Abstract
Disease development in human T-cell leukemia virus type 1 (HTLV-1)–infected individuals is positively correlated with the level of integrated viral DNA in T cells. HTLV-1 replication is positively regulated by Tax and Rex and negatively regulated by the p30 and HBZ proteins. In the present study, we demonstrate that HTLV-1 encodes another negative regulator of virus expression, the p13 protein. Expressed separately, p13 localizes to the mitochondria, whereas in the presence of Tax, part of it is ubiquitinated, stabilized, and rerouted to the nuclear speckles. The p13 protein directly binds Tax, decreases Tax binding to the CBP/p300 transcriptional coactivator, and, by reducing Tax transcriptional activity, suppresses viral expression. Because Tax stabilizes its own repressor, these findings suggest that HTLV-1 has evolved a complex mechanism to control its own replication. Further, these results highlight the importance of studying the function of the HTLV-1 viral proteins, not only in isolation, but also in the context of full viral replication.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma (ATLL)1-3 and the neurologic disease HTLV-1–associated myelopathy/tropical spastic paresis (HAM/TSP).4,5 In addition to the Gag, Pol, and Env proteins, HTLV-1 encodes through alternative splicing mRNAs for at least 7 additional viral proteins. The 2 essential positive regulators of HTLV-1 replication, the Tax and Rex proteins, are encoded by a unique, double-spliced mRNA and are expressed through ribosomal leaky scanning in orf-III and orf-IV, respectively.6 Tax is an enhancer of viral transcription by its interaction with CREB/ATF, CBP/p300, and the Tax response element (TRE) located within the viral promoter.7 In addition, Tax has been reported to interact with more than 100 different cellular proteins that affect gene expression, cell signaling, and proliferation.8 Tax is also thought to be a promoter of T-cell transformation, because Tax expression immortalizes T cells in vitro and induces tumors in transgenic animals.8,9 Rex interacts with a viral mRNA secondary structure, designated Rex responsive element (RxRE) located at the 3′–long terminal repeat (3′-LTR). Rex controls viral production by regulating the transport of unspliced and incompletely spliced viral mRNAs from the nucleus to the cytoplasm.10 HTLV-1 also encodes 2 negative regulators of viral expression. The first, the p30 protein, is encoded by orf-II, interacts with tax/rex mRNA, and retains it in the nucleus.11,12 At low concentrations, p30 also represses HTLV-1 LTR–driven transcription, whereas at high concentrations,13,14 it activates transcription. The second negative regulator of HTLV-1 expression is the HTLV-1 basic zipper factor (HBZ) that is encoded by the antisense strand of the HTLV-1 genome.15,16 HBZ interacts with CREB and CBP/p300, thereby blocking Tax-dependent transcription and viral expression.17,18 HBZ also inhibits the activity of JunB and c-Jun by sequestering them in transcriptional inactive nuclear bodies and affects activating protein 1 (AP-1)–dependent cellular and viral transcription.19,20 More recently, the HBZ antisense mRNA has been shown to induce T-cell proliferation.21,22
p1323,24 is a mitochondrial protein encoded by a distinct, singly spliced mRNA from orf-II. It accumulates in the inner membrane of the mitochondria and modulates K+ permeability and reactive oxygen species, thereby altering the morphology and function of mitochondria.25-27 Evidence of p13 expression in HTLV-1–infected cells is indirect, because only the presence of mRNA has been demonstrated in both in vitro and ex vivo studies.28-30 orf -II appears to be dispensable for viral infection and transformation of T cells in vitro; however, its requirement in HTLV-1 infection of rabbits remains uncertain because the mutation introduced into the HTLV-1 molecular clone to ablate p13 also affected the HBZ gene.31-33 Furthermore, the p13 protein has been reported to suppress cell growth in vitro, tumorigenicity in vivo, and to sensitize Jurkat T cells to Ras-mediated apoptosis.34,35 In the present study, we describe a novel function for p13, demonstrating that it is a negative regulator of HTLV-1 replication in the presence of Tax. The p13 protein binds to Tax and interferes with Tax-CBP/p300 complex formation, thereby decreasing Tax transcriptional activity. Tax stabilizes p13 expression by inducing a posttranslational modification of p13. Modified p13 is rerouted to nuclear speckles and colocalizes with Tax. Therefore, the presence of Tax overcomes the low expression level of p13 by stabilizing the protein through posttranslational modification. The finding of an additional virus-encoded negative regulator underscores the importance of tight control of viral replication for HTLV-1 persistence in the host.
Methods
Plasmids and cell lines
The p13-hemagglutinin (p13-HA) construct encodes the mammalian codon optimized p13 gene in-frame with the HA tag in the pMH vector.11 The p13 gene was amplified by PCR using the primer p13-F 5′ AGCTAAGCTTACCATGCTGATCAGC 3′ and the reverse primer p13-R 5′ AATTGAATTCGCCAGATTCTCGGGGTGGGGC 3′. The HA-fused ubiquitin constructs (WT, K63 only, and K48 only) were kindly provided by Dr Edward Harhaj (University of Miami, Miami, FL) with permission from Dr Shao-Cong Sun (M. D. Anderson Cancer Center, University of Texas, Houston, TX).36 The HA tag in these 3 constructs was changed to an AU1 tag (DTYRYI) by PCR using the following primers flanked with KpnI and XbaI: AU1-Ub-F: 5′AGCTGGTACCACCATGGACACCTATCGCTATATATCTCCCGAATTCGCGGCCGCGTCG 3′ and AU1-Ub-R: 5′ AGCTTCTAGAGGAAGAAGTCCAAAGCTT 3′, creating AU1-Ub-WT, AU1-Ub-K63, and AU1-Ub-K48. The reporter constructs HTLV-1 LTR luciferase (LTR-Luc) and RxRE-CAT37 were kindly provided by Dr Madeleine Duc Dodon (Virologie Humaine, Inserm, Lyon, France), the molecular clone pcRex was kindly provided by Dr David Derse (now deceased), and pAB and pcTax have been described previously.38-40 We generated the p13KO mutant by replacing the initiating methionine with isoleucine using the primer p13KO F: 5′-TACAAGTTAACCATTCTTATTATCAGCCCA-3′. This mutation did not alter the HBZ gene.
HEK 293T and HeLa cells were grown in DMEM and MT-2 cells were grown in RPMI medium, both supplemented with 10% FCS and Pen/Strep with antimycotic (Invitrogen). 293T cells were transfected with FuGene6 (Roche) and HeLa cells with FuGeneHD (Roche) according to the manufacturer's instructions. MT-2 cells were electroporated by AMAXA using the program X-05 according to the manufacturer's instructions.
Viral protein detection
Cells were lysed with RIPA buffer (1% NP-40, 50mM Tris-HCl, pH 7.5, 150mM NaCl, and protease inhibitor mix; Roche). Protein concentration was measured using the Bradford method and immunoprecipitation was performed with the appropriate primary and secondary antibodies and 30 μL of protein G-agarose before elution with SDS sample buffer with 10% β-mercaptoethanol. Alternatively, immunoprecipitations were performed using μMACS Protein G MicroBeads and columns according to the manufacturer's protocol (Miltenyi Biotec). In some studies, 293T cells were transfected and 48 hours later treated with 10μM cycloheximide. Samples were collected at the time points indicated. Proteins were transferred to nitrocellulose using the iBlot system (Invitrogen) or through conventional blotting. Membranes were blocked in 5% milk and incubated overnight at 4°C with primary antibody, followed by washing with TBS-Tween, incubation with secondary antibody, washing, and protein detection using ECL.
The anti-HA antibody clones 12CA5 and 3F10-hrp (dilution 1:1000 for Western blot) were obtained from Roche Applied Science; the 6E2 clone with Alexa Fluor 488 conjugation was obtained from Cell Signaling Technology. The monoclonal IgG2a anti-Tax antibody (dilution 1:100 for immunofluorescence) was prepared from the Tax hybridoma 168A51-2 obtained from AIDS Research and Reference Program, NIAID, National Institutes of Health, whereas the monoclonal anti-Tax antibody clone Tab172 IgG2a (dilution 1:1000 for Western blot) was kindly provided by Dr John Brady (recently deceased). The polyclonal Rex antibody (dilution 1:1000 by Western blot) was a gift from Dr David Derse. Peroxidase (POD)–conjugated anti–mouse and POD-conjugated anti–rabbit were used at a dilution of 1:10 000. The polyclonal rabbit anti-Cox IV (Abcam), the mouse IgG1 anti-SC35 (Sigma-Aldrich), and the polyclonal goat anti-sp100 (Sigma-Aldrich) antibodies were all used for immunofluorescence. Secondary antibodies used for immunofluorescence (1:5000) were: Alexa Fluor 568–anti-mouse, Alexa Fluor 568–anti-rabbit, Alexa Fluor 594–anti-goat, Alexa Fluor 488–anti-IgG1-mouse, and Alexa Fluor 568–anti-IgG2a. The luciferase reporter assay system was obtained from Promega and the luciferase values were normalized to the protein concentration determined using the Bio-Rad protein assay. Quantification of p19 Gag from the cell supernatant was performed using the HTLV p19 Gag-capturing assay from Zeptometrix. Cell supernatants were diluted to obtain concentrations within the standard range.
GST pull-down
GST and GST-Tax protein were purified as described previously.41 The p13 protein was expressed from the pMH vector under the T7 promoter. The TNT T7 coupled reticulocyte lysate system (Promega) was used to in vitro translate the p13 protein. A total of 400 ng of GST or GST-Tax was incubated with 35S-labeled p13 protein in 50 μL of GST-binding buffer (50mM Tris-HCl, 150mM NaCl, 0.5% NP-40, 1mM PMSF, 1mM DTT, and 5% glycerol) at 4°C for 4 hours. Fifty microliters of glutathione-Sepharose (50% slurry; Amersham) precoated with 2 μg/μL of BSA was added and incubated overnight at 4°C. Complexes bound on glutathione-Sepharose beads were then washed with GST washing buffer (50mM Tris-HCl, 150mM NaCl, 1.0% NP-40, 1mM PMSF, 1mM DTT, and 5% glycerol) 4 times and eluted in loading buffer by boiling for 4 minutes. Complexes were separated on 4%-20% SDS-PAGE gels by electrophoresis and analyzed by Western blotting using anti-HA antibody.
RT-PCR
Total RNA obtained by TRIzol (Invitrogen) was converted to cDNA using the SuperScript III First-Strand Synthesis Supermix (Invitrogen) and oligodT primers. Cytoplasmic RNA was isolated by lysing transfected cells in hypotonic lysis buffer (10mM HEPES, pH 7.9, 1.5mM MgCl2, 10mM KCl, and 0.5mM DTT) for 10 minutes on ice. The cytoplasmic and nuclear fractions were separated by centrifugation at 700g for 10 minutes. The cytoplasmic fraction was cleared by centrifugation at 3300g for 5 minutes and the supernatants treated with proteinase K (50 μg/mL) at 37°C for 30 minutes. Samples were treated with RNase-free DNase and extracted with chloroform. The following primers were used to amplify orf-II, gag and tax, and β-actin: p13 (283 bp) forward-5′ CTGCCTAGAGTGTGGACCGAG and reverse 5′ GGACGTCGTATGGGTACACGT; β-actin forward-5′CGGTTGGCCTTGGGGTTCAGGGGG and reverse-5′ ATCGTGGGGGCGCCCCAGGCACCA; gapdh forward-5′GGTCTCCTCCGACTTCAACA and reverse-5′TGCTGTAGCCAAATTCGTTG; tax (286 bp) forward-5′ TCAACCCTCACCACTCCAGGC and reverse 5′ GGGGCTCATGGTCATTGTCAT; taxrex (159bp) forward-5′GTCCGCCGTCTAGCTTCC and reverse 5′CTGGGAAGTGGGCCATGG; gag (201 bp) forward-5′ GCTGGCCGCTCATCACTG and reverse 5′ GGGACGGGATCTGGGCTT. Real-time quantitative RT-PCR was performed on a Stratagene Mx3000P. Briefly, RNA was reverse transcribed to cDNA. The cDNA was then used for PCR reactions to detect gag, tax/rex, actin, or gapdh mRNA. The accumulation of product was monitored with SYBR green dye. All values used were from the logarithmic phase of amplification.
Immunofluorescence and microscopy
HeLa cells were seeded on coverslips and transfected the following day by FuGENE HD. MT-2 cells were seeded on fibronectin-precoated coverslips for 30 minutes at 37°C 48 hours after transfection. HeLa cells on coverslips were fixed 24 hours after transfection with 4% paraformaldehyde (PFA) in PBS for 20 minutes, washed in PBS, and permeabilized in ice-cold methanol for 20 minutes at −20°C. MT-2 cells on coverslips were fixed with 4% PFA for 5 minutes in the medium, washed once with PBS, further fixed for 10 minutes with 4% PFA in PBS, washed with PBS, and treated with ice-cold methanol for 20 minutes at −20°C. Fixed cells were washed and incubated for 1 hour in 25-μL droplets containing primary antibodies, followed by washing and incubation with the appropriate Alexa Fluor–conjugated secondary antibodies. HeLa cells were mounted in 5 μL of the mounting medium Vectashield with DAPI (Vector Laboratories) and MT-2 cells were hard-mounted with DAPI (EMS) on a glass slide. Slides were examined with a laser scanning confocal microscope (LSM 510; Carl Zeiss MicroImaging) with a 63×/1.3 numerical aperture Plan Apochromat oil objective.
Results
The p13 protein inhibits HTLV-1 replication by down-regulating Tax activity
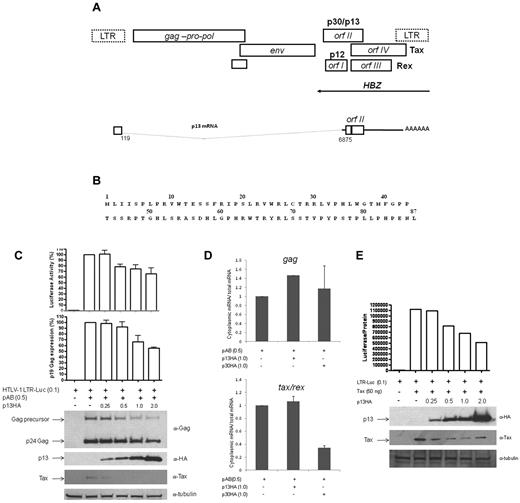
A singly spliced mRNA from the HTLV-1 orf-II (Figure 1A) encodes the mitochondria-localized, 87–amino acid p13 protein (Figure 1B).23,24 We investigated the role of p13 in viral replication and found that increasing amounts of p13-HA decreased the expression of the proviral clone pAB38,40 in a dose-dependent manner. Both extracellular p19 Gag production and intracellular Gag expression were inhibited by p13 (Figure 1C). Tax activity on the HTLV-1 LTR-Luc reporter construct was also reduced by p13 (Figure 1C).
p13 expression decreases viral production and Tax activity. (A) Schematic representation of the HTLV-1 genome that encodes the enzymatic genes gag-pro-pol and env and the regulatory proteins p12, p30, p13, Rex, Tax, and HBZ (arrow indicates antisense direction of the HBZ gene) at the 3′ of the genome. Box under env indicates the second exon that contains the ATG-initiating codons for the envelope p30, Tax, and Rex proteins. The singly spliced orf-II p13 mRNA is illustrated below. (B) Single-letter amino acid code of p13 from HTLV-1LAF. (C) 293T cells transfected with the HTLV-1 molecular clone pAB (0.5 μg) without and with increasing amounts of p13-HA and the reporter construct HTLV-1 LTR-Luc (0.1 μg). DNA concentrations were equalized using the backbone vector pMH. Viral replication was measured 48 hours after transfection by extracellular p19 Gag ELISA (pg/mL) and intracellular anti-p24 Gag immunoblotting. Lysates were examined for p13 and Tax expression. Tubulin was used as a loading control. Tax-dependent LTR-Luc activity was measured and adjusted for protein concentration. p19 Gag concentration and luciferase activity were set to 100% for pAB alone, and the standard deviation represents an average of 3 independent experiments. (D) Quantitative RT-PCR was performed on cytoplasmic and total RNA isolated from 293T cells transfected with pAB (0.5 μg) and p30, p13, or vector control (pMH) (1.0 μg). RNA levels of cytoplasmic over total for gag (top panel) and tax/rex (bottom panel) are shown. The graphs correspond to gag or tax signals adjusted for gapdh level. Graphs represent values from duplicate experiments. (E) 293T cells were transfected with pcTax (50 ng) without (−) or with (+) increasing amounts of p13-HA and the reporter construct HTLV-1 LTR-Luc (0.1 μg). Luciferase activity is normalized for protein concentration and is the result presented is representative of > 3 independent experiments. Lysates were examined by immunoblotting for expression of p13, Tax, and tubulin.
p13 expression decreases viral production and Tax activity. (A) Schematic representation of the HTLV-1 genome that encodes the enzymatic genes gag-pro-pol and env and the regulatory proteins p12, p30, p13, Rex, Tax, and HBZ (arrow indicates antisense direction of the HBZ gene) at the 3′ of the genome. Box under env indicates the second exon that contains the ATG-initiating codons for the envelope p30, Tax, and Rex proteins. The singly spliced orf-II p13 mRNA is illustrated below. (B) Single-letter amino acid code of p13 from HTLV-1LAF. (C) 293T cells transfected with the HTLV-1 molecular clone pAB (0.5 μg) without and with increasing amounts of p13-HA and the reporter construct HTLV-1 LTR-Luc (0.1 μg). DNA concentrations were equalized using the backbone vector pMH. Viral replication was measured 48 hours after transfection by extracellular p19 Gag ELISA (pg/mL) and intracellular anti-p24 Gag immunoblotting. Lysates were examined for p13 and Tax expression. Tubulin was used as a loading control. Tax-dependent LTR-Luc activity was measured and adjusted for protein concentration. p19 Gag concentration and luciferase activity were set to 100% for pAB alone, and the standard deviation represents an average of 3 independent experiments. (D) Quantitative RT-PCR was performed on cytoplasmic and total RNA isolated from 293T cells transfected with pAB (0.5 μg) and p30, p13, or vector control (pMH) (1.0 μg). RNA levels of cytoplasmic over total for gag (top panel) and tax/rex (bottom panel) are shown. The graphs correspond to gag or tax signals adjusted for gapdh level. Graphs represent values from duplicate experiments. (E) 293T cells were transfected with pcTax (50 ng) without (−) or with (+) increasing amounts of p13-HA and the reporter construct HTLV-1 LTR-Luc (0.1 μg). Luciferase activity is normalized for protein concentration and is the result presented is representative of > 3 independent experiments. Lysates were examined by immunoblotting for expression of p13, Tax, and tubulin.
Because p13 corresponds to a truncated carboxyterminal form of the viral protein p30 that inhibits HTLV-1 replication through a posttranscriptional mechanism,23,24 there was the possibility that these 2 proteins inhibited HTLV-1 replication via a common mechanism. However, coexpression of pAB with p13 or p30 revealed that they use different mechanisms of action. As expected, expression of p30 was associated with a decrease in the level of tax/rex mRNA, but not gag mRNA, in the cytoplasm over the total mRNA11 (Figure 1D). No change in tax/rex or gag cytoplasmic/total mRNA was found for p13, suggesting that p13 works to inhibit HTLV-1 production differently than p30 (Figure 1D). We next cotransfected 293T cells with the LTR-Luc reporter plasmid and the Tax cDNA in the absence or presence of increasing amounts of p13-HA plasmid. We found that p13 decreased LTR-Luc activity in a dose-dependent manner (Figure 1E), suggesting that p13 may affect Tax at the transcriptional level.
The p13 protein interacts with Tax and decreases its association with CBP/p300
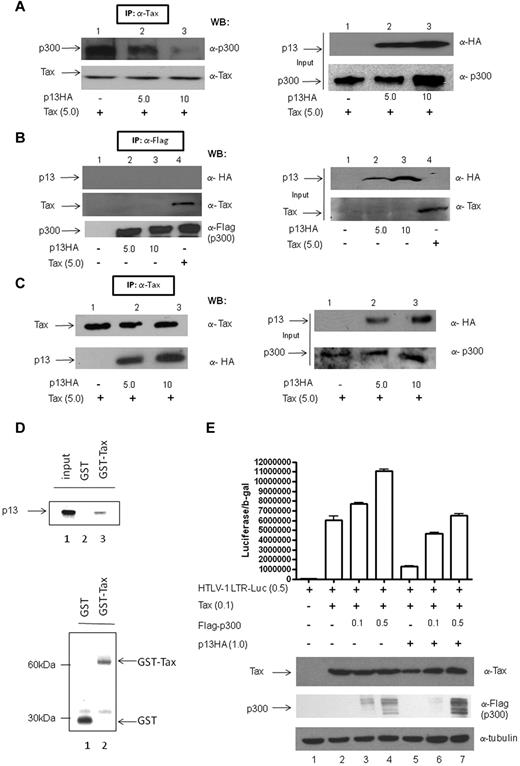
Tax transactivation of the LTR occurs through the recruitment of the coactivators CBP and p300 to the CREB/ATF-Tax complex. To determine whether the negative effect of p13 on Tax transcriptional activity could be due to its interference with the Tax/CBP/p300 interaction, we transfected 293T cells with Tax and increasing amounts of p13-HA. The cell lysates, normalized for equal amount of Tax protein, were immunoprecipitated with anti-Tax antibody. As expected, p300 coimmunoprecipitated with Tax (Figure 2A lane 1).Coexpression of increasing amounts of p13 resulted in a reduction of the amount of p300 that coimmunoprecipitated with Tax (Figure 2A top panel lanes 2 and 3). This was not because of a difference in p300 input levels (Figure 2A right panel), suggesting that p13 may interfere with Tax CBP/p300 interaction. To determine whether p13 competes with Tax for p300 binding, we measured complex formation by transfecting 293T cells with either p13-HA or Tax, followed by immunoprecipitation using anti-p300 antibody (Figure 2B). As expected, Tax coprecipitated with p30042 and we found that p300 does not interact with p13 (Figure 2B compare lane 4 with lanes 2 and 3). Unexpectedly, however, we observed that p13 coimmunoprecipitated with Tax (Figure 2C lanes 2 and 3). This interaction was further verified using an anti-HA antibody that coimmunoprecipitated p13 and Tax in the context of Tax expression from the HTLV-1 molecular clone pAB (data not shown). Using GST pull-down assays, we also showed that p13 directly interacts with Tax (Figure 2D). The expression of an increasing amount of p300 rescued, although not completely, Tax-mediated activation of HTLV-1 LTR-Luc (Figure 2E). These findings demonstrate that p13 binds to Tax, competes with p300 for Tax interaction, and reduces Tax-mediated transcriptional activity on the viral promoter.
p13 competes with p300 for Tax interaction. (A) 293T cells were transfected with (+) or without (−) Tax and with increasing amounts of p13-HA. Tax-normalized lysates were immunoprecipitated using anti-Tax antibody and immunoblotted with anti-p300 and anti-Tax antibodies. The right panel shows immunoblots of total cell lysates (input) with anti-HA and anti-p300 antibodies. (B) 293T cells were transfected with empty vector, without (−) and with (+) pcTax and increasing amounts of p13-HA and with and without p300-Flag (5 μg). Lysates were immunoprecipitated with anti-p300 antibody and both immunoprecipitate and input (right panel) were immunoblotted with anti-HA, anti-Tax, and anti-Flag. (C) 293T cells were transfected with empty vector, p13-HA without (−) and with (+) Tax, or Tax alone. Cell lysates were immunoprecipitated using anti-Tax antibody. Immunoprecipitates (left panel) and total cell lysates (input, right panel) were immunoblotted with anti-HA and anti-Tax antibodies. (D) In vitro association of Tax and p13. In vitro transcribed and translated35 S-methionine–labeled p13 protein was incubated with GST (top panel lane 2) or GST-Tax (top panel lane 3) immobilized on glutathione-agarose beads. Radiolabeled p13 bound to GST-Tax was visualized with a PhosphorImager. The bottom panel is a Coomassie blue staining of the same gel as in the top panel showing GST (lane 1) and GST-Tax (lane 2) protein present in each reaction. (E) 293T cells were transfected with vector control, Tax without and with increasing amounts of p300, or Tax with p13 and increasing amounts of p300. The reporter construct HTLV-1 LTR-Luc was included and luciferase activity adjusted for transfection efficiency using RSV-βgal. DNA concentrations were equalized using the appropriate backbone vectors. SD is given for the average of 3 independent experiments. Lysates were examined by immunoblotting for expression of Tax, Flag-p300, and tubulin.
p13 competes with p300 for Tax interaction. (A) 293T cells were transfected with (+) or without (−) Tax and with increasing amounts of p13-HA. Tax-normalized lysates were immunoprecipitated using anti-Tax antibody and immunoblotted with anti-p300 and anti-Tax antibodies. The right panel shows immunoblots of total cell lysates (input) with anti-HA and anti-p300 antibodies. (B) 293T cells were transfected with empty vector, without (−) and with (+) pcTax and increasing amounts of p13-HA and with and without p300-Flag (5 μg). Lysates were immunoprecipitated with anti-p300 antibody and both immunoprecipitate and input (right panel) were immunoblotted with anti-HA, anti-Tax, and anti-Flag. (C) 293T cells were transfected with empty vector, p13-HA without (−) and with (+) Tax, or Tax alone. Cell lysates were immunoprecipitated using anti-Tax antibody. Immunoprecipitates (left panel) and total cell lysates (input, right panel) were immunoblotted with anti-HA and anti-Tax antibodies. (D) In vitro association of Tax and p13. In vitro transcribed and translated35 S-methionine–labeled p13 protein was incubated with GST (top panel lane 2) or GST-Tax (top panel lane 3) immobilized on glutathione-agarose beads. Radiolabeled p13 bound to GST-Tax was visualized with a PhosphorImager. The bottom panel is a Coomassie blue staining of the same gel as in the top panel showing GST (lane 1) and GST-Tax (lane 2) protein present in each reaction. (E) 293T cells were transfected with vector control, Tax without and with increasing amounts of p300, or Tax with p13 and increasing amounts of p300. The reporter construct HTLV-1 LTR-Luc was included and luciferase activity adjusted for transfection efficiency using RSV-βgal. DNA concentrations were equalized using the appropriate backbone vectors. SD is given for the average of 3 independent experiments. Lysates were examined by immunoblotting for expression of Tax, Flag-p300, and tubulin.
The p13 protein is stabilized by Tax and colocalizes with Tax in nuclear speckles
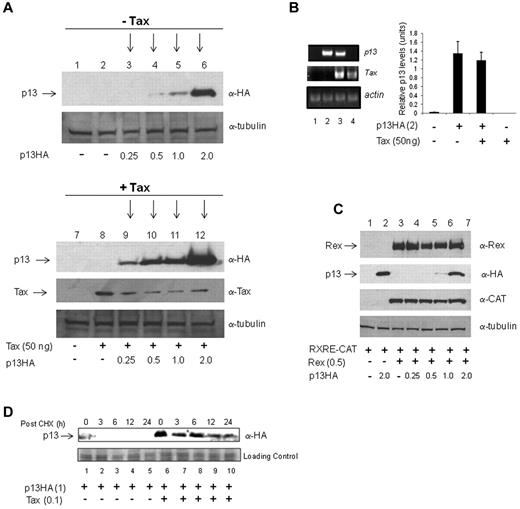
We observed that the level of the p13 protein was increased in the presence of Tax. To substantiate this observation, we expressed increasing amounts of p13 in the absence (Figure 3A top panel) or in the presence (Figure 1E and Figure 3A bottom panel) of Tax. When 0.25 and 0.5 μg of the p13 cDNA was transfected into 293T cells, the p13 protein was barely detectable in the absence of Tax (Figure 3A top panel). In contrast, in the presence of Tax, p13 was clearly visible even at the lowest concentration of 0.25μg (Figure 3A compare lanes 3 and 4 with lanes 9 and 10 of top and bottom panels). The difference in the level of the p13 protein was not because of Tax-mediated increased transcription from the CMV promoter that drives p13-HA expression, because no significant differences in mRNA expression of p13 was observed in the presence of Tax (Figure 3B). The effect of Tax on p13 stabilization was specific, because Rex did not stabilize p13, and p13 did not affect the ability of Rex to promote CAT expression from the responsive reporter construct RxRE-CAT (Figure 3C). Stabilization of the p13 protein by Tax was further confirmed in a time-course experiment, in which protein expression was blocked by cycloheximide (Figure 3D).
p13 is stabilized by Tax. (A) 293T cells were transfected without (−) and with increasing amounts of p13 and lysates immunoblotted by anti-HA and anti-tubulin antibodies (top panel, −Tax). The +Tax (bottom panel) is the same experiment presented in Figure 1E and added here for comparison. (B) RT-PCR was performed on 293T cells transfected with vector control DNA, p13 alone, p13 and Tax, or Tax alone. Total RNA was collected 48 hours after transfection and subjected to real-time PCR using primers specific for p13 (top panel), Tax (middle panel), or actin (bottom panel). The graph represents p13 mRNA levels from 3 independent experiments. (C) 293T cells were transfected with the CMV-driven, Rex-dependent reporter plasmid pRXRE-CAT and Rex without and with increasing amounts of p13-HA. Whole-cell lysates were immunoblotted for expression of Rex, p13-HA, CAT, and tubulin. (D) To measure the half-life of the p13 protein, 293T cells were transfected with p13 in the absence or presence of Tax. The cells were treated with 10μM cycloheximide 48 hours after transfection. Whole-cell lysates were prepared at the indicated times and the protein levels were examined by Western blot analysis for p13 (anti-HA). The bottom panel is a Coomassie blue–stained gel shown as a loading control.
p13 is stabilized by Tax. (A) 293T cells were transfected without (−) and with increasing amounts of p13 and lysates immunoblotted by anti-HA and anti-tubulin antibodies (top panel, −Tax). The +Tax (bottom panel) is the same experiment presented in Figure 1E and added here for comparison. (B) RT-PCR was performed on 293T cells transfected with vector control DNA, p13 alone, p13 and Tax, or Tax alone. Total RNA was collected 48 hours after transfection and subjected to real-time PCR using primers specific for p13 (top panel), Tax (middle panel), or actin (bottom panel). The graph represents p13 mRNA levels from 3 independent experiments. (C) 293T cells were transfected with the CMV-driven, Rex-dependent reporter plasmid pRXRE-CAT and Rex without and with increasing amounts of p13-HA. Whole-cell lysates were immunoblotted for expression of Rex, p13-HA, CAT, and tubulin. (D) To measure the half-life of the p13 protein, 293T cells were transfected with p13 in the absence or presence of Tax. The cells were treated with 10μM cycloheximide 48 hours after transfection. Whole-cell lysates were prepared at the indicated times and the protein levels were examined by Western blot analysis for p13 (anti-HA). The bottom panel is a Coomassie blue–stained gel shown as a loading control.
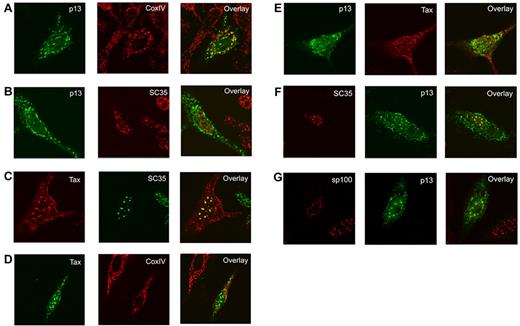
We next investigated the cellular localization of p13 in the presence of Tax by expressing the p13 and Tax proteins alone or together in HeLa cells. As expected,23,24 when expressed in the absence of Tax, p13 localized to the cytoplasm and colocalized with the CoxIV protein in the mitochondria (Figure 4A). The p13 protein localized exclusively to the cytoplasm and no colocalization was observed with the essential splicing factor SC35, which localizes to nuclear speckles (Figure 4B). As reported previously, Tax localized diffusely or in dots in the cytoplasm and in a speckled pattern in the nuclei,43,44 and colocalized with SC35 (Figure 4C) but not with CoxIV (Figure 4D). Surprisingly, coexpression of Tax with p13 resulted in the colocalization of a portion of both proteins with Tax in the nuclear speckles (Figure 4E). Furthermore, the p13-Tax speckles colocalized with SC35 (Figure 4F) but not with the POD/PML body-localizing protein sp100 (Figure 4G). These data demonstrate that in the presence of Tax, a portion of the p13 protein is routed to nuclear speckles.
p13 translocates to the nucleus when coexpressed with Tax. HeLa cells were transfected with p13-HA (2μg) or Tax (0.5μg) and fixed 24 hours after transfection (A-D). DNA concentrations were adjusted using pMH plasmid. Subcellular localization of p13 was examined by immunofluorescence with anti-HA and anti-CoxIV (A) and anti-HA and anti-SC35 (B). Tax localization with anti-Tax and anti-SC35 (C) and anti-Tax and anti-CoxIV (D) antibodies is shown. (E-G) HeLa cells transfected with the p13-HA (2μg) and Tax (0.5μg) constructs and fixed 24 hours after transfection. Subcellular localization of Tax and p13 was examined by immunofluorescence with anti-HA and anti-Tax antibodies (E). Subcellular localization of p13 was analyzed with anti-HA and anti-SC35 (F) and with anti-HA and anti-sp100 and (G). An overlay of these images is shown on the right.
p13 translocates to the nucleus when coexpressed with Tax. HeLa cells were transfected with p13-HA (2μg) or Tax (0.5μg) and fixed 24 hours after transfection (A-D). DNA concentrations were adjusted using pMH plasmid. Subcellular localization of p13 was examined by immunofluorescence with anti-HA and anti-CoxIV (A) and anti-HA and anti-SC35 (B). Tax localization with anti-Tax and anti-SC35 (C) and anti-Tax and anti-CoxIV (D) antibodies is shown. (E-G) HeLa cells transfected with the p13-HA (2μg) and Tax (0.5μg) constructs and fixed 24 hours after transfection. Subcellular localization of Tax and p13 was examined by immunofluorescence with anti-HA and anti-Tax antibodies (E). Subcellular localization of p13 was analyzed with anti-HA and anti-SC35 (F) and with anti-HA and anti-sp100 and (G). An overlay of these images is shown on the right.
In the presence of Tax, p13 is stabilized and rerouted to the nucleus
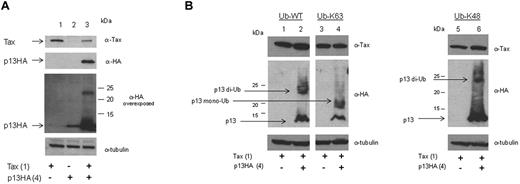
Overexposure of protein blots in which p13 was coexpressed with Tax resulted in the detection of protein smears by the α-HA antibody, suggesting posttranslational modification of p13 (Figure 5A). To investigate whether, in the presence of Tax, p13 could be ubiquitinated, Tax and p13 were coexpressed with wild-type ubiquitin (Ub-WT), K-63 ubiquitin in which all lysines except K-63 were mutated to arginines (Ub-K63), or ubiquitin K48 in which all lysines except K-48 were mutated to arginines (Ub-K48) (Figure 5B). When p13 was coexpressed with Tax and Ub-WT, immunoblotting analysis demonstrated the presence of a protein of the expected size for p13 and an additional band of approximately 23 kDa (Figure 5B lane 2). Coexpression of p13, Tax, and Ub-K63 yielded a lower–molecular weight band around 18 kDa that reacted with the anti-HA antibody (Figure 5B lane 4). In contrast, coexpression of Ub-K48 with p13 and Tax resulted in the appearance of a larger protein band similar to that from coexpression of Ub-WT (Figure 5B lane 6). The level of p13 was higher in the presence of Ub-K48 than in the presence of Ub-K63, suggesting that stabilization of p13 could be ubiquitin K-48 dependent (Figure 5B compare lanes 2, 4, and 6). The p13 protein does not contain lysines (Figure 1B), the canonical target for ubiquitin, raising the possibility that ubiquitination of p13 may occur on other amino acid residues such as cysteine through thioester bonds45 or on serine /threonine.46 The unique cysteine at position 27 of p13 is likely not the target of ubiquitination, because the p13C27A-HA mutant was stabilized by Tax and a protein smear was observed by Western blot analysis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). N-ethylmaleimide (NEM) treatment preserved mono-ubiquitination of p13C27A and Tax. No difference in Tax mono-ubiquitination was observed in the presence or absence of p13C27A (supplemental Figure 1 top panel compare lanes 7 and 8). These data suggest that in the presence of Tax, p13 undergoes a posttranslational modifications in part because of mono-ubiquitination on residues other than cysteine in position 27.
p13 is ubiquitinated in the presence of Tax. (A) 293T cells were transfected with p13 (4.0 μg; lanes 2 and 3) in the absence or presence of Tax (1.0 μg). Lysates were prepared with buffer containing NEM (10μM) and immunoblots were made with antibodies to Tax, HA, and tubulin. (B) 293T cells were transfected with Tax (1.0 μg) and Ub-WT (2.0 μg), Ub-K63 (2.0 μg), or Ub-K48 (2.0 μg) and p13-HA (4.0 μg) as indicated. Lysates were immunoblotted for the expression of Tax, p13-HA, and tubulin. Arrows indicate ubiquitinated forms of p13.
p13 is ubiquitinated in the presence of Tax. (A) 293T cells were transfected with p13 (4.0 μg; lanes 2 and 3) in the absence or presence of Tax (1.0 μg). Lysates were prepared with buffer containing NEM (10μM) and immunoblots were made with antibodies to Tax, HA, and tubulin. (B) 293T cells were transfected with Tax (1.0 μg) and Ub-WT (2.0 μg), Ub-K63 (2.0 μg), or Ub-K48 (2.0 μg) and p13-HA (4.0 μg) as indicated. Lysates were immunoblotted for the expression of Tax, p13-HA, and tubulin. Arrows indicate ubiquitinated forms of p13.
p13 modulates HTLV-1 expression in infected cells
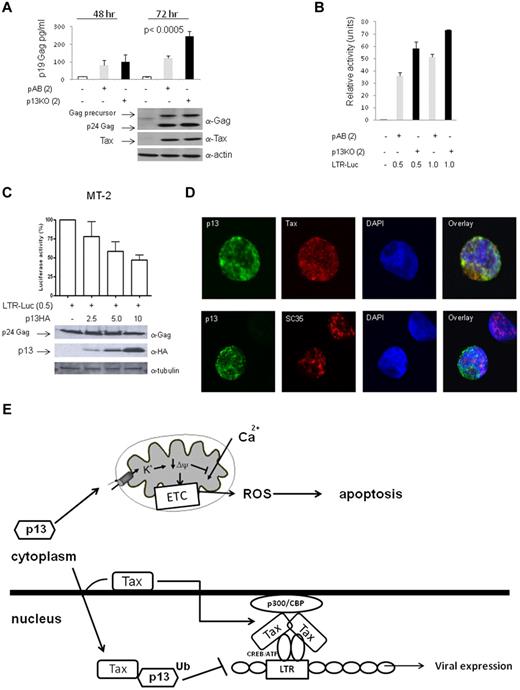
In HTLV-1–infected cells, the mRNA that encodes p13 is expressed at much lower levels than Tax. Therefore, even though Tax stabilizes p13 and likely changes the stoichiometry of the 2 proteins, the relevance to HTLV-1 infection reported in Figure 1, could be questioned because of the artificial overexpression of proteins in transfection systems. To address this point, we generated a proviral clone in which the initiating ATG of the p13 codon was mutated to ATT to generate the p13-knockout clone (p13KO). This nucleotide change does not result in a change of the amino acid sequence of the overlapping genes HBZ or p12; however, it introduces a change in the internal methionine of p30 (the initiation codon of p13) to an isoleucine. This amino acid change does not affect p30 function (our unpublished results). Transient expression of wild-type HTLV-1 and the p13KO virus demonstrated that the absence of p13 was associated with significantly elevated virus production over time (P < .0005 at 72 hours after transfection; Figure 6A). Whereas we did not observe a significant change in the expression of Tax from the 2 molecular clones (Figure 6A bottom panels), Tax activity was increased in cells transfected with the p13KO clone (Figure 6B). To investigate whether p13 also suppresses viral replication in a chronically infected T cell, we transfected the HTLV-1 LTR-Luc reporter construct together with increasing amounts of p13-HA in the HTLV-1 producer T-cell line MT-2. p13 decreased Tax-dependent luciferase activity in MT-2 cells in a dose-dependent manner (Figure 6C). Confocal microscopy of the p13-HA–transfected MT-2 cells revealed that p13 colocalized with Tax in the cytoplasm and partially colocalized with Tax and SC35 in nuclear speckles (Figure 6D). These results demonstrated that p13-mediated suppression of viral replication occurs when p13 is expressed naturally from a HTLV-1 molecular clone, as was indirectly suggested by the finding that p13 ablation results in an increased virus production. Furthermore, p13 also appears to be stabilized and rerouted to the nucleus in T cells, the natural target of HTLV-1 infection.
p13 modulates virus expression in HTLV-1–infected cells. (A) 293T cells were transfected with HTLV-1 molecular clones pAB (gray bars) or p13KO (black bars). Culture supernatants were harvested 48 and 72 hours after transfection and extracellular p19 Gag levels measured by ELISA assay. Western blot analysis for intracellular p24 Gag, Tax, and actin was performed on whole-cell lysates from 72-hour cultures. The values given are an average of 4 independent experiments. (B) Luciferase activity for the reporter construct LTR-Luc (0.5 μg or 1.0 μg) was measured for 293T cells transfected with HTLV-1 molecular clones pAB (gray bars) or p13KO (black bars). Values are an average of 2 independent experiments and are adjusted for transfection efficiency using pRLTK-Luc. (C) MT-2 cells were transfected with the reporter construct HTLV-1 LTR-Luc and without (−) and with increasing amounts of p13-HA. Lysates were immunoblotted for the expression of p24 Gag, p13-HA, and tubulin. Luciferase activity was measured 48 hours after transfection and normalized for protein concentration. Luciferase activity was set at 100% for cells transfected in the absence of p13-HA. SD is given for the average of 3 independent experiments. (D) MT-2 cells were transfected with p13-HA and, 48 hours after transfection, cells were added to fibronectin-precoated coverslips, fixed, and stained with anti-HA, anti-Tax, or anti-SC35 antibodies for analysis by confocal microscopy. DAPI staining identifies the cell nucleus. (E) Schematic representation of the predicted interplay between Tax and p13. Tax binds to the viral LTR in the nucleus through interaction with CREB. Tax activates transcription through the recruitment of basal transcription machinery and coactivators such as p300/CBP. The p13 protein expressed alone resides in the mitochondrial membrane, affects the electron transport chain (ETC), the membrane potential (Δψ), and the production of reactive oxygen species (ROS). In the presence of Tax, however, p13 is stabilized, becomes ubiquitinated, and part of it interacts with Tax, colocalizes with Tax to nuclear speckles, and reduces viral expression.60
p13 modulates virus expression in HTLV-1–infected cells. (A) 293T cells were transfected with HTLV-1 molecular clones pAB (gray bars) or p13KO (black bars). Culture supernatants were harvested 48 and 72 hours after transfection and extracellular p19 Gag levels measured by ELISA assay. Western blot analysis for intracellular p24 Gag, Tax, and actin was performed on whole-cell lysates from 72-hour cultures. The values given are an average of 4 independent experiments. (B) Luciferase activity for the reporter construct LTR-Luc (0.5 μg or 1.0 μg) was measured for 293T cells transfected with HTLV-1 molecular clones pAB (gray bars) or p13KO (black bars). Values are an average of 2 independent experiments and are adjusted for transfection efficiency using pRLTK-Luc. (C) MT-2 cells were transfected with the reporter construct HTLV-1 LTR-Luc and without (−) and with increasing amounts of p13-HA. Lysates were immunoblotted for the expression of p24 Gag, p13-HA, and tubulin. Luciferase activity was measured 48 hours after transfection and normalized for protein concentration. Luciferase activity was set at 100% for cells transfected in the absence of p13-HA. SD is given for the average of 3 independent experiments. (D) MT-2 cells were transfected with p13-HA and, 48 hours after transfection, cells were added to fibronectin-precoated coverslips, fixed, and stained with anti-HA, anti-Tax, or anti-SC35 antibodies for analysis by confocal microscopy. DAPI staining identifies the cell nucleus. (E) Schematic representation of the predicted interplay between Tax and p13. Tax binds to the viral LTR in the nucleus through interaction with CREB. Tax activates transcription through the recruitment of basal transcription machinery and coactivators such as p300/CBP. The p13 protein expressed alone resides in the mitochondrial membrane, affects the electron transport chain (ETC), the membrane potential (Δψ), and the production of reactive oxygen species (ROS). In the presence of Tax, however, p13 is stabilized, becomes ubiquitinated, and part of it interacts with Tax, colocalizes with Tax to nuclear speckles, and reduces viral expression.60
Discussion
HTLV-1 propagates by both clonal expansion of infected T cells and de novo viral infection.40 Therefore, tempering viral expression in human and nonhuman primates may be essential to escape immune surveillance. The negative regulators of viral replication, the p30 and HBZ proteins, limit tax/rex RNA export and Tax and Rex expression47 and Tax activity through attenuation of CREB and AP-1 activation,17 respectively. In the present study, we report that the HTLV-1 protein p13 is an additional negative regulator of viral replication that acts by interfering with Tax transcriptional activity through a mechanism distinct from that of HBZ.
p13 contains a mitochondrial targeting signal spanning amino acids 21-3025,26 and localizes to mitochondria when expressed alone. However, we found here that when p13 was coexpressed with Tax, a portion of p13 colocalized with Tax in SC35-positive nuclear speckles. Furthermore, p13 directly interacted with Tax, competed with p300 for Tax binding, and decreased viral gene expression. The p13 protein was stabilized in the presence of Tax, likely by ubiquitination.
Ubiquitin conjugation influences diverse cellular processes, including protein degradation, endocytic trafficking, histone activation, DNA repair, and cell survival.48 The p13 protein does not contain any lysines, the canonical target for ubiquitin, suggesting that unconventional ubiquitination occurs. Ubiquitination has been shown to be fused linearly to the α-NH2 group at the α-NH2 group at the N-terminal of a substrate,49 to internal cysteine residues through thioester bonds,45 or to serines or threonines.46,50 This has been demonstrated for both proteins that contain lysines and proteins that do not. The p13 protein is not ubiquitinated on cysteine through a thioester bond, because the p13C27A mutant, which lacks the unique cysteine residue at position 27, was also ubiquitinated. However, as demonstrated by Wang et al,46 ubiquitination of serine and threonine residues can occur on the C-terminal of MHC-1 by the viral E3 ligase mK3, supporting the hypothesis that p13 could be ubiquitinated on any of its 11 serines and 8 threonines. More recently, Tokarev et al51 showed that serine-threonine ubiquitination of the restriction factor Bst-2/tetherin plays a role in vpu's counteraction of innate defenses. In the absence of Tax, neither p13 nor the p13C27A mutant are ubiquitinated, stabilized, or localized to the nucleus. There have been many studies conducted on the posttranslational modification of the viral Tax protein. Tax has been shown to be both mono- and polyubiquitinated.36,44,52-55 Ubiquitination of Tax is important for both activation of the canonical and noncanonical NF-κB pathway and localization to the cytoplasm.44 Conversely, Tax is also sumoylated, which directs its nuclear localization and is important for both NF-κB activation and HTLV-1 LTR activity. Interestingly, sumoylation and ubiquitination occur on the overlapping lysines 280 and 284, making them mutually exclusive.44 Recently, it was demonstrated that Tax is mainly K63-linked polyubiquitinated, and that this ubiquitination is dependent on the E2 ubiquitin-conjugation enzyme Ubc13, which is critical for NEMO interaction and NF-κB activation.36 The Tax mutant M22 is deficient in NF-κB activation, is not ubiquitinated, and does not interact with Ubc13.36,53 In the cytoplasm, Tax also concentrates in perinuclear speckles that localize near the centrosome, the microtubule-organizing center, and the cis-Golgi.54,56,57 Recently, K63-polyubiquitinated Tax was shown to recruit inhibitor κB kinase complex to this perinuclear compartment, supporting a model in which Tax activates inhibitor κB kinase in a centrosome-dependent manner.56 However, preliminary data suggest that Tax itself may not need to be ubiquitinated to stabilize the p13 protein. We found that the Tax mutant M22, which has been shown to be poorly ubiquitinated, is still capable of stabilizing p13 (data not shown).
Recently, Yan et al identified PDLIM2, a novel ubiquitin E3 ligase that induces K63- and K48-dependent ubiquitination of Tax and redirects Tax to the nuclear matrix, where Ub-Tax is degraded.58 In our studies, we found that whereas Tax expression stabilized p13, Tax levels were modestly reduced in the presence of p13. In addition, we observed that p13 and Tax colocalized to nuclear speckles. It is possible that p13 participates in PDLIM2-regulated Tax ubiquitination and that PDLIM2 may participate in p13 ubiquitination. Further studies are required to address the role of PDLIM2 in p13 regulation.
Our data demonstrate that posttranslational modifications are likely crucial for p13 cellular localization and function. A possible model for the interplay between Tax and p13 is shown in Figure 6E. Briefly, Tax recruits K48-specific E2 and E3 enzymes that result in serine/threonine ubiquitination and possibly other posttranslational modifications of p13, or, as suggested by others, in Tax functioning as an E3 ligase.36 The p13 protein is stabilized through K48-dependent di-ubiquitination of serine and/or threonine residues, resulting in rerouting of p13 to the nucleus. Ghorbel et al identified a nuclear localization signal at the C-terminus of p30,59 a region that corresponds to the C-terminus of p13. It is possible that p13 ubiquitination leads to a conformational change resulting in exposure of this nuclear localization sequence. In addition, p13 interaction with Tax may mask the mitochondria-targeting signal of p13. Expression of the p13 protein appears to reduce the access of Tax to the LTR promoter and its association with CBP/p300. Our results are consistent with a model that, during active viral replication, which requires Tax expression, the p13 protein is modified and becomes a negative regulator of viral replication. Our finding that selective ablation of p13 from a biologically active molecular clone of HTLV-1 enhances Tax activity and virus production further supports this model. At this point, we cannot rule out that the mutation in p13KO completely knocks out p13 expression and that alternative initiation does not occur at methionine 39. However, the fact that more virus is produced in the transfection of p13KO suggests that p13 function has been impaired. Tax mRNA is expressed at a 100- to 1000-fold higher level than the p13 mRNA. However, we show that the stoichiometry of the 2 proteins is regulated as follows: by increasing p13 stability, Tax decreases its own activity. Therefore, feedback regulation of virus expression is not only mediated by p30 and HBZ, but also by Tax in concert with p13. We have demonstrated that the mechanism of negative regulation of HTLV-1 replication, which is mediated by p13, is relevant in vitro for virus replication. However, further studies will be needed to investigate the relevance of p13 in vivo in the infected host.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Teresa Habina for editorial assistance; V. Ciminale and D. D'Agostino for helpful discussions; and Nancy Van Prooyen, Dustin Edwards, and Cody Buchmann for critical reading of the manuscript.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: V.A. and C.A.P.-M. designed and performed research, analyzed the data, and wrote the manuscript; V.V. generated the p13KO virus; U.S.-D., M.B., R.W.P., V.C., R.F. and C.N. performed the research and analyzed the data; and G.F. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Genoveffa Franchini, National Cancer Institute, Bldg 41, Rm D-804, Bethesda, MD 20892; e-mail: franchig@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal