA10; IgG1), mouse anti–human PSGL-1 (KPL-1; IgG1), rat anti–mouse CD44 (KM114 and IM7; IgG1), mouse anti–human CD44 (515; IgG1), rat anti–mouse CD43 (S7; IgG2a), mouse anti–human CD43 (1G10; IgG1), rat anti–mouse CD11a (2D7; IgG2a), rat anti–mouse CD11b (M1/70; IgG2b), rat anti–mouse CD18 (GAME-46; IgG1), rat anti–human CD29 (mAb 13; IgG2a), mouse anti–human CD49b (12F1; IgG2a), rat anti–mouse CD62L (MEL-14; IgG2a), mouse anti–human CD62L (DREG-56; IgG1), mouse IgG1 κ isotype, mouse IgG2a isotype, mouse IgM isotype, rat IgG isotype, rat IgM isotype, and human IgG1 isotype. The following secondary antibodies were also purchased from BD Biosciences PharMingen: PE streptavidin, biotin anti–rat IgM, and goat anti–mouse Ig-horseradish peroxidase (HRP). Mouse anti–human CD49 days (HP-2/1; IgG1), mouse anti–human CD11a (25.3; IgG1), mouse anti–human CD11b (BEAR1; IgG1), and mouse anti–human CD18 (7E4; IgG1) were purchased from Beckman Coulter. Anti–human L-selectin mAb (LAM 1–116) was a gift from Dr T.F. Tedder (Duke University, Durham, NC). Recombinant mouse E-selectin/human Ig chimera (E-Ig) and mouse anti–human CD44 (2C5; IgG1) were purchased from R&D Systems. Rat anti–mouse CD43 (1B11; IgG2a) was purchased from BioLegend. The following secondary antibodies were purchased from Southern Biotechnology: rabbit anti–human IgG-biotin, goat anti–mouse IgM-PE, goat anti–rat IgG-PE, goat anti–mouse Ig-biotin, goat anti–rat Ig-HRP, and goat anti–human Ig-HRP.
Vibrio cholerae neuraminidase, which cleaves sialic acid in α(2-3,6,8)-linkage, was purchased from Roche Diagnostics. O-sialoglycoprotein endopeptidase (OSGE) was from Accurate Chemical and Scientific.
Flow cytometry
Aliquots of cells (2 × 105) were washed with PBS/2% FBS and incubated with primary mAbs or with isotype control mAbs (either unconjugated or fluorochrome conjugated). The cells were washed in PBS/2% FBS and, for indirect immunofluorescence, incubated with appropriate secondary fluorochrome-conjugated antibody to isotype antibodies. After washing cells, fluorescence intensity was determined using a Cytomics FC 500 MPL flow cytometer (Beckman Coulter). For E-Ig (10 μg/mL) staining, chimera buffer (Hanks balanced salt solution [HBSS]/5mM HEPES/2mM CaCl2/5% FBS) was used for all incubations, dilutions, and washing. The secondary antibodies used were a biotinylated antihuman IgG (1:200 dilution in chimera buffer) followed by streptavidin-PE (5 μg/mL) staining in chimera buffer. As a control for E-Ig staining, 20mM EDTA was added to the chimera buffer.
IP studies
Human CD34+ and KG1a cells and mouse LSK cells were lysed using 2% NP-40 in 150mM NaCl, 50mM Tris-HCI, pH 7.4, 20 μg/mL PMSF, 0.02% sodium azide, and protease inhibitor cocktail tablet (Roche Molecular Biochemicals). For immunoprecipitation (IP) using E-Ig, lysis buffer contained 2mM CaCl2; otherwise, 1mM EDTA was added to all lysates. Lysates were cleared by centrifugation at 14 000g, then precleared by incubation with protein G-agarose (Invitrogen) for 1 hour at 4°C. Lysates were then incubated with antibodies or E-Ig (each at 3 μg) for 2 hours at 4°C, followed by incubation with protein G-agarose beads for 2 hours at 4°C under constant rotation. Beads were washed with detergent lysis buffer (containing 2% NP-40/1% SDS) and then pelleted by centrifugation at 14 000g, diluted in reducing sample buffer, and boiled. Agarose beads were centrifuged, and the supernatant (released protein) was subjected to SDS-PAGE and transferred to PVDF membrane. The blots were immunostained with the specified antibodies or recombinant protein (E-Ig). For comparison of E-selectin ligand contribution(s) of PSGL-1, CD43, and CD44 within a given cell population, exhaustive IPs were performed for each product within a respective lysate. Supernatants from each round of antibody-lysate incubation were reincubated with the subsequent antibody in the series (3 μg of antibody in each of 3 rounds) and IPs from each round were prepared as described earlier in this paragraph. Between each antibody treatment, 2 rounds of protein G-agarose beads were used to remove any potential antibody residuals from the previous IP treatment; Western blot of these samples yielded no signal with any antibody sequences tested, suggesting that the prior IP was complete with no residual antibody. In addition, no detectable signal for PSGL-1, CD43, CD44, HECA-452, or E-Ig was observed in the lysate after the removal of all 3 glycoproteins, suggesting that each IP was performed to completion and that no other glycoprotein(s) contributed to E-selectin ligand activity in these immunoprecipitated samples.
Western blot analysis
For SDS-PAGE and Western blots of quantified protein lysates or of immunoprecipitated protein, samples were diluted in reducing sample buffer, boiled, and then separated on 4% to 20% or 7.5% Criterion Tris-HCl SDS-PAGE gels (Bio-Rad) as described previously.7 SDS-PAGE–resolved proteins were transferred to Sequi-blot PVDF membrane (Bio-Rad), and the membrane was blocked with milk/Tris-buffered saline. Membranes were then immunostained with primary antibodies of interest followed by incubation with appropriate HRP- or AP-conjugated secondary antibodies. Blots were visualized with chemiluminescence using Lumi-Light Western Blotting Substrate (Roche Diagnostics) for HRP or with Western Blue AP substrate (Promega) for AP.
Lentiviral CD44 siRNA transduction
CD44/HCELL expression in KG1a cells was silenced using lentiviral CD44 siRNA constructs as previously described.28 After lentiviral infection with siRNA, vector alone, or a scrambled sequence, cells were selected with puromycin. CD44/HCELL expression in all cells was monitored routinely. Cells transduced with the scrambled sequence or vector alone were equivalent in terms of expression of all surface molecules tested and adhesion to all substrates tested; results are reported for scrambled sequence-transduced cells as the negative control for all experiments.
HA binding assay
To analyze CD44 binding to hyaluronic acid (HA), KG1a adhesion to immobilized HA (rooster comb, Sigma-Aldrich) was performed in multiwell plates as described previously.29
Blot rolling assay
The blot rolling assay was used to detect selectin-binding activity of membrane proteins of KG1a cells, human CD34+ cells, and mouse LSK cells resolved by SDS-PAGE. This assay has been described previously.30,31 Briefly, Western blots of immunoprecipitated CD44, CD43, and PSGL-1 membrane proteins were stained with anti-CLA (HECA-452), and the membrane was rendered translucent by immersion in DMEM with 10% glycerol. CHO-E cells were resuspended (5 × 106/mL) in DMEM containing 2mM CaCl2 and 10% glycerol. The blots were placed within a parallel plate flow chamber, and CHO-E cells were perfused at a physiologically relevant shear stress of 1.0 dyn/cm2, with an adjustment in the volumetric flow rate made to account for the increase in viscosity because of the presence of 10% glycerol in the flow medium. Molecular weight markers were used as guides to aid placement of the flow chamber over stained bands of interest. The number of interacting cells per square millimeter was tabulated as a function of the molecular weight region and compiled into an adhesion histogram. Nonspecific adhesion was assessed by perfusing CHO-E cell suspensions containing 5mM EDTA (which eliminates divalent cation-dependent E-selectin adhesion) in the flow medium. CHO-M were used to assess nonspecific adhesive interactions of CHO cells on each glycoprotein studied; the observed binding interactions were used to establish the background levels of binding, and only binding above this threshold was reported for CHO-E cells.
Competitive short-term homing studies of mouse LSK cells after exofucosylation to enforce cell surface HCELL expression
Enforced exofucosylation of murine LSK cells was performed by glycosyltransferase-programmed stereosubstitution (GPS) using fucosyltransferase VI (FTVI) and donor nucleotide sugar (GDP-fucose) as previously described.9 Briefly, mouse LSK cells were washed with HBSS, counted, and resuspended in 60 mU/mL FTVI in HBSS (without Ca2+ or Mg2+) containing 20mM HEPES, 0.1% human serum albumin, and 1mM GDP-fucose for 40 minutes at 37°C. These FTVI-treated cells are referred to as GPS-LSKs. For control, LSK cells were resuspended in the same HBSS buffer without the FTVI enzyme (buffer-treated LSK [BT-LSK]). Mouse BT-LSK and GPS-LSK were labeled with either 5mM CFDA-SE (Invitrogen) or Cell Tracker Orange (CTO) for 5 minutes at room temperature in RPMI containing 10% FBS and injected intravenously into C57BL6. A total of 1 million BT-LSK + 1 million GPS-LSK in a volume of 0.2 mL of HBSS were injected into each mouse. HBSS buffer alone was used to determine background signals. For each experiment, each cell type was treated with reciprocal dyes: in one set of experiments, recipient mice received 1 × 106 BT-LSK (CFDA-SE) in competition with 1 × 106 GPS-LSK (CTO), whereas in another set of experiments recipient mice received 1 × 106 BT-LSK (CTO) in competition with 1 × 106 GPS-LSK (CFDA-SE). Sixteen hours after LSK transfer, mice were killed and perfused with 1× PBS without Ca2+ and Mg2+. The bone marrow was harvested, and single-cell suspensions were assessed for frequencies of CFDA-SE and CTO-positive cells by flow cytometry. Flow cytometric data were analyzed and expressed as percentage of dye-positive events detected in 200 000 cells scanned within a narrow gate that is set to include LSK cells. This gate was determined based on mixing dye-labeled mouse LSK with suspensions of cells isolated from bone marrow of C57BL/6 mice.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical significance of differences between means was determined by one-way analysis of variance. If means were shown to be significantly different, multiple comparisons were performed post-hoc by the Tukey t test. Statistical significance was defined as P < .05.
Results
Species-specific differences in expression of glycoprotein E-selectin ligands among marrow cells enriched for HSPCs
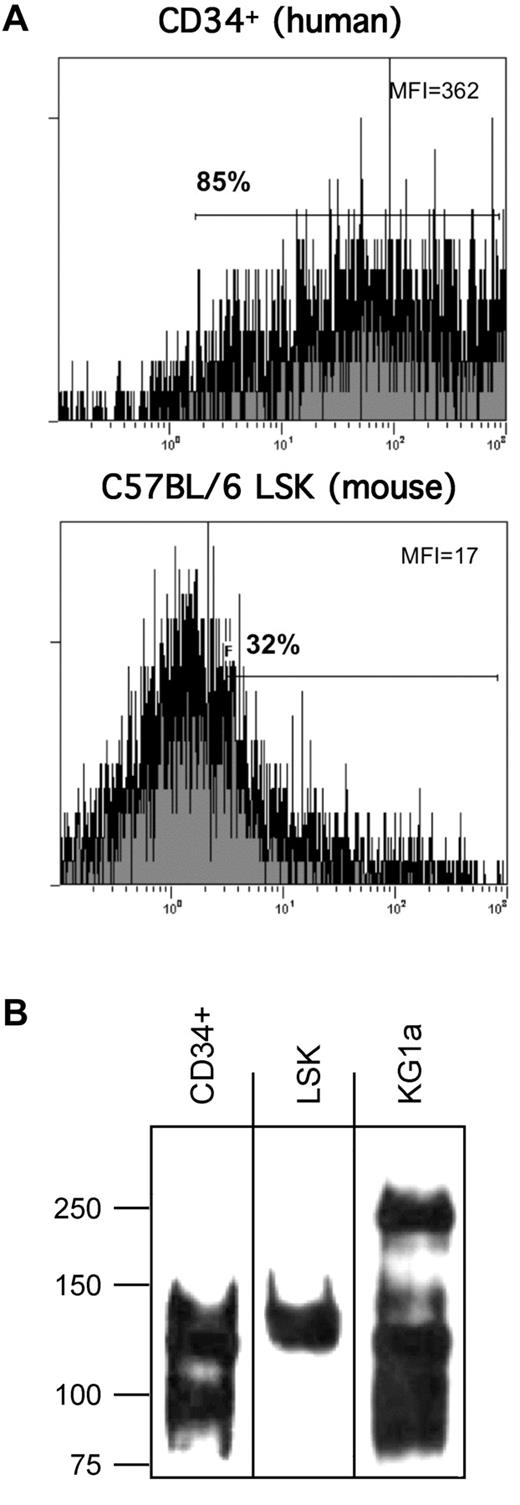
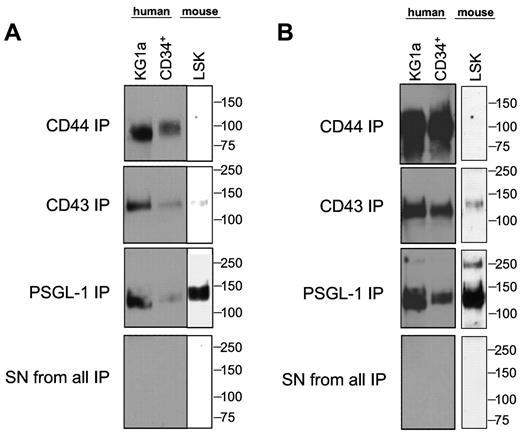
Using E-Ig as a probe in flow cytometry, we consistently observed that human CD34+ cells express higher E-selectin ligand expression than murine LSK cells (Figure 1A). Based on this observed difference, flow cytometry was performed to assess whether mouse and human cells differ in expression of scaffolding proteins reported to display relevant sialofucosylated E-selectin binding determinants: PSGL-1, CD44, CD43, ESL-1, L-selectin, MAC-1 (CD11b/CD18), and LFA-1 (CD11a/CD18). As shown in Table 1, marrow cells enriched for HSPC from both human and mouse express CD43, CD44, PSGL-1, L-selectin, CD11a, and CD18, and neither human nor mouse cells express ESL-1 and MAC-1. To define the relevant glycoprotein E-selectin ligands expressed on human and mouse cells, lysates of human CD34+ cells, mouse LSK cells, and of the primitive human hematopoietic cell line KG1a, were immunoprecipitated using E-Ig resolved under reducing conditions by SDS-PAGE, and then exposed by Western blot using E-Ig. As shown in Figure 1B, native human CD34+ cells, as well as KG1a cells, express prominent E-Ig reactive bands at ∼ 100 kDa and at ∼ 120 to 130 kDa, whereas mouse LSK cells express E-Ig reactive bands only at ∼ 120 to 130 kDa. This molecular weight migration profile is not typical of L-selectin (MW ∼ 70-100 kDa) nor of the α-chain of LFA-1 (CD11a, MW ∼ 180 kDa) but is consistent with that of several characterized E-selectin ligands, including CD43 (MW ∼ 130 kDa), the monomer form (ie, reduced heterodimer) of PSGL-1 (MW ∼ 120-130 kDa), and CD44 (MW ∼ 100 kDa). Accordingly, to further characterize the E-selectin ligands migrating at these MW ranges, we exhaustively immunoprecipitated lysates of human and murine cells using antibodies to CD43, PSGL-1, and CD44, and then probed for E-Ig and HECA-452 reactivity by Western blot. As illustrated in Figure 2, the major E-Ig– and HECA-452–reactive species in human CD34+ cells and KG1a cells is at ∼ 100 kDa, and this band entirely consists of the CD44 glycoform known as HCELL. CD44 immunopreciptated from mouse LSK lysates was not HECA-452 nor E-Ig reactive showing that HCELL is not an E-selectin ligand on mouse cells (Figure 2). However, within the range of ∼ 120 to 130 kDa, CD43 and PSGL-1 each contribute as glycoprotein E-selectin ligands on human and mouse cells. Notably, in mouse LSK cells, E-Ig and HECA-452 reactivity was primarily observed on PSGL-1 (CLA) with a minor contribution provided by CD43. Importantly, in both human and mouse cell lysates, 2 rounds of protein G-agarose clearance after each antibody treatment, with subsequent Western blot of these protein G samples, showed no residual antibody signal indicating that all immunoprecipitates were collected in the initial protein G treatment. In addition, no detectable signal for PSGL-1, CD43, and CD44 was observed in any lysates after the removal of all 3 glycoproteins, further indicating that each immunoprecipitation was performed to completion. Notably, after immunoprecipitation of the 3 molecules PSGL-1, CD43, and CD44, there was no residual HECA-452 and E-Ig reactivity in respective lysates, indicating that no detectable glycoprotein(s) other than CLA (PSGL-1) and CD43 in mouse LSK cells, and other than CLA (PSGL-1), CD43, and CD44 in human CD34+ cells, contributed to E-selectin ligand activity (Figure 2; supplemental Figure 2). Moreover, in each case, immunoprecipitates of L-selectin and CD11a from lysates of mouse and human cells did not stain with E-Ig, indicating that neither L-selectin nor LFA-1 serves as E-selectin ligands on either mouse or human marrow cells enriched for HSPCs (supplemental Figure 3).
Analysis of E-selectin ligand expression on human CD34+ cells and mouse LSK cells. (A) Representative flow cytometric histograms of E-Ig staining of mouse LSK cells and human CD34+ cells. (B) E-Ig was used to immunoprecipitate E-selectin ligands from cell lysates of human CD34+ cells and mouse LSK cells. SDS-PAGE–resolved material was subsequently immunoblotted with E-Ig.
Analysis of E-selectin ligand expression on human CD34+ cells and mouse LSK cells. (A) Representative flow cytometric histograms of E-Ig staining of mouse LSK cells and human CD34+ cells. (B) E-Ig was used to immunoprecipitate E-selectin ligands from cell lysates of human CD34+ cells and mouse LSK cells. SDS-PAGE–resolved material was subsequently immunoblotted with E-Ig.
Expression of proteins* reported to serve as E-selectin ligands on mouse and human HSPC-enriched cells
| . | CD43 . | CD44 . | PSGL-1 . | L-selectin . | ESL-1 . | CD11a . | CD11b . | CD18 . |
|---|---|---|---|---|---|---|---|---|
| Mouse LSK | ++++ | ++++ | ++++ | +++ | − | +++ | − | +++ |
| Human CD34+ | ++++ | ++++ | +++ | ++ | − | +++ | − | +++ |
| . | CD43 . | CD44 . | PSGL-1 . | L-selectin . | ESL-1 . | CD11a . | CD11b . | CD18 . |
|---|---|---|---|---|---|---|---|---|
| Mouse LSK | ++++ | ++++ | ++++ | +++ | − | +++ | − | +++ |
| Human CD34+ | ++++ | ++++ | +++ | ++ | − | +++ | − | +++ |
Flow cytometric analysis of CD43, CD44, PSGL-1, L-selectin, ESL-1, CD11a, CD11b, and CD18 on human CD34+ cells and mouse LSK cells from freshly harvested bone marrow. All results displayed are representative of n = 5 flow cytometric experiments performed on both human (CD34+) and mouse (LSK) HSPCs.
− indicates 0%-5% positive; +, 6%-35% positive; ++, 36%-65% positive; +++, 66%-95% positive; and ++++, ≥ 96% positive.
Percentage of positive cells as determined by flow cytometric analysis for the specific proteins expressed above their respective isotype controls on mouse LSK cells and human CD34+ cells from freshly harvested bone marrow. All results are representative of n = 3 flow cytometric experiments.
HCELL is the predominant E-selectin ligand on human CD34+ cells, whereas CLA is the predominant E-selectin ligand on mouse LSK cells. Western blot analysis of CD44, CD43, and PSGL-1 immunoprecipitated from cell lysates of human KG1a cells, human CD34+ cells, and mouse LSK cells, stained with E-Ig (A) and HECA-452 mAb (B). As shown here, whole cell lysates of human and mouse cells were initially exhaustively immunoprecipitated using mAb directed to PSGL-1; after clearance of PSGL-1, the residual lysate was exhaustively immunoprecipitated with anti-CD43 mAb and then followed by anti-CD44 mAb. Similar results were obtained when the order of the glycoproteins immunoprecipitated was varied. No detectable signal for PSGL-1, CD43, or CD44 was observed in lysate(s) after removal of the target glycoprotein(s) indicating that immunoprecipitation depleted the respective glycoprotein(s) from lysate(s). The absence of residual HECA-452 and E-Ig reactivity after exhaustive immunoprecipitation indicates that no other glycoprotein(s) appear to be detected as E-selectin ligands within the respective HSPC-enriched populations. Data shown in supplemental Figure 2 corroborate this conclusion. Results shown are representative of 6 separate experiments.
HCELL is the predominant E-selectin ligand on human CD34+ cells, whereas CLA is the predominant E-selectin ligand on mouse LSK cells. Western blot analysis of CD44, CD43, and PSGL-1 immunoprecipitated from cell lysates of human KG1a cells, human CD34+ cells, and mouse LSK cells, stained with E-Ig (A) and HECA-452 mAb (B). As shown here, whole cell lysates of human and mouse cells were initially exhaustively immunoprecipitated using mAb directed to PSGL-1; after clearance of PSGL-1, the residual lysate was exhaustively immunoprecipitated with anti-CD43 mAb and then followed by anti-CD44 mAb. Similar results were obtained when the order of the glycoproteins immunoprecipitated was varied. No detectable signal for PSGL-1, CD43, or CD44 was observed in lysate(s) after removal of the target glycoprotein(s) indicating that immunoprecipitation depleted the respective glycoprotein(s) from lysate(s). The absence of residual HECA-452 and E-Ig reactivity after exhaustive immunoprecipitation indicates that no other glycoprotein(s) appear to be detected as E-selectin ligands within the respective HSPC-enriched populations. Data shown in supplemental Figure 2 corroborate this conclusion. Results shown are representative of 6 separate experiments.
Sialofucosylated glycans conferring E-selectin binding to CD43 on human CD34+ cells and mouse LSK cells are clustered on mucin-like O-linked glycans
The E-selectin binding determinants of CD44 on human cells7 and of PSGL-1 on human and mouse cells are well characterized.27,32,33 Recent studies have shown that CD43 is an E-selectin ligand on neutrophils and T cells21-23 and that this property is the result of expression HECA-452–reactive sialofucosylated determinants presented on O-glycans of the core protein. To determine whether O-glycans similarly direct CD43 E-selectin ligand activity on enriched HSPC populations from human and mouse marrow, CD43 was immunoprecipiated and then treated with neuraminidase or with the enzyme OSGE and analyzed by Western blot using mAb HECA-452. As shown in Figure 3, HECA-452 reactivity of CD43 from both human and mouse cells is eliminated after neuraminidase digestion and by OSGE digestion. These findings indicate that, in contrast to HCELL, which displays HECA-452–reactive selectin ligand determinants on N-glycans,7 CD43, like PSGL-1/CLA, presents relevant sLex-like determinants as O-linked clusters (ie, on mucin-like O-linked glycans)34,35 (Figure 2).
CD43 on native human CD34+ and mouse LSK cells displays sialofucosylated O-glycans. CD43 was immunoprecipitated from membrane preparations of human CD34+ cells and mouse LSK cells, followed by treatment with enzymes as shown. Respective immunoprecipitates, normalized in each case for amount of input protein, were resolved on 4% to 20% SDS-PAGE, blotted, and stained with mAb HECA-452. Absence of staining after neuraminidase digestion confirms that ∼ 130 kDa CD43 is decorated with sialic acid. Abrogation of HECA-452 reactivity after OSGE digestion demonstrates that sialofucosylations of CD43 on respective human and mouse cells are displayed predominantly on O-linked glycans. Results are representative of at least 3 separate experiments.
CD43 on native human CD34+ and mouse LSK cells displays sialofucosylated O-glycans. CD43 was immunoprecipitated from membrane preparations of human CD34+ cells and mouse LSK cells, followed by treatment with enzymes as shown. Respective immunoprecipitates, normalized in each case for amount of input protein, were resolved on 4% to 20% SDS-PAGE, blotted, and stained with mAb HECA-452. Absence of staining after neuraminidase digestion confirms that ∼ 130 kDa CD43 is decorated with sialic acid. Abrogation of HECA-452 reactivity after OSGE digestion demonstrates that sialofucosylations of CD43 on respective human and mouse cells are displayed predominantly on O-linked glycans. Results are representative of at least 3 separate experiments.
Differential E-selectin ligand activity of CLA and CD43 on human CD34+ cells and mouse LSK cells under physiologic flow conditions
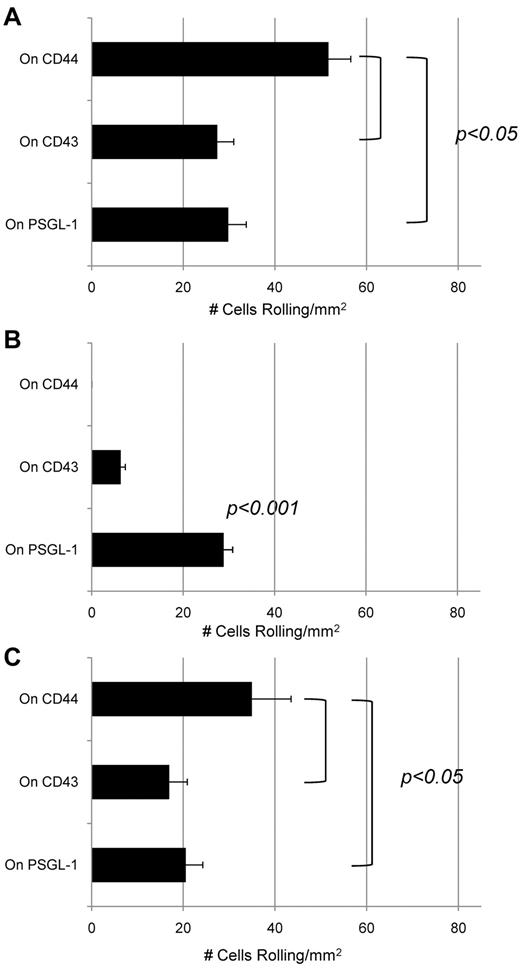
To examine whether CD43 and CLA on mouse LSK cells and human CD34+ cells display functional E-selectin ligand activity under physiologic flow conditions, we performed blot rolling assays30,31 on HECA-452–stained blots of CD43 and PSGL-1 immunoprecipitated from lysates of these cells and of human KG1a cells. As shown previously for HCELL by blot rolling assay,7,26 E-selectin transfected CHO cells (CHO-E) displayed rolling interactions on CD43 and PSGL-1 (CLA) immunoprecipitated from human CD34+ cells (Figure 4A). In contrast, only CLA supported avid CHO-E cell rolling on blot rolling assay of mouse LSK cells (Figure 4B). Blot rolling assay performed on immunoprecipitated CD43 and PSGL-1 (CLA) from KG1a cells revealed that E-selectin ligand activity was reproducibly observed on both CD43 and CLA (Figure 4C).7 Specificity for E-selectin binding was confirmed by the observed elimination of binding in presence of EDTA and by treatment of CHO-E cells with a function-blocking mAb to E-selectin (data not shown).
CD43 is a functional E-selectin ligand on human CD34+ cells but not on mouse LSK cells. Blot rolling assays were performed on immunoprecipitates of CD44, CD43, and PSGL-1 from human CD34+ and mouse LSK cells, and from KG1a cells. In each case, immunoprecipitated protein was resolved by SDS-PAGE and blotted for HECA-452 reactivity. CHO-E cells were perfused over immunoprecipitated glycoproteins from (A) human CD34+ cells, (B) mouse LSK cells, or (C) KG1a cells at 0.6 dyne/cm2. After cell perfusion, the numbers of rolling cells/mm2 in 4 distinct fields of view were counted. To control for specificity of CHO-E binding to membrane glycoproteins, EDTA was added to the buffer containing CHO-E cells before use in adhesion assays; no cells bound in the presence of EDTA (data not shown). Nonspecific adhesion was assessed by perfusing mock-transfected CHO cells (CHO-M) over the same region of the blot (at CD44, CD43, and PSGL-1). Low levels of binding were observed using CHO-M cells, and these values were subtracted from the values measured using CHO-E cells. Results shown reflect multiple assays (n = 4) on HECA-452 blots of multiple (n = 3) membrane preparations. Data are mean ± SEM.
CD43 is a functional E-selectin ligand on human CD34+ cells but not on mouse LSK cells. Blot rolling assays were performed on immunoprecipitates of CD44, CD43, and PSGL-1 from human CD34+ and mouse LSK cells, and from KG1a cells. In each case, immunoprecipitated protein was resolved by SDS-PAGE and blotted for HECA-452 reactivity. CHO-E cells were perfused over immunoprecipitated glycoproteins from (A) human CD34+ cells, (B) mouse LSK cells, or (C) KG1a cells at 0.6 dyne/cm2. After cell perfusion, the numbers of rolling cells/mm2 in 4 distinct fields of view were counted. To control for specificity of CHO-E binding to membrane glycoproteins, EDTA was added to the buffer containing CHO-E cells before use in adhesion assays; no cells bound in the presence of EDTA (data not shown). Nonspecific adhesion was assessed by perfusing mock-transfected CHO cells (CHO-M) over the same region of the blot (at CD44, CD43, and PSGL-1). Low levels of binding were observed using CHO-M cells, and these values were subtracted from the values measured using CHO-E cells. Results shown reflect multiple assays (n = 4) on HECA-452 blots of multiple (n = 3) membrane preparations. Data are mean ± SEM.
Analysis of HCELL contribution to E-selectin ligand activity of human cells
To date, mAb that can block E-selectin ligand activity of CLA, CD43, or HCELL have not been reported. Accordingly, to assess HCELL-specific contributions to E-selectin ligand activity of human cells, we sought to dampen HCELL expression by targeted gene silencing of CD44. Because technical manipulations associated with lentiviral transduction of native HSPC-enriched cells led to procedure-associated changes in phenotype, including culture-associated loss of sialofucosylated structures that correlate with selectin ligand activity (ie, loss of HECA-452–reactive epitopes such as sLex), CD44 silencing was performed on the KG1a cell line, which displays no phenotypic drift on transduction.
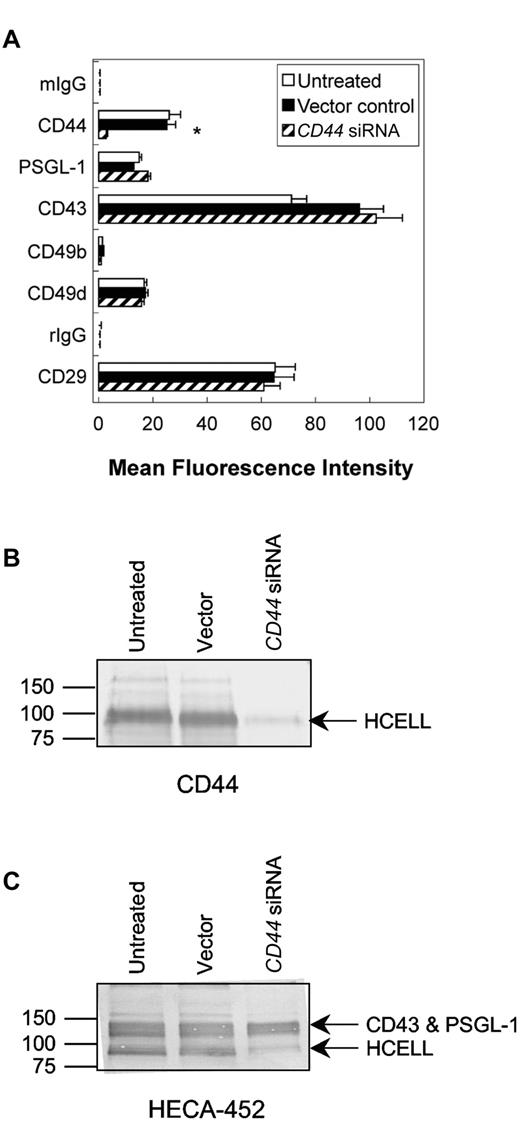
As measured by flow cytometry, CD44 siRNA transduction of KG1a cells resulted in ∼ 90% reduction in surface expression of CD44 relative to untreated and vector-transduced cells (Figure 5A). To assess the specificity of CD44 silencing, expression of CD43 and PSGL-1, as well as other adhesion molecules (integrin subunits α2 [CD49b], α4 [CD49d], and β1 [CD29]) were quantified by flow cytometry. No changes in expression were observed for these proteins compared with untreated cells or scrambled vector-transduced cells (Figure 5A). Western blots of proteins resolved by SDS-PAGE revealed that CD44 siRNA transduction abrogated total CD44 expression (Figure 5B). As expected, CD44 silencing correlated with a marked reduction of CD44 HECA-452 reactivity (HCELL)7,24,25 compared with untreated and scrambled vector-transduced cells (Figure 5C).
HCELL expression is specifically reduced on KG1a cells by CD44 siRNA transduction. (A) Flow cytometric analysis of CD44, PSGL-1, and integrin expression of untreated, vector-transduced (control), and CD44 siRNA-transduced KG1a cells. CD44 expression was reduced on CD44 siRNA transduction, as measured by anti-CD44 mAb 2C5 (used in Figure) and further confirmed using anti-CD44 mAb 515 (data not shown, which recognizes an epitope distinct from 2C5). PSGL-1, VLA-2 (CD49b/CD29) integrin, and VLA-4 (CD49d/CD29) integrin expression was unaffected by viral transduction. Values are mean fluorescence intensities ± SEM; n = 6. *P < .05 relative to untreated and vector controls. (B-C) Western blot analysis of HCELL on KG1a cells. Whole cell lysates equivalent to 2 × 105 (for anti-CD44 mAb, 2C5) and 1 × 105 (for HECA-452) untreated, vector-transduced, and CD44 siRNA-transduced KG1a cells were resolved on a 7.5% SDS-PAGE gel under reducing conditions and immunoblotted with the appropriate mAb. Note that HCELL expression is nearly eliminated by CD44 silencing (minimal staining of HECA-452 and CD44 mAb at ∼ 100 kDa in CD44-silenced cell lysates relative to untreated and vector-transduced cell lysates).
HCELL expression is specifically reduced on KG1a cells by CD44 siRNA transduction. (A) Flow cytometric analysis of CD44, PSGL-1, and integrin expression of untreated, vector-transduced (control), and CD44 siRNA-transduced KG1a cells. CD44 expression was reduced on CD44 siRNA transduction, as measured by anti-CD44 mAb 2C5 (used in Figure) and further confirmed using anti-CD44 mAb 515 (data not shown, which recognizes an epitope distinct from 2C5). PSGL-1, VLA-2 (CD49b/CD29) integrin, and VLA-4 (CD49d/CD29) integrin expression was unaffected by viral transduction. Values are mean fluorescence intensities ± SEM; n = 6. *P < .05 relative to untreated and vector controls. (B-C) Western blot analysis of HCELL on KG1a cells. Whole cell lysates equivalent to 2 × 105 (for anti-CD44 mAb, 2C5) and 1 × 105 (for HECA-452) untreated, vector-transduced, and CD44 siRNA-transduced KG1a cells were resolved on a 7.5% SDS-PAGE gel under reducing conditions and immunoblotted with the appropriate mAb. Note that HCELL expression is nearly eliminated by CD44 silencing (minimal staining of HECA-452 and CD44 mAb at ∼ 100 kDa in CD44-silenced cell lysates relative to untreated and vector-transduced cell lysates).
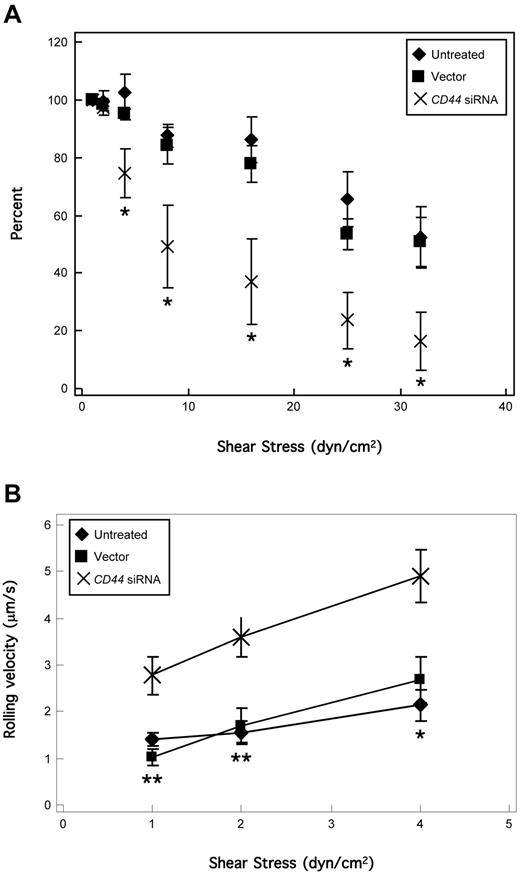
To fully assess the functional impact of CD44 silencing, two complementary assays were performed. First, CD44 is best recognized for mediating adherence to HA,29,36 and we thus tested the capacity of CD44-silenced KG1a cells to bind HA. We observed that untreated and scrambled vector-transduced KG1a cells equivalently bound to HA avidly (> 500 cells/mm2, n = 6), but that HA adherence was eliminated in CD44 siRNA-transduced cells (2.5 ± 1.7 cells/mm2, n = 6). To assess the role of CD44 silencing on the E-selectin ligand activity of HCELL, untreated, scrambled vector-transduced, and CD44 siRNA-transduced KG1a cells were perfused over CHO-E cell monolayers under physiologic shear stress levels. All KG1a cell binding to CHO-E cells was E-selectin–specific, as perfused cells did not bind to CHO-E cells pretreated with a function-blocking anti–E-selectin mAb (data not shown). Untreated and scrambled vector-transduced cells maintained high numbers of rolling interactions on CHO-E cells at a shear stress of 1 to 20 dyn/cm2, but CD44 siRNA-transduced cells displayed a strikingly decreased rolling capacity at a shear stress > 1 dyn/cm2 (Figure 6A). The contribution of HCELL to E-selectin ligand activity at ≤ 4 dyn/cm2 (postcapillary venule shear37 ) was further revealed in rolling velocity analysis, as CD44 siRNA-transduced cells rolled markedly faster than untreated and scrambled vector-transduced cells (Figure 6B). Thus, CD44-silenced cells show a dramatically reduced ability to engage E-selectin, highlighting the dominant contribution of HCELL over CLA and CD43 in mediating E-selectin–dependent rolling interactions.
HCELL on KG1a cells is the primary mediator of shear-resistant E-selectin binding. (A) KG1a cells were perfused over CHO-E cell monolayers for 2 minutes at wall shear stress = 1 dyn/cm2; then detachment assays were performed by increasing shear stress stepwise every 30 seconds. The number of rolling cells at the end of each interval was counted and calculated as the percentage relative to the number of cells rolling at the start of the detachment assay (ie, end of the attachment period at 1 dyn/cm2). All binding was E-selectin–dependent, confirmed by inhibition of attachment in the presence of function-blocking anti–E-selectin mAb 68-5H11 and by treatment with EDTA (data not shown), and lack of binding of KG1a cells to CHO-mock cells. HCELL-mediated E-selectin binding was most evident at shear stress levels > 4 dyn/cm2. Data are mean ± SEM; n = 5. *P < .05, relative to untreated and vector controls. Importantly, on CD44 silencing no differences were observed on HECA-452 reactivity of PSGL-1/CLA (Figure 5C), which typically correlates with selectin binding capacity. (B) Velocity of KG1a cell rolling on CHO-E cells at each shear stress was calculated as the distance traveled by the centroid of the cell divided by the time period of observation (5 seconds). Cells lacking HCELL (CD44 siRNA-transduced) rolled significantly faster than those expressing HCELL (untreated, vector-transduced). Data are mean ± SEM; n > 20 cells. *P < .05, relative to untreated and vector control. **P < .01 relative to untreated and vector control.
HCELL on KG1a cells is the primary mediator of shear-resistant E-selectin binding. (A) KG1a cells were perfused over CHO-E cell monolayers for 2 minutes at wall shear stress = 1 dyn/cm2; then detachment assays were performed by increasing shear stress stepwise every 30 seconds. The number of rolling cells at the end of each interval was counted and calculated as the percentage relative to the number of cells rolling at the start of the detachment assay (ie, end of the attachment period at 1 dyn/cm2). All binding was E-selectin–dependent, confirmed by inhibition of attachment in the presence of function-blocking anti–E-selectin mAb 68-5H11 and by treatment with EDTA (data not shown), and lack of binding of KG1a cells to CHO-mock cells. HCELL-mediated E-selectin binding was most evident at shear stress levels > 4 dyn/cm2. Data are mean ± SEM; n = 5. *P < .05, relative to untreated and vector controls. Importantly, on CD44 silencing no differences were observed on HECA-452 reactivity of PSGL-1/CLA (Figure 5C), which typically correlates with selectin binding capacity. (B) Velocity of KG1a cell rolling on CHO-E cells at each shear stress was calculated as the distance traveled by the centroid of the cell divided by the time period of observation (5 seconds). Cells lacking HCELL (CD44 siRNA-transduced) rolled significantly faster than those expressing HCELL (untreated, vector-transduced). Data are mean ± SEM; n > 20 cells. *P < .05, relative to untreated and vector control. **P < .01 relative to untreated and vector control.
Exofucosylation of mouse LSK cells creates HCELL, enhancing E-selectin ligand activity and LSK homing to marrow
As shown in Table 1, CD44 is strongly expressed among both mouse and human HSPCs, but, in contrast to human, CD44 on mouse LSK cells lacks reactivity with E-selectin (Figures 2A, 4B). Thus, mouse LSK cells do not natively express the E-selectin binding CD44 glycoform known as HCELL.1,7,24 We thus sought to determine whether GPS of CD44 glycans would enforce HCELL expression.9 To this end, we treated mouse LSK cells with the α(1,3)-linkage-specific fucosyltransferase, FTVI. This enzyme specifically places a fucose onto a terminal type 2 lactosamine unit; if that lactosamine is capped with an α(2,3)-linked sialic acid, sLex is created. After FTVI treatment of mouse LSK cells, (GPS-LSK) E-Ig binding profoundly increased (Figure 7A), correlated specifically with appearance of HCELL without changes in overall CD44 levels (Figure 7B). To determine whether the relevant sialofucosylations on HCELL were displayed on N-glycans, we tested E-Ig reactivity on Western blot of lysates of GPS-LSK after digestion with N-glycosidase F (Figure 7C); no evident staining with E-Ig following digestion was observed, indicating that the relevant E-selectin binding determinant(s) are displayed on N-glycans, as characteristic of HCELL on human HSPCs. Therefore, treatment of mouse LSK cells with FTVI creates the E-selectin binding glycoform of CD44, HCELL, likening E-selectin ligand activity of mouse LSK cells to that of human CD34+ cells.
Exofucosylation (GPS) of mouse LSK cells enforces HCELL expression, yielding higher E-selectin binding and increased homing to marrow. (A) Flow cytometric analysis of E-Ig binding on BT-LSK and GPS-treated LSK (GPS-LSK) cells. EDTA control displayed a mean fluorescence intensity = 3.1. Results displayed are representative of at least n = 5 flow cytometric experiments. (B) GPS-LSK and BT-LSK cells were lysed, and Western blot analysis was performed on whole cell lysates and blotted with E-selectin and CD44. (C) CD44 was immunoprecipitated (with IM7 and KM114) from equivalent amounts of cell lysates from GPS-treated (+) or untreated (−) mouse LSK cell lysates that had been either treated with N-glycosidase F (+) or not (−). Immunoprecipitates were then electrophoresed and blotted with E-Ig and CD44. Staining with E-Ig was performed in the presence of Ca2+. (D) GPS-LSK (black bars) or BT-LSK (white bars) were labeled reciprocally with CFDA-SE and/or CTO and injected intravenously in a 1:1 ratio competitively into C57BL6 mice. Bone marrow was analyzed 16 hours after the injection to determine the percentage of carboxyfluorescein succinimidyl ester and CTO-positive cells present within a defined gate-representing mouse LSK cells. Mice that did not receive cells were used to determine the background signal. Error bars represent the SEM. Data are representative of 6 mice. Parallel control experiments measuring the competition of BT cells to untreated cells did not show any advantage or disadvantage of either cell type (supplemental Figure 4).
Exofucosylation (GPS) of mouse LSK cells enforces HCELL expression, yielding higher E-selectin binding and increased homing to marrow. (A) Flow cytometric analysis of E-Ig binding on BT-LSK and GPS-treated LSK (GPS-LSK) cells. EDTA control displayed a mean fluorescence intensity = 3.1. Results displayed are representative of at least n = 5 flow cytometric experiments. (B) GPS-LSK and BT-LSK cells were lysed, and Western blot analysis was performed on whole cell lysates and blotted with E-selectin and CD44. (C) CD44 was immunoprecipitated (with IM7 and KM114) from equivalent amounts of cell lysates from GPS-treated (+) or untreated (−) mouse LSK cell lysates that had been either treated with N-glycosidase F (+) or not (−). Immunoprecipitates were then electrophoresed and blotted with E-Ig and CD44. Staining with E-Ig was performed in the presence of Ca2+. (D) GPS-LSK (black bars) or BT-LSK (white bars) were labeled reciprocally with CFDA-SE and/or CTO and injected intravenously in a 1:1 ratio competitively into C57BL6 mice. Bone marrow was analyzed 16 hours after the injection to determine the percentage of carboxyfluorescein succinimidyl ester and CTO-positive cells present within a defined gate-representing mouse LSK cells. Mice that did not receive cells were used to determine the background signal. Error bars represent the SEM. Data are representative of 6 mice. Parallel control experiments measuring the competition of BT cells to untreated cells did not show any advantage or disadvantage of either cell type (supplemental Figure 4).
To study the effect of GPS-engineered HCELL activity on short-term homing of mouse LSK cells in vivo, LSK cells were stained using a fluorochrome tracking dye, CFSA-SE or CTO and adoptively transferred into C57BL/6 mice. Within 16 hours after competitive injection of GPS-treated and BT-LSK cells, GPS-LSK cells accumulated in the marrow 3.5-fold more efficiently than BT-LSK cells (Figure 7D). The competitive advantage of GPS-LSK cells in infiltrating the bone marrow is a true difference in trafficking and not simply the preferential expansion of these cells in situ, as their average CFDA-SE and CTO fluorescence was not reduced relative to BT-LSK cells (data not shown). Thus, the creation of HCELL on mouse LSK cells after exofucosylation licenses immigration of these cells into the bone marrow, high-lighting the critical role of HCELL in driving osteotropism of cells in vivo.
Discussion
It is well established that marrow microvascular endothelial cells constitutively express E-selectin2,5 and that engagement of E-selectin promotes homing of circulating cells to marrow.9,17 In this study, we sought to elucidate the glycoprotein E-selectin ligands expressed on mouse and human bone marrow cells enriched for hematopoietic stem and progenitor cells. We used complementary biochemical approaches and functional assays to define the scope and breadth of these effectors of osteotropism. Our results reveal critical species-specific variations in the expression and function of the glycoprotein E-selectin ligands, providing novel perspectives on their distinct contributions to HSPC migration in mice and humans.
The data presented here show that, compared with leukocytes, HSPCs display a relatively restricted set of E-selectin ligands. Notably, both mouse LSK cells and human CD34+ cells do not express the glycoproteins MAC-1 or ESL-1, and although these cells express L-selectin38 and LFA-1,20 our studies reveal that these structures do not function as E-selectin ligands. We confirmed results of others indicating that CLA (PSGL-1) serves as an E-selectin ligand on human CD34+ cells and mouse LSK cells,1 but, importantly, our data are the first to show that CD43 also functions as an E-selectin ligand on mouse LSK cells and human CD34+ cells. CD43 is a major sialomucin expressed by most leukocytes.34 Two major glycoforms, 115- and 130-kDa, have been identified for both human and mouse CD43.34,35 CD43 contributes significantly to E-selectin ligand activity on leukocytes as revealed by studies in PSGL-1−/− mice showing substantial residual E-selectin ligand activity on neutrophils39,40 and T cells,21,22,41,42 and with direct biochemical studies in mouse and human T cells.22,23 Prior studies showed that the 130-kDa glycoform functions as an E-selectin ligand on leukocytes, and here we detected the 130-kDa form of CD43 as the E-selectin ligand binding glycoform for both human CD34+ cells and mouse LSK cells. Importantly, measured by E-Ig reactivity in Western blot of mouse LSK cell lysates, the contribution of CD43 to E-selectin binding was modest compared with that of CLA. Furthermore, in blot rolling assays of immunoprecipitated proteins from LSK cells, CLA supported E-selectin–mediated interactions significantly more than CD43. On the contrary, E-selectin binding by CD43 on human CD34+ cells was equivalent to that of CLA as measured by Western blot and by blot rolling assays.
Results of flow cytometry and of Western blot using E-Ig indicate that E-selectin ligand activity is more pronounced on human CD34+ cells than on mouse LSK cells. This critical species-specific difference could be attributable, at least in part, to the unique expression of HCELL on human CD34+ cells: although mouse LSK cells highly express CD44, the CD44 lacks requisite sialofucosylations conferring HCELL function. Moreover, all human hematopoietic cells that express HCELL, including native CD34+ cells and KG1a cells,7,24,25 de novo AML cells,7,24,25 and G-CSF–mobilized peripheral blood leukocytes,26 display higher binding to E-selectin than cells that lack HCELL.7,24-26 To more directly assess the contribution(s) of HCELL to E-selectin ligand activity, we performed complementary studies using Western blot and flow chamber assays. The potency of HCELL as an E-selectin ligand was evident in Western blots of native CD34+ cells and of KG1a cells showing that E-Ig reactivity, normalized for equivalent input cell number in lysates and in immunoprecipitated protein, was most prominent on HCELL (Figure 2A); indeed, CLA and CD43 combined did not show the same level of E-Ig reactivity as that displayed by HCELL. To further evaluate the contribution of HCELL to E-selectin binding activity on intact cells, CD44 siRNA was used to eliminate expression of HCELL on the surface of KG1a cells. For these studies, we used KG1a cells as we observed that lentiviral transduction induced phenotypic changes in native CD34+ cells, and, specifically, this primitive human hematopoietic cell line displays an E-Ig reactivity profile on Western blot similar to that of native CD34+ cells (Figure 2A). As observed in flow chamber assays, silencing of CD44 expression profoundly decreased E-selectin ligand activity as shown in both the dramatic decrease in number of rolling cells and the markedly increased E-selectin–specific rolling velocity over all physiologically relevant shear stress levels tested (Figure 6). Commensurate with these findings, enforced expression of HCELL on mouse LSK cells (normally devoid of HCELL) by exofucosylation markedly increases E-selectin ligand activity. Notably, exofucosylation of LSK cells only induced expression of HCELL, and not creation of other E-selectin ligands or heightened expression of sLex on the other E-selectin ligands (ie, CLA or CD43). The enforced HCELL expression alone significantly increased the efficiency of homing to bone marrow (> 3-fold). Collectively, these data indicate that HCELL is the most potent E-selectin ligand expressed on human CD34+ cells and, specifically, functions as a “bone marrow homing receptor.”
Our findings here offer unifying perspectives on data derived from both human and mouse studies regarding the molecular effectors of HSPC osteotropism. Transplantation studies using hematopoietic progenitor cells obtained from CD44−/− mice have shown no deficiency in homing of such cells to marrow,43 findings commensurate with the absence of HCELL expression on mouse LSK cells. The fact that human CD34+ cells uniquely express HCELL and possess markedly more E-selectin ligand activity than mouse LSK cells suggests that E-selectin–dependent adhesive interactions may play a greater role in human CD34+ cell homing to marrow. This notion is consistent with reports using mouse models showing that, although E-selectin–dependent interactions are certainly contributory, marrow homing of mouse HSPCs mainly depends on α4-integrin–dependent receptor/ligand interaction,3,4,27,44,45 and that integrins α4β1 (VLA-4) and α4β7 (LPAM-1) each mediate substantial rolling of murine HSPCs on marrow microvascular endothelium.46 In contrast, studies of human CD34+ cells have indicated that Step 1 rolling interactions on bone marrow endothelium (and subsequent extravasation into marrow) are overwhelmingly selectin-dependent, with the VLA-4/VCAM-1 adhesion pathway playing only a minor role in this activity.8
The present study offers novel insights into species-specific differences in the scaffold proteins that carry relevant sialofucosylations rendering E-selectin ligand activity among marrow cells enriched for HSPCs. Beyond significance in HSPC homing, the fact that human CD34+ cells and mouse LSK cells use different sets of glycoprotein E-selectin ligand(s) also has profound implications in the biology of hematopoiesis. Engagement of cell surface CD44, PSGL-1, and CD43 has each been associated with signal transduction and dramatic effects on cell growth and survival.32,33,47-53 E-selectin–dependent binding of HSPCs to marrow microvessels, either concurrent with transmigration or, subsequently, within “vascular niches,” could affect hematopoietic activity, with differential effects depending on the target glycoprotein ligand(s). Importantly, it is known that ligation of PSGL-1 can suppress hematopoiesis in mouse33 and human54 HSPCs. This fact, together with evidence that non–PSGL-1 E-selectin ligands can also trigger apoptosis and growth inhibition of HSPCs from human and mouse,54 highlights a broader role for E-selectin receptor/ligand interactions in hematopoiesis. The distinct expression of HCELL on human HSPCs, coupled with the fact that CD44 itself has multiple ligands within the marrow milieu (eg, hyaluronic acid36 and osteopontin55 ), suggests that hematopoietic effect(s) attributable to CD44 ligation in humans will reflect a balance among competing signals induced by E-selectin and other CD44 ligands. Further studies are thus warranted to define how the differences revealed here in respective HSPC E-selectin ligand expression license glycoprotein-specific signaling cascades modulating hematopoietic processes in humans and mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Sandra L. King for helpful discussions; Jack Lee, Rafael Jaimes, and Julia Chee Jia Chin for technical assistance; and the staff of the Cell Processing Laboratory of the Bone Marrow Transplantation Unit at the Massachusetts General Hospital (Boston, MA) for their assistance in procuring bone marrow harvest material.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute (grants RO1 HL60528 and RO1 HL073714, R.S.), the National Institutes of Health (NRSA Training Grant, M.M.B.), the American Medical Association (Research Seed Grant Award, J.T.C.), the American Society of Hematology (Training Fellowship, J.T.C.), and the Howard Hughes Medical Institute (Medical Student Research Training Fellowship, J.T.C.).
National Institutes of Health
Authorship
Contribution: J.S.M. designed and performed research, collected and analyzed data, and wrote the paper; M.M.B. performed research, collected and analyzed data, and wrote the paper; S.Z.G. and N.M.D. performed research and collected and analyzed data; J.T.C. performed research; R.C.F. analyzed data and wrote the paper; and R.S. conceived the study, designed research, analyzed data, supervised all experimentation, funded research, and wrote the paper.
Conflict-of-interest disclosure: In accordance with National Institutes of Health policies and procedures, intellectual property related to HCELL is retained by R.S. The remaining authors declare no competing financial interests.
The current affiliation for J.S.M. and S.Z.G. is Division of Chemical and Life Sciences and Engineering, King Abdullah University of Science and Technology, Jeddah, Saudi Arabia. The current affiliation for M.M.B. is Chemical and Biomolecular Engineering, Ohio University, Athens, OH. The current affiliation for N.M.D. is Project Management–Discovery and Alliances, Piramal Life Sciences Limited, Mumbai, India.
Correspondence: Robert Sackstein, Harvard Institutes of Medicine, 77 Ave Louis Pasteur, Rm 671, Boston, MA 02115 e-mail: rsackstein@rics.bwh.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal