Abstract
In adults with sickle cell disease (SCD), an increased tricuspid regurgitation velocity (TRV) by Doppler echocardiography is associated with increased morbidity and mortality. Although sildenafil has been shown to improve exercise capacity in patients with pulmonary arterial hypertension, it has not been evaluated in SCD. We therefore sought to determine whether sildenafil could improve exercise capacity in SCD patients with increased TRV and a low exercise capacity. A TRV ≥ 2.7 m/s and a 6-minute walk distance (6MWD) between 150 and 500 m were required for enrollment in this 16-week, double-blind, placebo-controlled sildenafil trial. After 74 of the screened subjects were randomized, the study was stopped early due to a higher percentage of subjects experiencing serious adverse events in the sildenafil arm (45% of sildenafil, 22% of placebo, P = .022). Subject hospitalization for pain was the predominant cause for this difference: 35% with sildenafil compared with 14% with placebo (P = .029). There was no evidence of a treatment effect on 6MWD (placebo-corrected effect −9 m; 95% confidence interval [95% CI] −56-38; P = .703), TRV (P = .503), or N-terminal pro-brain natriuretic peptide (P = .410). Sildenafil appeared to increase hospitalization rates for pain in patients with SCD. This study is registered at www.clinicaltrials.gov as NCT00492531.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1185.
Disclosures
Robyn J. Barst has received support for research grants and/or consulting from Actelion, Eli Lilly, Gilead, GlaxoSmithKline, Medtronics, Bayer, Ikaria, Pfizer, Novartis, United Therapeutics, and NHLBI. Kathryn L. Hassell has received funding from NHLBI as a site subinvestigator for the walk-PHaSST study. Gregory J. Kato has received research support from a cooperative research and development agreement between the NIH and Ikaria INO Therapeutics and from the Division of Intramural Research of the NIH. Victor R. Gordeuk has received research support from Biomarin, TRF-Pharma, and Emmaus Pharmaceuticals. J. Simon R. Gibbs has received research support from Actelion and Bayer and has served on advisory boards and/or received lecture fees from Actelion, Bayer, GlaxoSmithKline, Lilly, Pfizer, and United Therapeutics. Lakshmanan Krishnamurti has received research support from the NHLBI (HB-06-06). Reda E. Girgis has served as an advisory board member for Actelion, Gilead, and United Therapeutics and has received research support from Actelion, Gilead, United Therapeutics, Pfizer, and ICOS. Erika Berman Rosenzweig has received research support from Pfizer. David B. Badesch has received honoraria for service on steering committees or advisory boards (or as a consultant) to the following companies working in the area of PH: Actelion/CoTherix, Gilead/Myogen, Encysive, Pfizer, Mondobiotech/Mondogen, Biogen IDEC, United Therapeutics/Lung Rx, GlaxoSmithKline, Lilly/ICOS, Bayer, Ikaria, and Arena, and has received grant support for clinical studies from GlaxoSmithKline, Actelion/CoTHerix, Gilead/Myogen, Pfizer/Encysive, United Therapeutics/Lung Rx, Lilly/ICOS, Bayer, and Novartis. Sophie Lanzkron received award K23HL083089-03 from the NHLBI. Mark T. Gladwin has received research support in the form of a Collaborative Research and Development Agreement between the US Government and INO Therapeutics and is listed as a co-inventor on a US Government Patent for the use of nitrite salts for cardiovascular indications. The remaining authors; the Associate Editor Narla Mohandas; and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the rationale for use of sildenafil in patients with SCD and an elevated TRV
Describe the safety and tolerability of sildenafil in patients with SCD and an elevated TRV, based on findings from a randomized, controlled trial
Describe the efficacy of sildenafil in these patients for increasing low exercise capacity in that trial
Release date: July 28, 2011; Expiration date: July 28, 2012
Introduction
In adult patients with sickle cell disease (SCD), an increased tricuspid regurgitation velocity (TRV) as assessed by Doppler echocardiography is associated with a high risk of death.1-6 Similarly, an increase in pulmonary artery systolic pressure, also as assessed by Doppler echocardiography, is associated with an increased risk of death in individuals older than 45 years without SCD.7 In SCD, the risk of developing an elevated TRV increases with age, hemolysis (defined by indirect markers of hemolysis), renal insufficiency (defined as an elevated creatinine level), iron overload (defined as an elevated ferritin level), and systemic hypertension (defined as an elevated systolic systemic blood pressure).2,5,6 Chronic intravascular hemolysis has been shown to cause endothelial dysfunction (defined as a resistance to nitric oxide–mediated vasodilation or impaired responses to L-NMMA or acetylcholine8,9 ) secondary to the nitric oxide–scavenging effects of plasma hemoglobin and catabolism of L-arginine by erythrocyte arginase 1.10 These mechanisms suggest that therapeutic interventions enhancing the effects of nitric oxide could be beneficial.9-11
The phosphodiesterase-5 (PDE5) inhibitor sildenafil has been shown to improve pulmonary hemodynamics and exercise capacity in adults with group 1 pulmonary hypertension (PH) (defined by right heart catheterization [RHC] as a mean pulmonary artery pressure ≥ 25 mmHg, a pulmonary artery occlusion pressure or a left ventricular end diastolic pressure ≤ 15 mmHg, and an increased pulmonary vascular resistance)12 : idiopathic or heritable pulmonary arterial hypertension (PAH), or PAH associated with connective tissue diseases or repaired congenital systemic to pulmonary shunts.13 Further, sildenafil has been shown to improve exercise capacity in patients with left ventricular systolic dysfunction and pulmonary venous hypertension (defined via cardiac catheterization as a mean pulmonary artery pressure ≥ 25 mmHg and a pulmonary artery occlusion pressure or a left ventricular end diastolic pressure ≥ 15 mmHg)14,15 and in healthy volunteers exposed to experimental hypoxia or high altitude,16,17 suggesting a beneficial role of PDE5 inhibition in patients with group 2 PH (PH with left heart disease) and group 3 PH (PH associated with lung diseases and/or hypoxemia). PDE5 inhibition also prevents and reverses cardiac hypertrophy in mice exposed to chronic left ventricular pressure overload induced by transverse aortic constriction.18 Two uncontrolled case series have suggested that sildenafil treatment for patients with SCD and an increased right ventricular systolic pressure estimated by an increased TRV appeared to be well tolerated, improved exercise capacity, and decreased TRV.19,20
Based on these observations, we sought to determine whether sildenafil would also be efficacious in patients with SCD and an elevated TRV. We designed a double-blind, placebo-controlled trial to assess the safety, tolerability, and efficacy of sildenafil in patients with SCD who had both an elevated TRV and decreased exercise capacity. Because this study was not designed as a trial for patients with cardiac catheterization–confirmed group 1 PAH,12 RHC was not required in all study subjects. Our objective was to evaluate the effects of sildenafil on the wider SCD population with low exercise capacity associated with an increased Doppler-estimated pulmonary artery systolic pressure (as assessed by the TRV).2,5,6,21 We hypothesized that sildenafil might offer benefit to this large subpopulation of at-risk patients even in the absence of PH confirmed by cardiac catheterization.
Methods
Selection of subjects
Subjects with sickle cell hemoglobinopathy, ≥ 12 years of age were eligible for the screening study, and those with a Doppler-defined elevated estimated right ventricular systolic pressure (peak TRV ≥ 2.7 m/s obtained by Doppler echocardiography on 2 separate measurements) and a 6MWD of 150-500 m were eligible for the interventional trial. For a complete list of inclusion/exclusion criteria, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Subjects receiving hydroxyurea therapy were required to be on a stable dose for at least 1 month before the start of the study. Exclusion criteria included prior or current treatment with prostacyclin analogs, endothelin receptor antagonists, other phosphodiesterase inhibitors, or L-arginine. During the treatment portion of the study, subjects who required initiation of hydroxyurea, chronic transfusion therapy, protease inhibitor therapy for human immunodeficiency virus, or other treatments for PH were removed from the study, but remained in the intention-to-treat analysis. Local institutional review boards or ethics committees approved the protocol, and written informed consent was obtained from all study subjects in accordance with the Declaration of Helsinki.
Study design
The study was designed to screen approximately 1000 subjects to enroll 132 subjects in a nested intervention trial. Based on a priori sample-size calculations, a sample of 132 subjects (n = 66 sildenafil; n = 66 placebo) with an assumed 10% dropout rate was deemed to afford the primary analysis 90% power. These calculations included a 2-sided α of 0.05 to detect a 16-week change in 6MW distance of 40 m between the 2 treatment groups because this is considered a clinically meaningful change in 6MW distance.
In the screening trial, subjects were evaluated by self-reported history modeled after the NIH-PH screening survey,6 physical examination, laboratory screening, transthoracic Doppler echocardiography, and 6MW test. The intervention trial was a 16-week, double-blind, placebo-controlled trial conducted in 10 centers (9 in the United States and 1 in the United Kingdom). Sites were recruited for their combined expertise as referral centers in SCD and PH as well as access to the target population. The randomization used an adaptive algorithm,22 balancing treatment group assignment across both TRV strata (2.7-2.9 m/s and ≥ 3.0 m/s) and clinical sites. Subjects were randomized 1:1 to sildenafil or placebo via an Internet and voice randomization system. Investigators, subjects, and the pharmacy were blinded to study treatment. Bottles of study medication were prepackaged with unique bottle numbers, and the Internet and voice randomization system assigned bottle numbers at the time of dosing. Subjects received 20 mg of oral sildenafil or matching placebo 3 times daily for 6 weeks, followed by 40 mg 3 times daily for 4 weeks, followed by 80 mg 3 times daily for 6 weeks (as tolerated). A maximum study drug dose of 80 mg 3 times daily was used because it achieved the most significant improvement in hemodynamic parameters in the pivotal sildenafil trial in patients with PAH.13
Subjects with TRV ≥ 3.0 m/s underwent RHC at baseline (before randomization) and after 16 weeks of study drug therapy. At baseline, the acute hemodynamic effects of inhaled nitric oxide (40 ppm for at least 10 minutes) and open-label sildenafil (60 mg orally at 30 and 60 minutes postdose) were evaluated (see supplemental Methods for details of the acute hemodynamic protocol). After 16 weeks of therapy, the same procedure was repeated, but blinded study medication was administered instead of open-label sildenafil. Based on a priori sample size calculations, a sample of 66 subjects (n = 33 sildenafil; n = 33 placebo) was deemed to afford analysis 80% power. These calculations included a 2-sided α of 0.05 to detect a 16-week change in mean pulmonary artery pressure and pulmonary vascular resistance between the 2 treatment groups.
Outcome measures
The primary efficacy end point was change in exercise capacity (assessed by 6MWD) from baseline to week 16. Additional efficacy measures included changes in TRV, hemodynamic parameters (for subjects undergoing RHC), Borg Dyspnea Score (BDS), World Health Organization (WHO) functional classification for PH, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, quality of life (as assessed by the SF-36), and 3 adjudicated secondary end points (right heart failure, acute chest syndrome, and time to clinical deterioration of PH), self-reported clinical PH outcomes, initiation of additional PH therapies, and assessments of subjective pain using the Brief Pain Inventory daily for 7 days around each study visit. Safety assessments included adverse event reporting, clinical laboratory assessments, physical examination, and vital signs. Safety and efficacy assessments were conducted at baseline and at weeks 6, 10 (excluding echocardiography), and 16.
Statistical analysis
All efficacy and safety analyses were conducted on the intention-to-treat population, defined as all randomized subjects, regardless of therapy received. One subject in the sildenafil group was randomized but never received therapy. All other subjects received the therapy to which they were randomized.
The primary efficacy analysis was an ANCOVA on the change in 6MWD from baseline to week 16, comparing treatment groups, and controlling for baseline 6MWD, site, and TRV stratum. Predefined imputation rules for the week-16 6MWD were as follows: a value of 0 m was imputed for subjects who died during the intervention trial. Otherwise, subjects without a week-16 assessment had their last observation carried forward. Once the study was stopped, the week-16 RHCs were not performed for subjects with TRV ≥ 3.0 m/s.
The study was stopped early by the NHLBI following the unanimous recommendation from the Data and Safety Monitoring Board (DSMB) because significantly more sildenafil-treated subjects experienced a serious adverse event (SAE) compared with placebo. In addition, there was no observed improvement in the primary efficacy measure of 6MWD or by an estimation of results per futility analysis. These interim efficacy analyses were provided at the request of the Data and Safety Monitoring Board and were not prespecified a priori.
All prespecified analyses were conducted according to the protocol and statistical analysis plan, despite the reduced number of subjects (n = 74 randomized vs 132 planned) and the resultant reduced power to detect treatment group differences. Treatment group comparisons for continuous measures were assessed via ANCOVA, controlling for the TRV stratum. Treatment group differences in adverse events and SAEs were evaluated via Cochran-Mantel-Haenszel χ2 tests, controlling for TRV stratum. Post hoc analyses, conducted to help clarify this study's unexpected results and generate hypotheses for future research, are clearly noted.
To assess the possibility that subjective experiences of pain may have differed between treatment groups, we added an additional post hoc Brief Pain Inventory analysis: the outcomes were simultaneously modeled using a mixed effect repeated measures model. A random subject intercept was used that gives all the tests for a subject an equal correlation. An autoregressive structure was applied across the visits for a given test, allowing measurements for that test to be more highly correlated if they were collected at adjacent visits. Global P values indicate whether there was a treatment difference across all Brief Pain Inventory items at all time points and at each time point. We also included a post hoc propensity score analysis designed to evaluate whether uncontrolled baseline imbalances between treatment groups may have influenced the effect of the treatment group on SAEs. In general, propensity score analyses are intended to balance groups on potential confounding factors to get a more accurate estimate of the effect of treatment. This is accomplished by building a model to predict the probability that a particular subject will receive one particular treatment instead of the other. This propensity score is then included as a covariate in a separate model to predict treatment effect. In this analysis, we first created a propensity score using relevant baseline parameters (ie, age, sex, SCD genotype, baseline hydroxyurea use, creatinine and NT-proBNP, and self-reported history of episodes of severe pain requiring hospital admission in the previous year) to predict treatment group by a logistic regression. The resultant score was used in a second logistic regression, including site and stratum, to determine whether treatment group differences in SAEs still existed after adjusting for each subject's propensity score.
Results
Subject characteristics
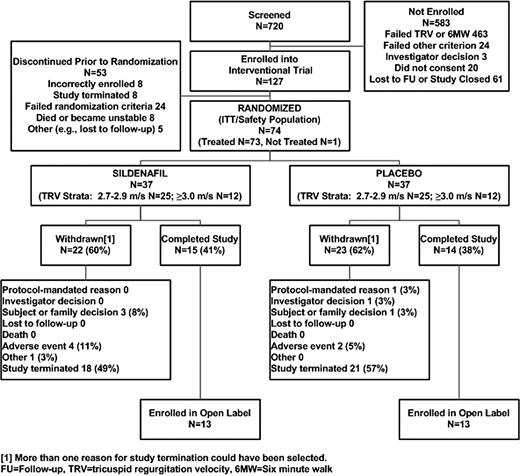
Of the 720 subjects who were screened, 74 were randomized to receive placebo (n = 37) or sildenafil (n = 37; Figure 1) before the study was prematurely stopped. The entry characteristics of the 626 (87%) screened subjects with detectable TRV by the screening echocardiography are categorized according to screening TRV (< 2.7 m/s, 2.7-2.9 m/s, and ≥ 3.0 m/s) in Table 1. An elevated estimated right ventricular systolic pressure was more frequent in older subjects with a higher systolic blood pressure, lower systemic arterial oxygen saturation, more severe hemolysis (increased LDH), worse renal function (elevated creatinine), and a higher self-reported prevalence of cutaneous leg ulcerations. Subjects with elevated estimated right ventricular systolic pressure had a higher reported prevalence of current tobacco use and had worse exercise capacity as measured by 6MWD and higher NT-proBNP levels despite normal left ventricular systolic function (assessed by transthoracic echocardiography, data not shown). We also performed an additional analysis including all nondetectable TRV values in the lower group, and this did not change any of the significant relationships presented in Table 1.
Characteristics of screened patients with SCD according to TRV*
| Characteristic . | TRV . | P† . | ||
|---|---|---|---|---|
| < 2.7 m/s . | 2.7-2.9 m/s . | ≥ 3.0 m/s . | ||
| Age, y | 34 ± 12 (403) | 41 ± 13 (141) | 44 ± 14 (82) | < .001 |
| Female sex, n (%) | 54 (403) | 56 (141) | 41 (82) | .08 |
| SCD genotype, SS/SC/SB+ or SB0/Other‡ | 68/23/5/4 (403) | 78/17/4/1 (141) | 85/14/0/1 (81) | .09 |
| 6MWD, m | 450 ± 96 (401) | 425 ± 103 (141) | 398 ± 91 (79) | < .001 |
| NT-proBNP, pg/mL | 108 ± 210 (380) | 276 ± 742 (132) | 2954 ± 8469 (75) | < .001** |
| NT-proBNP, log10 | 1.7 ± 0.5 (380) | 1.9 ± 0.7 (132) | 2.5 ± 0.9 (75) | < .001 |
| Blood pressure, mmHg | ||||
| Systolic | 118 ± 13 (403) | 121 ± 15 (140) | 123 ± 17 (81) | .01 |
| Diastolic | 69 ± 10 (403) | 68 ± 11 (140) | 68 ± 12 (81) | .56 |
| Mean arterial§ | 86 ± 10 (403) | 86 ± 11 (140) | 86 ± 12 (81) | .76 |
| Heart rate, bpm | 78 ± 12 (403) | 77 ± 13 (140) | 78 ± 12 (81) | .92 |
| Oxygen saturation, % | 97 ± 3 (402) | 96 ± 3 (138) | 95 ± 4 (81) | < .001 |
| Body surface area,¶ m2 | 1.78 ± 0.22 (399) | 1.82 ± 0.21 (139) | 1.83 ± 0.23 (77) | .09 |
| Current smoking, % | 15 (403) | 13 (140) | 27 (81) | .01 |
| History of hydroxyurea therapy,# % | 49 (403) | 54 (141) | 49 (81) | .59 |
| History of acute chest syndrome,# % | 60 (403) | 71 (141) | 58 (81) | .05 |
| History of stroke,# % | 9 (403) | 13 (141) | 17 (81) | .08 |
| History of asthma,# % | 19 (403) | 24 (141) | 22 (81) | .46 |
| History of cardiovascular problems,# % | 37 (403) | 65 (141) | 72 (81) | < .001 |
| History of renal problems# | 38 (403) | 50 (141) | 58 (81) | .001 |
| History of leg ulcers,# % | 15 (403) | 28 (141) | 31 (81) | < .001 |
| > 20 blood transfusions/lifetime,# % | 27 (403) | 40 (141) | 54 (81) | < .001 |
| History of priapism,# % of men | 29 (185) | 34 (62) | 30 (47) | .74 |
| Hemoglobin, g/dL | 9.7 ± 1.9 (397) | 8.9 ± 1.9 (137) | 8.4 ± 2.1 (78) | < .001 |
| White blood cell count, ×103/μL | 9 ± 4 (397) | 10 ± 4 (137) | 11 ± 4 (78) | .002 |
| Platelet count, ×103/μL | 359 ± 147 (396) | 346 ± 116 (137) | 351 ± 122 (78) | .99 |
| Blood urea nitrogen, mg/dL | 9 ± 6 (398) | 11 ± 7 (138) | 17 ± 12 (79) | < .001 |
| Creatinine, mg/dL | 0.8 ± 0.4 (397) | 0.9 ± 0.7 (138) | 1.5 ± 2.0 (80) | < .001 |
| Total bilirubin, mg/dL | 2.8 ± 2.9 (396) | 3.2 ± 2.6 (137) | 3.5 ± 2.6 (80) | .002 |
| Alkaline phosphatase, U/L | 98 ± 57 (397) | 100 ± 47 (137) | 118 ± 58 (80) | .008 |
| Alanine aminotransferase, U/L | 27 ± 23 (397) | 31 ± 22 (137) | 28 ± 19 (80) | .02 |
| Aspartate aminotransferase, U/L | 41 ± 34 (388) | 49 ± 27 (137) | 54 ± 34 (78) | < .001 |
| Lactate dehydrogenase, IU/L | 420 ± 288 (369) | 477 ± 340 (129) | 522 ± 264 (74) | .003 |
| Characteristic . | TRV . | P† . | ||
|---|---|---|---|---|
| < 2.7 m/s . | 2.7-2.9 m/s . | ≥ 3.0 m/s . | ||
| Age, y | 34 ± 12 (403) | 41 ± 13 (141) | 44 ± 14 (82) | < .001 |
| Female sex, n (%) | 54 (403) | 56 (141) | 41 (82) | .08 |
| SCD genotype, SS/SC/SB+ or SB0/Other‡ | 68/23/5/4 (403) | 78/17/4/1 (141) | 85/14/0/1 (81) | .09 |
| 6MWD, m | 450 ± 96 (401) | 425 ± 103 (141) | 398 ± 91 (79) | < .001 |
| NT-proBNP, pg/mL | 108 ± 210 (380) | 276 ± 742 (132) | 2954 ± 8469 (75) | < .001** |
| NT-proBNP, log10 | 1.7 ± 0.5 (380) | 1.9 ± 0.7 (132) | 2.5 ± 0.9 (75) | < .001 |
| Blood pressure, mmHg | ||||
| Systolic | 118 ± 13 (403) | 121 ± 15 (140) | 123 ± 17 (81) | .01 |
| Diastolic | 69 ± 10 (403) | 68 ± 11 (140) | 68 ± 12 (81) | .56 |
| Mean arterial§ | 86 ± 10 (403) | 86 ± 11 (140) | 86 ± 12 (81) | .76 |
| Heart rate, bpm | 78 ± 12 (403) | 77 ± 13 (140) | 78 ± 12 (81) | .92 |
| Oxygen saturation, % | 97 ± 3 (402) | 96 ± 3 (138) | 95 ± 4 (81) | < .001 |
| Body surface area,¶ m2 | 1.78 ± 0.22 (399) | 1.82 ± 0.21 (139) | 1.83 ± 0.23 (77) | .09 |
| Current smoking, % | 15 (403) | 13 (140) | 27 (81) | .01 |
| History of hydroxyurea therapy,# % | 49 (403) | 54 (141) | 49 (81) | .59 |
| History of acute chest syndrome,# % | 60 (403) | 71 (141) | 58 (81) | .05 |
| History of stroke,# % | 9 (403) | 13 (141) | 17 (81) | .08 |
| History of asthma,# % | 19 (403) | 24 (141) | 22 (81) | .46 |
| History of cardiovascular problems,# % | 37 (403) | 65 (141) | 72 (81) | < .001 |
| History of renal problems# | 38 (403) | 50 (141) | 58 (81) | .001 |
| History of leg ulcers,# % | 15 (403) | 28 (141) | 31 (81) | < .001 |
| > 20 blood transfusions/lifetime,# % | 27 (403) | 40 (141) | 54 (81) | < .001 |
| History of priapism,# % of men | 29 (185) | 34 (62) | 30 (47) | .74 |
| Hemoglobin, g/dL | 9.7 ± 1.9 (397) | 8.9 ± 1.9 (137) | 8.4 ± 2.1 (78) | < .001 |
| White blood cell count, ×103/μL | 9 ± 4 (397) | 10 ± 4 (137) | 11 ± 4 (78) | .002 |
| Platelet count, ×103/μL | 359 ± 147 (396) | 346 ± 116 (137) | 351 ± 122 (78) | .99 |
| Blood urea nitrogen, mg/dL | 9 ± 6 (398) | 11 ± 7 (138) | 17 ± 12 (79) | < .001 |
| Creatinine, mg/dL | 0.8 ± 0.4 (397) | 0.9 ± 0.7 (138) | 1.5 ± 2.0 (80) | < .001 |
| Total bilirubin, mg/dL | 2.8 ± 2.9 (396) | 3.2 ± 2.6 (137) | 3.5 ± 2.6 (80) | .002 |
| Alkaline phosphatase, U/L | 98 ± 57 (397) | 100 ± 47 (137) | 118 ± 58 (80) | .008 |
| Alanine aminotransferase, U/L | 27 ± 23 (397) | 31 ± 22 (137) | 28 ± 19 (80) | .02 |
| Aspartate aminotransferase, U/L | 41 ± 34 (388) | 49 ± 27 (137) | 54 ± 34 (78) | < .001 |
| Lactate dehydrogenase, IU/L | 420 ± 288 (369) | 477 ± 340 (129) | 522 ± 264 (74) | .003 |
Values within parentheses are percentages, unless otherwise indicated.
Percentages are based on the number of subjects (n) in a given group for the population analyzed. ± values are means ± SD. Log values (on a base 10 scale) were used to analyze laboratory measurements. Values in parentheses are total numbers.
Stratum differences were assessed using ANOVA for continuous variables and χ2 tests for categorical variables.
SB+ or SB0 = S beta+ thalassemia or S beta0 thalassemia, respectively.
Mean arterial pressure was calculated with the use of the following equation: mean arterial pressure = 1/3 systolic blood pressure + 2/3 diastolic blood pressure.
The body surface area was calculated using the Mosteller formula: body surface area = square root of (height in cm × weight in kg)/3600.
Medical history was primarily captured via self-report.
This analysis is of the log10 value and is a duplicate of the one noted in the next line of the table. Because the actual values were especially non-normal, we have reported the transformed means ± SD as well as the original.
The baseline characteristics of subjects enrolled in the intervention trial are listed in Table 2. Twenty-five percent of the screened population had both a TRV ≥ 2.7 m/s and a 6MWD between 150 and 500 m (Figure 1). The subjects randomized to sildenafil had similar 6MWD and TRV assessments, but significantly higher NT-proBNP (P = .03) and creatinine levels (P = .04) compared with the placebo arm. The sildenafil group also demonstrated nonsignificantly lower hemoglobin levels (P = .07) compared with the placebo group (Table 2) and a higher yearly self-reported rate of severe pain requiring hospitalization (3.6 ± 7 vs 1.4 ± 2 episodes per year, P = .02 evaluated by Poisson regression with correction for overdispersion to examine treatment group differences in counts of hospitalizations for the year before study enrollment). There were no other differences in baseline SCD-related variables between groups: sickle cell genotype (P = .45), history of hydroxyurea use (P = .33), hydroxyurea dose at study start (P = .62), prior transfusion experience (> 20 transfusions in a lifetime; P > .99), or number of subjects on transfusion regimens at study start (P > .99) (supplemental Methods and supplemental Figure 2).
Baseline characteristics of intervention trial subjects*
| Characteristic . | Sildenafil (n = 37) . | Placebo (n = 37) . | P† . |
|---|---|---|---|
| Age, y | 47 ± 13 | 44 ± 14 | .33 |
| Female sex, % | 62 | 62 | > .999 |
| Black, % | 97 | 100 | > .999 |
| Not Hispanic, % | 87 | 100 | .05 |
| SCD genotype, SS/SC/SB+ or SB0/other,‡ % | 84/13/0/3 | 76/22/3/0 | .45 |
| 6MWD distance, m | 381 ± 75 | 386 ± 75 | .76 |
| Tricuspid regurgitant jet velocity, m/s | 3.0 ± 0.5 | 3.0 ± 0.3 | .38 |
| NT-proBNP, pg/L | 527 ± 852 | 243 ± 367 | .03§ |
| NT-proBNP, log10 | 2.3 ± 0.6 | 2.0 ± 0.6 | .03 |
| BDS | 2.5 ± 2.10 | 2.1 ± 2.02 | .33 |
| NYHA functional class (I/II/III/IV), % | 32/41/27/0 | 40/38/22/0 | .77 |
| Left ventricular ejection fraction, % | 61 ± 6 | 62 ± 6 | .56 |
| Blood pressure, mmHg | |||
| Systolic | 125 ± 21 | 119 ± 13 | .18 |
| Diastolic | 69 ± 11 | 70 ± 9 | .69 |
| Heart rate, bpm | 74 ± 13 | 78 ± 10 | .11 |
| Oxygen saturation, % | 95 ± 4 | 96 ± 3 | .27 |
| Body surface area, m2 | 1.87 ± 0.34 | 1.84 ± 0.21 | .70 |
| Current smoking, % | 16 | 14 | .89 |
| History of hydroxyurea therapy, % | 57 | 70 | .33 |
| Hydroxyurea dosing-current, mg/kg (n) | 8.7 ± 7.9 (12) | 6.8 ± 9.0 (12) | .62 |
| History of acute chest syndrome, % | 62 | 70 | .62 |
| History of asthma, % | 16 | 38 | .07 |
| On chronic transfusion therapy, % | 5 | 8 | > .999 |
| > 20 blood transfusions during lifetime, % | 41 | 41 | > .999 |
| History of priapism, % of men | 14 | 14 | > .999 |
| Hemoglobin, g/dL | 8.1 ± 1.6 | 8.7 ± 1.6 | .07 |
| White blood cell count, ×103/μL | 9.0 ± 3.4 | 9.0 ± 3.6 | .95 |
| Platelet count, ×103/μL | 344 ± 120 | 328 ± 110 | .57 |
| Blood urea nitrogen, mg/dL | 15 ± 11 | 11 ± 6 | .29 |
| Creatinine, mg/dL | 1.0 ± 0.5 | 0.8 ± 0.3 | .04 |
| Total bilirubin, mg/dL | 3.1 ± 2.6 | 3.3 ± 2.4 | .64 |
| Alkaline phosphatase, U/L | 98 ± 47 | 104 ± 52 | .39 |
| Alanine aminotransferase, U/L | 22 ± 12 | 25 ± 18 | .40 |
| Aspartate aminotransferase, U/L | 44 ± 27 | 45 ± 25 | .90 |
| Lactate dehydrogenase, IU/L | 491 ± 293 | 482 ± 348 | .75 |
| Characteristic . | Sildenafil (n = 37) . | Placebo (n = 37) . | P† . |
|---|---|---|---|
| Age, y | 47 ± 13 | 44 ± 14 | .33 |
| Female sex, % | 62 | 62 | > .999 |
| Black, % | 97 | 100 | > .999 |
| Not Hispanic, % | 87 | 100 | .05 |
| SCD genotype, SS/SC/SB+ or SB0/other,‡ % | 84/13/0/3 | 76/22/3/0 | .45 |
| 6MWD distance, m | 381 ± 75 | 386 ± 75 | .76 |
| Tricuspid regurgitant jet velocity, m/s | 3.0 ± 0.5 | 3.0 ± 0.3 | .38 |
| NT-proBNP, pg/L | 527 ± 852 | 243 ± 367 | .03§ |
| NT-proBNP, log10 | 2.3 ± 0.6 | 2.0 ± 0.6 | .03 |
| BDS | 2.5 ± 2.10 | 2.1 ± 2.02 | .33 |
| NYHA functional class (I/II/III/IV), % | 32/41/27/0 | 40/38/22/0 | .77 |
| Left ventricular ejection fraction, % | 61 ± 6 | 62 ± 6 | .56 |
| Blood pressure, mmHg | |||
| Systolic | 125 ± 21 | 119 ± 13 | .18 |
| Diastolic | 69 ± 11 | 70 ± 9 | .69 |
| Heart rate, bpm | 74 ± 13 | 78 ± 10 | .11 |
| Oxygen saturation, % | 95 ± 4 | 96 ± 3 | .27 |
| Body surface area, m2 | 1.87 ± 0.34 | 1.84 ± 0.21 | .70 |
| Current smoking, % | 16 | 14 | .89 |
| History of hydroxyurea therapy, % | 57 | 70 | .33 |
| Hydroxyurea dosing-current, mg/kg (n) | 8.7 ± 7.9 (12) | 6.8 ± 9.0 (12) | .62 |
| History of acute chest syndrome, % | 62 | 70 | .62 |
| History of asthma, % | 16 | 38 | .07 |
| On chronic transfusion therapy, % | 5 | 8 | > .999 |
| > 20 blood transfusions during lifetime, % | 41 | 41 | > .999 |
| History of priapism, % of men | 14 | 14 | > .999 |
| Hemoglobin, g/dL | 8.1 ± 1.6 | 8.7 ± 1.6 | .07 |
| White blood cell count, ×103/μL | 9.0 ± 3.4 | 9.0 ± 3.6 | .95 |
| Platelet count, ×103/μL | 344 ± 120 | 328 ± 110 | .57 |
| Blood urea nitrogen, mg/dL | 15 ± 11 | 11 ± 6 | .29 |
| Creatinine, mg/dL | 1.0 ± 0.5 | 0.8 ± 0.3 | .04 |
| Total bilirubin, mg/dL | 3.1 ± 2.6 | 3.3 ± 2.4 | .64 |
| Alkaline phosphatase, U/L | 98 ± 47 | 104 ± 52 | .39 |
| Alanine aminotransferase, U/L | 22 ± 12 | 25 ± 18 | .40 |
| Aspartate aminotransferase, U/L | 44 ± 27 | 45 ± 25 | .90 |
| Lactate dehydrogenase, IU/L | 491 ± 293 | 482 ± 348 | .75 |
Percentages are based on the number of subjects (n) in a given group for the population analyzed. ± values are means ± SD. Log values (on a base 10 scale) were used to analyze laboratory measurements.
For categorical variables, treatment group differences were assessed using a Fisher exact test. For age, TRV, NT-proBNP, 6MWD, hemoglobin electrophoresis, and BDS treatment group differences were assessed via t test. For left ventricular ejection fraction and other laboratory values, treatment group differences were assessed via ANOVA including stratum.
SB+ or SB0 = S beta+ thalassemia or S beta0 thalassemia, respectively.
This analysis is of the log10 value and is a duplicate of the one noted in the next line of the table. Because the actual values were especially nonnormal, we have reported the transformed means ± SD as well as the original.
Acute hemodynamic effects of inhaled nitric oxide and oral sildenafil
Twenty-four subjects underwent RHC at baseline and 8 subjects underwent repeat RHC at week 16. At baseline, a statistically significant decrease in mean pulmonary artery pressure and pulmonary vascular resistance and an increase in mixed venous oxygen saturation were observed with acute inhalation of nitric oxide (Table 3), with no significant effects on systemic blood pressure or pulmonary capillary wedge pressure. In addition, at baseline, a statistically significant decrease in mean systemic blood pressure, right atrial pressure, mean pulmonary artery pressure, and pulmonary capillary wedge pressure occurred with the acute sildenafil administration (60 mg orally; Table 3). PH (defined as mean pulmonary artery pressure ≥ 25 mmHg) was confirmed in 13 of the 24 subjects (54%) who underwent an initial RHC for a baseline TRV ≥ 3.0 m/s. Because this study was designed to explore the effects of sildenafil in patients with low exercise capacity and high TRV, all patients with both low exercise capacity and increased TRV were randomized to therapy (regardless of their measured hemodynamics).
Baseline hemodynamics assessed via right heart catheterization
| . | First baseline . | Inhaled nitric oxide* . | Second baseline* . | 30 min after sildenafil† . | 60 min after sildenafil† . |
|---|---|---|---|---|---|
| Mean pulmonary artery pressure, mmHg | 28 ± 11 | 26 ± 10‡ | 28 ± 12 | 27 ± 11 | 26 ± 12§ |
| Mean right atrial pressure, mmHg | 8 ± 4 | 8 ± 5 | 9 ± 5 | 8 ± 4§ | 8 ± 4§ |
| Mean systemic arterial pressure, mmHg | 88 ± 14 | 86 ± 14 | 90 ± 10 | 83 ± 13‡ | 84 ± 10‡ |
| Mean pulmonary capillary wedge pressure, mmHg | 13 ± 4 | 14 ± 6 | 14 ± 5 | 13 ± 5 | 12 ± 5§ |
| Cardiac output, L/min | 8.6 ± 3.3 | 8.5 ± 2.5 | 8.2 ± 2.8 | 9.0 ± 3.6 | 8.5 ± 2.9 |
| Pulmonary vascular resistance, dyn/s/cm5 | 157 ± 122 | 120 ± 73‡ | 161 ± 120 | 143 ± 122 | 150 ± 119 |
| Mixed venous oxygen saturation, % | 65 ± 13 | 68 ± 9‡ | 66 ± 14 | 66 ± 12 | 65 ± 13 |
| . | First baseline . | Inhaled nitric oxide* . | Second baseline* . | 30 min after sildenafil† . | 60 min after sildenafil† . |
|---|---|---|---|---|---|
| Mean pulmonary artery pressure, mmHg | 28 ± 11 | 26 ± 10‡ | 28 ± 12 | 27 ± 11 | 26 ± 12§ |
| Mean right atrial pressure, mmHg | 8 ± 4 | 8 ± 5 | 9 ± 5 | 8 ± 4§ | 8 ± 4§ |
| Mean systemic arterial pressure, mmHg | 88 ± 14 | 86 ± 14 | 90 ± 10 | 83 ± 13‡ | 84 ± 10‡ |
| Mean pulmonary capillary wedge pressure, mmHg | 13 ± 4 | 14 ± 6 | 14 ± 5 | 13 ± 5 | 12 ± 5§ |
| Cardiac output, L/min | 8.6 ± 3.3 | 8.5 ± 2.5 | 8.2 ± 2.8 | 9.0 ± 3.6 | 8.5 ± 2.9 |
| Pulmonary vascular resistance, dyn/s/cm5 | 157 ± 122 | 120 ± 73‡ | 161 ± 120 | 143 ± 122 | 150 ± 119 |
| Mixed venous oxygen saturation, % | 65 ± 13 | 68 ± 9‡ | 66 ± 14 | 66 ± 12 | 65 ± 13 |
Second baseline was taken 10 minutes after completion of the INO hemodynamic assessment; P value is a comparison to first baseline.
P compared with second baseline
P < .01; and
§P < .05; P values are derived from a repeated measures mixed model including step/intervention as the effect of interest and the first baseline value as a covariate.
Exercise capacity, BDS, WHO functional class, and clinical worsening
There was no difference in the change in 6MWD from baseline to week 16 in sildenafil subjects (−16 ± 20 m) compared with placebo subjects (−7 ± 20 m; Table 4). The mean placebo-corrected treatment effect using prespecified imputation rules was −9 m at week 16 (difference estimate = −9; 95% confidence interval [95% CI] −56, 38; P = .70). Post hoc adjustment for baseline differences in NT-proBNP, creatinine, and hemoglobin levels did not change the results. The primary efficacy results did not change when analyzing, post hoc, the small subgroup (n = 13) of subjects with RHC confirmed PH (ie, mean pulmonary artery pressure ≥ 25 mmHg measured at RHC). Neither did the results differ when evaluating the per-protocol population or when performing a post hoc analysis controlling for any hydroxyurea use during the treatment phase.
Results for 6MWD, TRV, NT-proBNP, and BDS by time point
| . | Sildenafil . | Placebo . | P* . | ||||
|---|---|---|---|---|---|---|---|
| N . | Mean (SD) . | Median (min, max) . | N . | Mean ± SD . | Median (min, max) . | ||
| Baseline | 37 | 381 ± 75 | 383 (198, 494) | 37 | 386 ± 75 | 390 (175, 492) | .76 |
| Week 6 | 25 | 372 ± 89 | 368 (176, 504) | 24 | 428 ± 81 | 440 (240, 530) | .16 |
| Week 10 | 19 | 374 ± 86 | 366 (180, 485) | 16 | 423 ± 82 | 433 (248, 539) | .16 |
| Week 16 | 15 | 364 ± 101 | 363 (190, 515) | 14 | 410 ± 105 | 431 (205, 564) | .34 |
| Imputed last visit | 37 | 361 ± 103 | 388 (176, 15) | 37 | 375 ± 122 | 408 (0, 564) | .73 |
| Primary analysis: ANCOVA model (LS mean SE) | −16 (20) | −7 (20) | |||||
| Difference sildenafil − placebo (95% CI) | −9 (−56, 38) | .70 | |||||
| Tricuspid regurgitant jet velocity, m/s | |||||||
| Baseline | 37 | 3.1 ± 0.5 | 2.8 (2.4, 4.3) | 37 | 3.0 ± 0.3 | 2.8 (2.7, 3.7) | .15 |
| Week 6 | 25 | 3.2 ± 0.7 | 2.9 (2.5, 4.7) | 24 | 2.9 ± 0.3 | 2.8 (2.6, 3.6) | .06 |
| Week 16 | 15 | 2.9 ± 0.5 | 2.7 (2.3, 4.1) | 14 | 2.9 ± 0.3 | 2.8 (2.5, 3.4) | .45 |
| Mixed effects regression (LS mean SE) | 3.00 (0.046) | 2.96 (0.045) | |||||
| Difference sildenafil − placebo (95% CI) | 0.04 (−0.08, 0.15) | .50† | |||||
| Brain natriuretic peptide (log10), pg/L | |||||||
| Baseline | 35 | 2.3 ± 0.6 | 2.3 (0.6, 3.6) | 35 | 2.0 ± 0.6 | 1.9 (0.6, 3.2) | .03 |
| Week 6 | 22 | 2.4 ± 0.4 | 2.4 (1.8, 3.1) | 22 | 2.0 ± 0.7 | 1.8 (0.6, 3.6) | .08 |
| Week 10 | 17 | 2.3 ± 0.5 | 2.2 (1.6, 3.5) | 14 | 2.2 ± 0.6 | 2.1 (1.6, 3.7) | .67 |
| Week 16 | 14 | 2.5 ± 0.7 | 2.4 (1.6, 3.7) | 12 | 2.3 ± 0.6 | 2.1 (1.8, 3.8) | .41 |
| Mixed effects regression (LS mean SE) | 2.40 (0.087) | 2.30 (0.093) | |||||
| Difference sildenafil − placebo (95% CI) | 0.11(−0.15, 0.36) | .41† | |||||
| BDS | |||||||
| Baseline | 37 | 2.5 ± 2.1 | 2.0 (0.0, 7.0) | 37 | 2.1 ± 2.0 | 2.0 (0.0, 9.0) | .28 |
| Week 6 | 25 | 3.4 ± 2.3 | 3.0 (0.0, 8.0) | 24 | 1.8 ± 1.3 | 2.0 (0.0, 4.0) | .003 |
| Week 10 | 19 | 2.7 ± 2.0 | 3.0 (0.0, 8.0) | 16 | 2.7 ± 1.7 | 3.0 (0.0, 6.0) | .67 |
| Week 16 | 15 | 2.0 ± 1.6 | 2.0 (0.0, 5.0) | 14 | 2.8 ± 2.4 | 2.5 (0.0, 7.0) | .51 |
| Mixed effects regression (LS mean SE) | 2.24 (0.396) | 2.62 (0.408 | |||||
| Difference sildenafil − placebo (95% CI) | 0.37 (−1.51, 0.77) | ||||||
| . | Sildenafil . | Placebo . | P* . | ||||
|---|---|---|---|---|---|---|---|
| N . | Mean (SD) . | Median (min, max) . | N . | Mean ± SD . | Median (min, max) . | ||
| Baseline | 37 | 381 ± 75 | 383 (198, 494) | 37 | 386 ± 75 | 390 (175, 492) | .76 |
| Week 6 | 25 | 372 ± 89 | 368 (176, 504) | 24 | 428 ± 81 | 440 (240, 530) | .16 |
| Week 10 | 19 | 374 ± 86 | 366 (180, 485) | 16 | 423 ± 82 | 433 (248, 539) | .16 |
| Week 16 | 15 | 364 ± 101 | 363 (190, 515) | 14 | 410 ± 105 | 431 (205, 564) | .34 |
| Imputed last visit | 37 | 361 ± 103 | 388 (176, 15) | 37 | 375 ± 122 | 408 (0, 564) | .73 |
| Primary analysis: ANCOVA model (LS mean SE) | −16 (20) | −7 (20) | |||||
| Difference sildenafil − placebo (95% CI) | −9 (−56, 38) | .70 | |||||
| Tricuspid regurgitant jet velocity, m/s | |||||||
| Baseline | 37 | 3.1 ± 0.5 | 2.8 (2.4, 4.3) | 37 | 3.0 ± 0.3 | 2.8 (2.7, 3.7) | .15 |
| Week 6 | 25 | 3.2 ± 0.7 | 2.9 (2.5, 4.7) | 24 | 2.9 ± 0.3 | 2.8 (2.6, 3.6) | .06 |
| Week 16 | 15 | 2.9 ± 0.5 | 2.7 (2.3, 4.1) | 14 | 2.9 ± 0.3 | 2.8 (2.5, 3.4) | .45 |
| Mixed effects regression (LS mean SE) | 3.00 (0.046) | 2.96 (0.045) | |||||
| Difference sildenafil − placebo (95% CI) | 0.04 (−0.08, 0.15) | .50† | |||||
| Brain natriuretic peptide (log10), pg/L | |||||||
| Baseline | 35 | 2.3 ± 0.6 | 2.3 (0.6, 3.6) | 35 | 2.0 ± 0.6 | 1.9 (0.6, 3.2) | .03 |
| Week 6 | 22 | 2.4 ± 0.4 | 2.4 (1.8, 3.1) | 22 | 2.0 ± 0.7 | 1.8 (0.6, 3.6) | .08 |
| Week 10 | 17 | 2.3 ± 0.5 | 2.2 (1.6, 3.5) | 14 | 2.2 ± 0.6 | 2.1 (1.6, 3.7) | .67 |
| Week 16 | 14 | 2.5 ± 0.7 | 2.4 (1.6, 3.7) | 12 | 2.3 ± 0.6 | 2.1 (1.8, 3.8) | .41 |
| Mixed effects regression (LS mean SE) | 2.40 (0.087) | 2.30 (0.093) | |||||
| Difference sildenafil − placebo (95% CI) | 0.11(−0.15, 0.36) | .41† | |||||
| BDS | |||||||
| Baseline | 37 | 2.5 ± 2.1 | 2.0 (0.0, 7.0) | 37 | 2.1 ± 2.0 | 2.0 (0.0, 9.0) | .28 |
| Week 6 | 25 | 3.4 ± 2.3 | 3.0 (0.0, 8.0) | 24 | 1.8 ± 1.3 | 2.0 (0.0, 4.0) | .003 |
| Week 10 | 19 | 2.7 ± 2.0 | 3.0 (0.0, 8.0) | 16 | 2.7 ± 1.7 | 3.0 (0.0, 6.0) | .67 |
| Week 16 | 15 | 2.0 ± 1.6 | 2.0 (0.0, 5.0) | 14 | 2.8 ± 2.4 | 2.5 (0.0, 7.0) | .51 |
| Mixed effects regression (LS mean SE) | 2.24 (0.396) | 2.62 (0.408 | |||||
| Difference sildenafil − placebo (95% CI) | 0.37 (−1.51, 0.77) | ||||||
Statistics for the 6MWD test were based on an ANCOVA model with treatment as a fixed effect and baseline 6MWD distance, TRV stratum, and study site as covariates. The P value for TRV by time point corresponds to an ANOVA model with treatment by strata as cells testing the hypothesis that the average values do not differ between the 2 treatment groups.
The P values for the repeated measures analyses are based on a linear mixed effects model with treatment, baseline value, time, and study site as fixed effects, and subject as a random effect using all available data from the randomized population, testing the hypotheses of no difference between the treatment groups after 16 weeks of therapy.
LS mean SE indicates least squares mean standard error.
There were no differences in changes in BDS (difference estimate = −0.37; 95% CI −1.51, 0.77; P = .51) or the ordinal outcome of WHO functional class between treatment groups at week 16 compared with baseline (data not shown). There was a treatment difference in the BDS at week 6: sildenafil subjects had greater breathlessness as assessed by the BDS (3.4 ± 2.3) than placebo subjects (1.8 ± 1.3; P = .003). There were no reported events of symptoms suggestive of worsening PH. Median overall study drug adherence was 74%. The adherence computation was limited to pill count data, which may have been imprecise.
Estimated right ventricular systolic pressure (via TRV) and NT-proBNP levels
There was no difference in change from baseline to week 16 in TRV in the sildenafil group compared with placebo (estimate of the difference = 0.04; 95% CI −0.08, 0.15; P = .50, Table 4). At week 6, the sildenafil subjects had a nonsignificantly higher TRV (3.2 ± 0.7 m/s) compared with placebo (2.9 ± 0.3 m/s; P = .06). Likewise, although there was no difference in change from baseline to week 16 in NT-proBNP levels (log10 transformed values; estimate of the difference = 0.11; 95% CI −0.15, 0.36; P = .41); at week 6 (after controlling for the baseline imbalance in NT-proBNP), there was a nonsignificantly higher NT-proBNP in the sildenafil group (2.4 ± 0.4 pg/mL) compared with the placebo group (2.0 ± 0.7 pg/mL; P = .08).
Safety and tolerability
The NHLBI stopped the study early because of the higher percentage of subjects experiencing an SAE in the sildenafil arm compared with the placebo arm (Table 5). Forty-six percent of the sildenafil group compared with 22% of the placebo group experienced an SAE (P = .02). Hospitalization for SCD pain episodes was the predominant cause for this overall SAE effect (35% sildenafil vs 14% placebo; P = .03). Headaches (none serious) occurred in 35% of the sildenafil group compared with 11% of the placebo group (P = .01) (supplemental Table 3). One SAE (fever leading to hospitalization) was considered possibly related to RHC; this subject also had a concurrent respiratory tract illness. No acute episodes of priapism in the males with SCD were reported during the interventional trial; one episode occurred during the open-label extension phase. One death occurred during the interventional trial: a subject assigned to the placebo arm died of acute chest syndrome 12 days after discontinuation from the study.
Number and percentage of subjects with treatment-emergent SAEs
| System organ class preferred term* . | Sildenafil (n = 37), n % . | Placebo (n = 37), n % . | P† . |
|---|---|---|---|
| Any treatment-emergent SAE | 17 (46) | 8 (22) | .02 |
| Congenital, familial, and genetic disorder sickle cell anemia with crisis | 13 (35) | 5 (14) | .03 |
| Blood and lymphatic system disorders | 3 (8) | 4 (11) | .69 |
| Acute chest syndrome | 1 (3) | 3 (8) | .28 |
| Anemia | 2 (5) | 1 (3) | .56 |
| Infections and infestations | 2 (5) | 0 (0) | .16 |
| Bronchitis | 1 (3) | 0 (0) | .32 |
| Lower respiratory tract infection | 1 (3) | 0 (0) | .32 |
| Metabolism and nutrition disorders | |||
| Hyperkalemia | 0 (0) | 2 (5) | .16 |
| Vascular disorders | 1 (3) | 1 (3) | > .999 |
| Hypertension | 0 (0) | 1 (3) | .32 |
| Hypotension | 1 (3) | 0 (0) | .32 |
| Cardiac disorders | 1 (3) | 0 (0) | .32 |
| Atrial fibrillation | 1 (3) | 0 (0) | .32 |
| Cardiac failure congestive | 1 (3) | 0 (0) | .32 |
| Eye disorders | |||
| Vitreous hemorrhage | 1 (3) | 0 (0) | .32 |
| General disorders and administration site conditions | |||
| Pyrexia | 1 (3) | 0 (0) | .32 |
| Injury, poisoning, and procedural complications | |||
| Traumatic brain injury | 1 (3) | 0 (0) | .32 |
| Psychiatric disorders | |||
| Suicide attempt | 1 (3) | 0 (0) | .32 |
| Respiratory, thoracic, and mediastinal disorders | |||
| Acute pulmonary edema | 1 (3) | 0 (0) | .32 |
| System organ class preferred term* . | Sildenafil (n = 37), n % . | Placebo (n = 37), n % . | P† . |
|---|---|---|---|
| Any treatment-emergent SAE | 17 (46) | 8 (22) | .02 |
| Congenital, familial, and genetic disorder sickle cell anemia with crisis | 13 (35) | 5 (14) | .03 |
| Blood and lymphatic system disorders | 3 (8) | 4 (11) | .69 |
| Acute chest syndrome | 1 (3) | 3 (8) | .28 |
| Anemia | 2 (5) | 1 (3) | .56 |
| Infections and infestations | 2 (5) | 0 (0) | .16 |
| Bronchitis | 1 (3) | 0 (0) | .32 |
| Lower respiratory tract infection | 1 (3) | 0 (0) | .32 |
| Metabolism and nutrition disorders | |||
| Hyperkalemia | 0 (0) | 2 (5) | .16 |
| Vascular disorders | 1 (3) | 1 (3) | > .999 |
| Hypertension | 0 (0) | 1 (3) | .32 |
| Hypotension | 1 (3) | 0 (0) | .32 |
| Cardiac disorders | 1 (3) | 0 (0) | .32 |
| Atrial fibrillation | 1 (3) | 0 (0) | .32 |
| Cardiac failure congestive | 1 (3) | 0 (0) | .32 |
| Eye disorders | |||
| Vitreous hemorrhage | 1 (3) | 0 (0) | .32 |
| General disorders and administration site conditions | |||
| Pyrexia | 1 (3) | 0 (0) | .32 |
| Injury, poisoning, and procedural complications | |||
| Traumatic brain injury | 1 (3) | 0 (0) | .32 |
| Psychiatric disorders | |||
| Suicide attempt | 1 (3) | 0 (0) | .32 |
| Respiratory, thoracic, and mediastinal disorders | |||
| Acute pulmonary edema | 1 (3) | 0 (0) | .32 |
System organ class and preferred term were based on MedDRA Version 10.1. If a subject experienced more than one episode of an adverse event, the subject was counted once for that preferred term. If a subject had more than one adverse event in a system organ class, the subject was counted once for that system organ class.
P value corresponds to a Cochran-Mantel-Haenszel χ2 test of no difference between treatments while controlling for strata.
To address the impact of treatment group imbalances in baseline factors on the treatment effect in SAEs, we applied a post hoc propensity score analysis. The likelihood of experiencing any SAE in the sildenafil group compared with placebo was not attenuated when controlling for these covariates, including baseline self-reported yearly rate of pain events.
Effects of therapy on brief pain inventory
As a result of the global analysis, there was no overall treatment group difference in the Brief Pain Inventory across the intervention trial (P = .62; supplemental Figure 2). At week 10, sildenafil subjects had higher pain-related scores than placebo subjects (P = .04) on all but the pain relief scale. These effects were no longer present at week 16 (P = .99).
Among the individual domains of the Brief Pain Inventory, at 10 weeks, sildenafil subjects had more pain that interfered with normal work (5.1 ± 3.68 vs 1.3 ± 2.18; P = .04) and enjoyment of life (4.9 ± 3.97 vs 2.4 ± 3.88; P = .03) compared with placebo subjects; the increases in pain that interfered with the ability to walk (4.6 ± 3.57 vs 1.4 ± 1.19; P = .19) were not significantly higher in the sildenafil arm versus the placebo group. Of subjects who participated in the scheduled visits, daily assessments were not returned by 24 of 74 (32%) of subjects at baseline, by 12 of 49 (24%) at week 6, by 10 of 35 (29%) at week 10, and by 5 of 29 (17%) at week 16.
Discussion
In this multicenter, double-blind, placebo-controlled trial evaluating the effects of sildenafil in subjects with SCD, elevated TRV, and decreased exercise capacity, administration of sildenafil was unexpectedly associated with an increased rate of hospitalizations for pain episodes compared with placebo. The NHLBI stopped the study for safety reasons because a greater proportion of subjects experienced SAEs in the sildenafil arm and based on a futility analysis of estimated efficacy results. This observation of an increased rate of subjects experiencing serious SCD pain episodes was not reported previously in 2 open-label studies, most likely due to the absence of control groups or to differences in patient selection.19,20 In one of the prior studies, subjects underwent maximization of SCD specific therapy with hydroxyurea and/or red blood cell transfusions before initiation of sildenafil. Whether this prevented or minimized the development of pain episodes requiring hospitalization is unknown.20
In the current 16-week trial, sildenafil therapy did not improve exercise capacity nor did it decrease Doppler-defined estimated right ventricular systolic pressure, as had been suggested in previous nonrandomized, uncontrolled studies. It is possible that by increasing pain, sildenafil interfered with subjects' ability to perform the 6MW test, although post hoc analysis did not demonstrate a relationship between report of pain by Brief Pain Inventory and 6MW distance. The treatment group differences in favor of placebo at week 6 for TRV, NT-proBNP, and dyspnea (BDS), also suggest that there may have been other factors that interfered with 6MWD improvement with sildenafil treatment. One limitation of this study is that the trial evaluated the effects of sildenafil in patients with exercise limitation and an elevated estimated right ventricular systolic pressure without hemodynamic confirmation of PH. Therefore, the potential lack of efficacy of sildenafil may not be applicable to patients with symptomatic hemodynamically confirmed PAH. Given its early termination, the study was underpowered to assess the effects of sildenafil therapy on the predetermined efficacy end points. However, at the time of study termination, a futility analysis revealed that there was only a 34% probability of detecting a significant difference in favor of sildenafil, based on the data available at the time of termination and on the purposefully liberal assumption that all future data conformed to the sildenafil superiority hypothesis. It is also possible that the PDE5 inhibition provided by sildenafil simply does not improve exercise capacity in patients with elevated TRV associated with SCD. This question can only be answered by further research.
The etiology of the increase in hospitalizations for SCD pain episodes in the group receiving sildenafil is unclear. The imbalance in self-reported rate of severe pain requiring hospitalizations between the treatment groups at baseline, as well as other baseline imbalances, may have contributed to these findings. However, statistical adjustment for the baseline imbalances did not change the significant effect of sildenafil on rates of hospitalization for pain. In addition, the baseline differences were not strong and no adjustments for multiple comparisons were made.
At the time the study was designed, it was not known that myalgias or back pain were side effects of chronic treatment with PDE5 inhibitors. Therefore, we were surprised by the apparent increase in pain episodes in the sildenafil-treated patients in this trial. There are several lines of evidence suggesting a role for nitric oxide and cGMP in the processing of inflammatory and neuropathic pain that could have played a role in the increase in pain episodes that we observed.23-29 Furthermore, inhibition of the de novo synthesis of tetrahydrobiopterin (BH4), an essential cofactor for nitric oxide production by NOS, attenuates inflammatory and neuropathic pain in rodents, whereas polymorphisms in the gene encoding GTP cyclohydrolase, the rate-limiting enzyme for BH4 synthesis, modulate human response to pain.30 Consistent with these observations, the use of PDE5 inhibitors in other PAH patient populations and in large clinical trials of patients with erectile dysfunction has been associated with an increase in the incidence of myalgias and back pain that could have contributed to the increase in the pain reported in the current study.13,31,32 It is increasingly evident that back pain and myalgias represent a class effect of PD5 inhibitors. Nevertheless, conflicting preclinical studies have also shown that augmentation of nitric oxide synthesis by L-arginine supplementation, the administration of cGMP analogs, or intrathecal PDE5 inhibition with sildenafil may reduce nociceptive behavior in animal models.33-37 Considering the results of this trial, the role of nitric oxide-cGMP signaling in SCD-related pain represents an important area for further study.
In conclusion, in this large screening study, an elevated TRV appeared to be frequent in SCD and was often associated with decreased exercise capacity and increased NT-proBNP. Treatment with sildenafil was associated with increased hospitalization rates for pain episodes. These observations should be considered when designing future studies in patients with SCD. Whereas we should be careful in interpreting efficacy data because the study was underpowered to evaluate the primary end point (because it was stopped early due to safety concerns), the sildenafil-treated patients did not have an improvement in exercise capacity nor did they have a decrease in estimated right ventricular systolic pressures. Based on these data, sildenafil therapy should not be recommended in SCD patients based solely on an elevated estimated right ventricular systolic pressure and a decreased exercise capacity. This study, however, does not address the question of whether sildenafil could be beneficial in specific subgroups of SCD patients, such as those fulfilling the group 1 PAH hemodynamic definition for the diagnosis of PAH and/or those optimally treated for their hemolysis before the initiation of PDE5 inhibition. Finally, these results highlight the importance of conducting multisite placebo-controlled studies in patient populations (such as those with hemolysis) that have not yet been evaluated in controlled PAH trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the walk-PHaSST clinical site support team: Albert Einstein College of Medicine: Verlene Davis; Columbia University: Margaret Lee and Daniela Brady; Children's Hospital Oakland: Ward Hagar, Lisa Lavrisha Howard Rosenfeld, and Elliott Vichinsky; Children's Hospital Pittsburgh: Regina McCollum; Hammersmith Hospital, London: Sally Davies, Gaia Mahalingam, Sharon Meehan, Ofelia Lebanto, and Ines Cabrita; Howard University: Alvin Thomas, Gladys Onojobi, Sharmin Diaz, Margaret Fadojutimi-Akinsiku, and Randa Aladdin; Johns Hopkins University: Durrant Barasa; NHLBI: James Taylor, Wynona Coles, Catherine Seamon, Mary Hall, Amy Chi, Cynthia Brenneman, Wen Li, and Erin Smith; University of Colorado: Deb McCollister and Julie McAfee; University of Illinois at Chicago: Robert Molokie, George Kondos, Patricia Cole-Saffold, and Lani Krauz. Thanks also to the data coordinating center team from Rho Inc: Jamie Spencer, Christopher Woods, Karen Kesler, Vickie Coble, and Ronald W. Helms. Special thanks to the volunteers who participated in the Walk-PHaSST study.
This work was supported in part by an NIH Clinical and Translational Science Award (UL1 RR024131 to C.R.M.) and a NIHR Biomedical Research Centre grant (to J.S.R.G.). Study drug and placebo were donated by Pfizer Inc (New York, NY). This project was funded with federal funds from the NHLBI, NIH, Department of Health and Human Services, under contract HHSN268200617182C.
National Institutes of Health
Authorship
Contribution: M.T.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; R.F.M., R.J.B., and M.T.G. conceived and designed the study; R.F.M., K.L.H., G.J.K., V.R.G., J.S.R.G., J.A.L., D.E.S., L.K., R.E.G., C.R.M., B.R., D.B.B., S.L., O.O., O.L.C., V.S., and M.T.G. collected data; R.F.M., R.J.B., N.A.Y., K.L.H., G.J.K., V.R.G., J.S.R.G., J.A.L., D.E.S., L.K., R.E.G., C.R.M., E.B.R., D.B.B., S.L., O.O., O.L.C., V.S., M.A.W., R.W., J.C.G., and M.T.G. analyzed and interpreted data; R.F.M., N.A.Y., and M.T.G. drafted the manuscript; R.F.M., R.J.B., N.A.Y., K.L.H., G.J.K., V.R.G., J.S.R.G., J.A.L., D.E.S., L.K., R.E.G., C.R.M., E.B.R., D.B.B., S.L., O.O., O.L.C., V.S., M.A.W., R.W., J.C.G., and M.T.G. critically revised the manuscript for important intellectual content; N.A.Y., M.A.W., and R.W. performed statistical analysis; R.F.M., R.J.B., and M.T.G. obtained funding; and N.A.Y. and J.C.G. provided administrative, technical, or material support and supervised the study. The NHLBI funded the conduct of the study and participated in the collection, management, analysis, and interpretation of the data. NHLBI scientific staff actively participated in the preparation, review, and approval of the manuscript. Pfizer supplied the active drug and matched placebo for the study, but did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Conflict-of-interest disclosure: R.J.B. has received support for research grants and/or consulting from Actelion, Eli Lilly, Gilead, GlaxoSmith-Kline, Medtronics, Bayer, Ikaria, Pfizer, Novartis, United Therapeutics, and NHLBI. K.L.H. has received funding from NHLBI as a site subinvestigator for the Walk-PHaSST study. G.J.K. has received research support from a cooperative research and development agreement between the NIH and Ikaria INO Therapeutics and from the Division of Intramural Research of the NIH. V.R.G. has received research support from Biomarin, TRF-Pharma, and Emmaus Pharmaceuticals. J.S.R.G. has received research support from Actelion and Bayer and has served on advisory boards and/or received lecture fees from Actelion, Bayer, GlaxoSmithKline, Lilly, Pfizer, and United Therapeutics. L.K. has received research support from the NHLBI (HB-06-06). R.E.G. has served as an advisory board member for Actelion, Gilead, and United Therapeutics and has received research support from Actelion, Gilead, United Therapeutics, Pfizer, and ICOS. E.B.R. has received research support from Pfizer. D.B.B. has received honoraria for service on steering committees or advisory boards (or as a consultant) to the following companies working in the area of PH: Actelion/CoTherix, Gilead/Myogen, Encysive, Pfizer, Mondo Biotech/Mondogen, Biogen IDEC, United Therapeutics/Lung Rx, GlaxoSmithKline, Lilly/ICOS, Bayer, Ikaria, and Arena, and has received grant support for clinical studies from GlaxoSmithKline, Actelion/CoTHerix, Gilead/Myogen, Pfizer/Encysive, United Therapeutics/Lung Rx, Lilly/ICOS, Bayer, and Novartis. S.L. received award K23HL083089-03 from the NHLBI. M.T.G. has received research support in the form of a Collaborative Research and Development Agreement between the US Government and INO Therapeutics and is listed as a co-inventor on a US Government Patent for the use of nitrite salts for cardiovascular indications. R.F.M., N.A.Y., J.A.L., D.E.S., C.R.M., O.O., O.L.C., V.S., M.A.W., R.W., and J.C.G. declare no competing financial interests.
The views expressed herein are those of the authors and do not reflect the official policy of the US government.
Correspondence: Dr Mark T. Gladwin, Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, UPMC Montefiore Hospital, NW628, 3459 Fifth Ave, Pittsburgh, PA 15213; e-mail: gladwinmt@upmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal