Abstract
Adoptive cell transfer of allogeneic tumor-specific T cells could potentially be used as a universal treatment for cancer. We present a novel approach for adoptive immunotherapy using fully MHC-mismatched allogeneic T cells redirected with tumor-specific, non-MHC–restricted antibody-based chimeric antigen receptor (T-bodies) in the absence of GVHD. Mice bearing systemic metastatic disease were lymphodepleted by irradiation and treated with Her2/neu re-directed T cells. Lymphodepletion created a ‘therapeutic window’, which allowed the allo–T-bodies to attack the tumor before their rejection. A single split dose administration of allogeneic T-bodies extended the survival of tumor-bearing mice similarly to syngeneic T-bodies, and to a significantly greater extent than nonspecific allogeneic T cells. Blocking egress of lymphocytes from lymphoid organs using the sphingosine-1-phosphate agonist, FTY720, extended the persistence of allogeneic T cells such that allogeneic T-bodies provided superior therapeutic benefit relative to syngeneic ones, and dramatically extended the median survival time of the treated mice for more than a year. Therefore, we suggest that ex-vivo generated MHC-mismatched T-bodies can be used universally for off-the-shelf cancer immunotherapy and that their graft-versus-host reactivity can be safely harnessed to potentiate adoptive cell therapy.
Introduction
Adoptive cell therapy (ACT) using tumor-specific T cells is a promising modality for the treatment of cancer.1 Recent clinical trials have demonstrated its therapeutic potential against both melanoma and neuroblastoma.2-4 While autologous tumor-specific T cells were able to induce cancer regression in some patients, generating tumor-specific T cells for each individual patient is logistically and economically challenging. Redirection of T cells through an antibody-based chimeric antigen receptor (CAR) can potentially create ‘universal effector T cells’ capable of recognizing targets independently of MHC restriction.5,6 Such redirected T cells could potentially be used as an ‘off-the-shelf’ immunotherapy-based treatment and may be administered to patients; however, allogeneic ACT is limited by host-versus-graft (HVG) rejection on one hand, and the graft-versus-host (GVH) response on the other.7
Allogeneic hematopoietic cell transplantation (HCT) is frequently used to treat hematologic malignancies, with donor T cells contained within the graft being mainly responsible for the GVL effect.8 Nevertheless, relapses often occur after allogeneic HCT, though they can be treated by transfer of additional T cells from the original donor at a later time point—a therapy known as donor leukocyte infusion (DLI).9,10 Because the host is already engrafted with the donor's hematopoietic system, no HVG reaction occurs, and the transferred cells are not rejected. However basing allogeneic ACT on allogeneic HCT is problematic for at least 2 reasons: first, MHC-matched donors are available for only a fraction of the patients, and even then, GVHD poses a significant problem.11 Second, because additional cells must be transferred from the original donor, this therapy could not be used as an ‘off-the-shelf’ universal treatment for cancer.
So far, there have been only a few studies based on treatment with allogeneic cells outside the context of allogeneic HCT. Zakrzewski et al showed that treatment of an experimental lymphoma with completely MHC-mismatched T cell precursors in conjunction with autologous HCT can provide some survival advantage.12 However, solid tumors are more resistant to allogeneic attack than hematologic tumors. Boni et al sought to address this issue, and showed that adoptive transfer of haploidentical naive transgenic T cells bearing a monoclonal TCR in combination with autologous HCT can induce regression of established melanomas.13 Both of these studies attempted to purge allo-reactivity from tumor-specific T cells, thereby avoiding GVHD.
Lymphocyte egress from the lymph nodes into the blood is a key step in the immune response.14 FTY720 is a compound capable of sequestering lymphocytes in the lymphoid organs by blocking signals from the sphingosine-1-phosphate receptor.15 FTY720 has been shown to inhibit GVHD in preclinical models,16,17 and conversely, has been shown to protect kidney allo-grafts in clinical trials18 demonstrating its ability to inhibit the HVG response. In addition, FTY720 has shown efficacy in the treatment of multiple sclerosis.19 However, modulation of migration using FTY720 can also be exploited for immunotherapy. In a model of allogeneic HCT against lymphoma, Kim et al showed that FTY720 inhibited GVHD without inhibiting the GVL response.20 By trapping the lymphocytes in the lymphoid organs together with the lymphoma, FTY720 inhibited GVHD against peripheral tissues but not against the tumor cells in the lymph nodes.
In this study, we sought to explore the therapeutic potential of fully MHC mismatched T cells, redirected using an antibody-based CAR specific for the tumor antigen Her2/neu in a model of systemic metastatic disease and in the absence of HCT. We hypothesized that controlled lymphodepletion could create a therapeutic window during which the allo–T-body cells could destroy the tumor before being themselves rejected, thus providing therapeutic benefit in the absence of GVHD. Furthermore, we proposed that the allo-reactivity of the allogeneic T cells could be harnessed to potentiate adoptive cell therapy and compensate for the limited persistence of these cells. Finally, we suggested that modulating migration of allogeneic T cells through the use of FTY720 could further potentiate ACT.
Methods
Materials and cell lines
FTY720 was purchased from Caymen Chemicals. Tumors were induced using the Renca cell line transduced with human Her2/neu kindly provided by Prof W. Wels (Chemotherapeutisches Forschungsinstitut Georg-Speyer-Haus).
Design of the CAR
The CAR was constructed as reported21 and schematically depicted in Figure 1. It is composed of scFv based on the anti-Her2/neu antibody fused to a CD28 and FcγR signaling moieties.
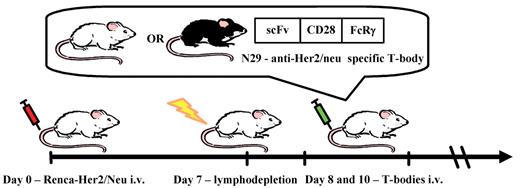
Schematic illustration of the experimental setup. Mice were injected intravenously with 105 Renca-Her2/Neu tumor cells on day 0. On day 7, mice were irradiated with 200 or 400 rads total body irradiation (TBI). A split dose of T-bodies (prepared by transduction of naive T cells with a Her2/neu-specific CAR, or isolated from transgenic mice expressing this CAR) was given on days 8 and 10. The CAR is composed of a scFv fused to CD28 and FcRγ signaling domains. T-bodies were derived from mice of either the C57BL/6 (allogeneic) or Balb/c (syngeneic) background.
Schematic illustration of the experimental setup. Mice were injected intravenously with 105 Renca-Her2/Neu tumor cells on day 0. On day 7, mice were irradiated with 200 or 400 rads total body irradiation (TBI). A split dose of T-bodies (prepared by transduction of naive T cells with a Her2/neu-specific CAR, or isolated from transgenic mice expressing this CAR) was given on days 8 and 10. The CAR is composed of a scFv fused to CD28 and FcRγ signaling domains. T-bodies were derived from mice of either the C57BL/6 (allogeneic) or Balb/c (syngeneic) background.
Proliferation assay
Renca-Her2/neu cells were incubated for 24 hours with preactivated C57Bl/6 T cells (either wild-type or N29 transgenic), and then 3H thymidine was added to the culture for an additional 24 hours. Proliferation was assessed by 3H incorporation.
Killing assay
Renca-Her2/neu cells were stably transduced with luciferase. Renca-Her2/neu-luciferase cells were incubated with preactivated C57Bl/6 T cells (either wild-type or N29 transgenic) at the indicated ratios. Viability of Renca-Her2/neu-luciferase cells was monitored 24 hours later by bioluminescence. Background luminescence was negligible (< 1% than the signal from the well with no effectors). Therefore viability was equal to experimental signal/maximal signal × 100, and killing was equal to 100 − viability percentage.
Flow cytometry
Anti–mouse CD3ϵ (145-2C11)-Percp-Cy5.5, anti–mouse CD62L (MEL14)-PE-Cy7, and anti–mouse H-2Kb (AF6-88.5)–Pacific Blue were purchased from Biolegend. PE–annexin V, Streptavidin-APC, and Streptavidin-APC- Cy5.5 were purchased from eBioscience. Polyclonal antibody preparation against the N29 CAR was generated in our laboratory and then biotinylated.
Before splenocyte staining, red cells were lysed using ACK buffer. Lymphocytes (1 × 106) were incubated with the appropriate antibodies in staining buffer (5% BSA, 0.05% sodium azide in PBS) for 30 minutes on ice. Alternatively annexin V staining buffer was used as indicated. CFSE labeling was performed according to the manufacturer's instructions (Molecular Probes). Cells were analyzed by flow cytometry (LSRII; Becton Dickinson) and FacsDiva software (Becton Dickinson).
Mice
Tumor bearing animals used in the experiments were generally 8- to 10-week-old BALB/c mice. Donor splenocytes were obtained from 6- to 16-week-old transgenic mice expressing the N29 CAR on either the C57BL/6 (allogeneic) or BALB/c (syngeneic) background. C57BL/6 and Balb/c Luciferase+ transgenic mice (kindly provided by Professor R. Negrin, Department of Medicine, Division of Blood and Marrow Transplantation, Stanford University22 ) were obtained by back-crossing FVB-Luciferase to these strains for at least 9 generations. Luciferase transgenic mice were then crossed to N29 transgenic mice, and F1 mice were used as donors in IVIS studies. All invasive procedures and imaging experiments were conducted under Ketamine and Xylazine general anesthesia (127.5 and 4.5 mg/kg, respectively). All animal studies were performed under protocols approved by the Weizmann Institute of Science Animal Care and Use Committee.
Retroviral transduction of T cells
Retroviral transduction of T cells was performed as described previously.23 Briefly, activated T cells were transduced using spin-infection on RetroNectin (Takara) coated plates in the presence of vector-containing supernatant and IL-2.
Adoptive transfer experiments
Mice were injected with 105 Renca-Her2/neu cells intravenously on day 1. All mice (including the control group) were irradiated on day 7 with 200 or 400 rads (137Cs source). Before transfer, all T cells were activated with Con-A 1μg/mL for 48 hours, and then cultured for 3-5 additional days with 350u/mL IL-2. Either 30 × 106 or 108 T cells were transferred in a split dose on days 8 and 10. In some experiments FTY720 was injected intraperitoneally (0.3 mg/kg) on days 8-18. Donor cells were either from wild-type mice (BALB/c or C57BL/6) or from N29 CAR transgenic mice (BALB/c or C57BL/6 backgrounds). In some cases, T cells from wild-type mice (BALB/c or C57Bl) were transduced with the CAR.
In vivo imaging
To follow trafficking of cells, the whole body cooled CCD camera system was used (IVIS 100 Series Imaging System; Xenogen). T-cells from C57BL/6-N29+/−/Luc+/− or Balb/c-N29+/−/Luc+/− mice were used in adoptive transfer. Luciferin was injected at 75μg/mouse and images were acquired at low resolution with a 3- to 5-minute exposure time. For ex vivo analysis, mice were first injected with luciferin at 150 mg/kg. Quantification of average radiance was performed using Living Image 2.5 software.
Statistical analysis
All results reported here were based on 2-sided test statistics. Survival analysis was done using the log-rank test. P values from independent experiments were combined using Fisher method. Bioluminescence signals were compared using the Mann-Whitney test. FACS analysis was done using the χ2 test. P < .05 was considered significant.
Results
Host preconditioning and transferred cell dose determines the antitumor effect of allogeneic T-bodies
To investigate the potential of allogeneic ACT, we used the BALB/c-derived mouse renal cell carcinoma (Renca) cell line expressing human Her2/neu. After intravenous inoculation of the tumor, experimental metastases mainly developed in the lungs, yet, as was reported in similar systems,24,25 extra-pulmonary metastases also developed. We lymphodepleted the tumor-bearing mice 7 days after tumor inoculation using sublethal irradiation (eg 200-400 rad, doses) that did not affect tumor development. Adoptive transfer of T cells redirected with a Her2/neu-specific CAR (T-bodies) was performed on days 8 and 10 (Figure 1). The CAR is composed of a scFv fragment derived from the Her2/Neu-specific N29 antibody fused to a CD28 coactivation moiety and FcRγ signaling sequences.21 This CAR design has been shown to activate naive T cells as well as to inhibit activation-induced death (AICD).21 T-bodies were obtained from transgenic (Tg) mice of BALB/c (Balb-N29) or C57BL/6 (Black-N29) background expressing the Her2/Neu-specific N29 CAR transgene,23 or by the transduction of wild-type T cells with a retrovector harboring the Her2/neu-specific CAR. Transduced T cells were activated as part of their transduction, while naive Tg T cells were also activated to enable comparison with the transduced T cells.
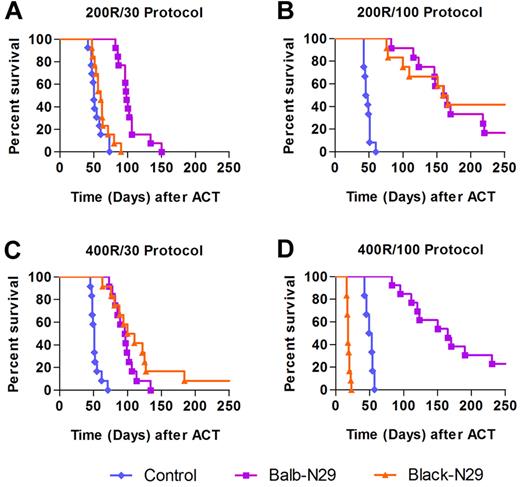
An adoptive transfer protocol consisting of 200 rad irradiation followed by transfer of 30 × 106 syngeneic transgenic T cells (Balb-N29) extended the median survival of tumor bearing mice to 97 days, compared with 50 days for the control group, which was also irradiated (P = .000004, Figure 2A), yet no complete cure of tumor-bearing mice was achieved. Using this protocol with allogeneic T-bodies (Black-N29) provided no survival advantage (Figure 2A). We postulated that under these conditions the HVG reaction was dominant, leading to rapid rejection of the allogeneic T-bodies. With an increased cell dose (200 rad and 100 × 106 T-bodies) allo–T-bodies extended median survival comparably to syngeneic T-bodies (162 and 160 days, respectively), compared with only 46 day survival for the control group (P = .000004 for both allogeneic and syngeneic T-bodies vs control, Figure 2B). Reducing graft rejection by increasing lymphodepletion (400 rads) and using the lower cell dose (30 × 106) increased median survival of tumor bearing mice treated with allogeneic T-bodies to 105 days, similarly to syngeneic T-bodies (median of 95 days) and more than the 50 day median of the control group (P = .000004 for both allogeneic and syngeneic T-bodies vs control, Figure 2C). Importantly, no GVHD-associated mortality occurred using either of the protocols, demonstrating that an allogeneic T-body response can provide therapeutic benefit without significant toxicity to the host. However increasing both the cell dose and the irradiation dose (400 rads and 100 × 106 T-bodies) caused lethal GVHD, which was preceded by weight loss (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) in all mice receiving allo–T-bodies (Figure 2D).
Balance between host preconditioning and transferred cell dose determines the antitumor benefit provided by allogeneic T-bodies. Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 12/group) were irradiated and then either left untreated as a control (blue diamonds), or systemically administered with syngeneic T-bodies (Balb-N29, purple squares) or allogeneic T-bodies (Black-N29, orange triangles). The results shown are pooled from 2 independent experiments. P values were computed for each experiment separately using the log-rank test, and combined using the Fisher method. (A) Protocol consisting of 200 rad irradiation and 30 × 106 T-bodies (200R/30). Balb-N29 or Black-N29 vs control P = .000004. (B) Protocol consisting of 200 rad irradiation and 100 × 106 T-bodies (200R/100). Balb-N29 or Black-N29 vs control P = .000004. (C) Protocol consisting of 400 rad irradiation and 30 × 106 T-bodies (400R/30). Balb-N29 or Black-N29 vs control P = .000004. (D) Protocol consisting of 400 rad irradiation and 100 × 106 T-bodies (400R/100). Balb-N29 vs control P = .000004. In this protocol, mice receiving the Black-N29 died from GVHD manifested by severe cachexia (supplemental Figure 1).
Balance between host preconditioning and transferred cell dose determines the antitumor benefit provided by allogeneic T-bodies. Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 12/group) were irradiated and then either left untreated as a control (blue diamonds), or systemically administered with syngeneic T-bodies (Balb-N29, purple squares) or allogeneic T-bodies (Black-N29, orange triangles). The results shown are pooled from 2 independent experiments. P values were computed for each experiment separately using the log-rank test, and combined using the Fisher method. (A) Protocol consisting of 200 rad irradiation and 30 × 106 T-bodies (200R/30). Balb-N29 or Black-N29 vs control P = .000004. (B) Protocol consisting of 200 rad irradiation and 100 × 106 T-bodies (200R/100). Balb-N29 or Black-N29 vs control P = .000004. (C) Protocol consisting of 400 rad irradiation and 30 × 106 T-bodies (400R/30). Balb-N29 or Black-N29 vs control P = .000004. (D) Protocol consisting of 400 rad irradiation and 100 × 106 T-bodies (400R/100). Balb-N29 vs control P = .000004. In this protocol, mice receiving the Black-N29 died from GVHD manifested by severe cachexia (supplemental Figure 1).
Both CAR and TCR-based allo-reactivity augment the antitumor response
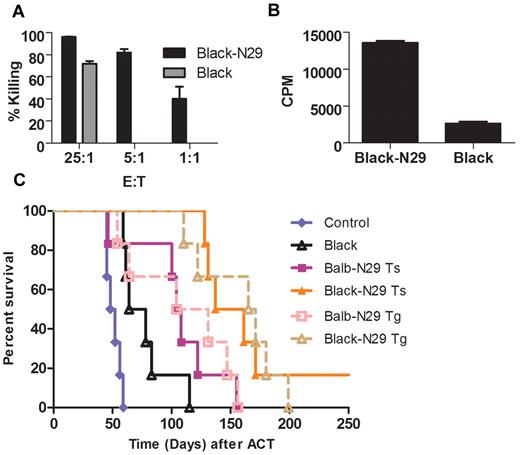
Because allo-reactive T cells can potentially directly recognize tumors through their TCR we compared the activity of nonredirected allogeneic T cells to that of tumor-specific allo–T-bodies (Figure 3A-B). In both proliferation and killing assays nonredirected T cells demonstrated some activity against the tumor, but in both cases were vastly inferior to allo–T-bodies. To evaluate the contribution of the CAR to the allogeneic T-body response in vivo under realistic conditions, we transduced wild-type BALB/c and C57BL/6 T cells (typically with 50% transduction efficiency) with the N29 CAR and compared their antitumor effect to that of mock-transduced C57BL/6 T cells in Renca-Her2/neu tumor bearing mice. Mock-transduced allogeneic T cells were able to extend the median survival of tumor bearing mice to 71 days compared with only 50 days for the control group (P = .001 for allogeneic T cells vs control, Figure 3C). Nevertheless, allo–T-bodies were much more effective than mock-transduced allogeneic T cells, and were able to extend median survival to 150 days (P = .0005 allogeneic T-bodies vs nonredirected allogeneic T cells, Figure 3); thus, redirection through the CAR potentiates allogeneic therapy. Allogeneic T-bodies were also superior to syngeneic T-bodies, which extended median survival to only 106 days (P = .012 for allogeneic T-bodies vs syngeneic T-bodies, Figure 3C). In the same experiment, we compared transduced T-bodies to transgenic T-bodies. Equal numbers of Tg T-bodies were transferred with addition of T cells from the wild-type strain in order simulate the 50% transduction efficiency of transduced T-bodies. There was no significant difference between the survival benefit of transduced versus transgenic T-bodies (Figure 3C). These data directly demonstrate that addition of nonredirected allogeneic T cells can potentiate allogeneic ACT.
Both antibody based chimeric receptor and TCR-based allo-reactivity contribute to the antitumor response. (A) Preactivated allogeneic T cells (either transgenic T-bodies—Black-N29 or wild-type—Black) were incubated with Renca-Her2/neu-luciferase cells at the indicated ratios. Killing activity was quantified by measuring the bioluminescent signal after 24 hours (no killing was observed using Black cells at 1:1 and 5:1 ratios). (B) Preactivated allogeneic T cells (either transgenic T-bodies—Black-N29 or wild-type—Black) were incubated with Renca-Her2/neu cells, and thymidine incorporation was measured 24 hours later. (C) Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 6/group) were irradiated with 200 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 100 × 106 T cells. The T cell populations used were either: allogeneic mock transduced T cells (Black, empty black triangles, P = .0011 vs control), syngeneic T cells transduced with the N29 CAR (Balb-N29 Ts, filled purple squares), allogeneic T cells transduced with the N29 CAR (Black-N29 Ts, filled orange triangles, P = .0005 vs Black, P = .012 vs Balb-N29), syngeneic T cells from N29 transgenic Balb/c mice (Balb-N29 Tg, open pink squares, no significant difference was seen vs transduced cells), or allogeneic T cells from N29 transgenic C57BL/6 mice (C57BL-N29 Tg, open brown triangles, no significant difference was seen vs transduced cells).
Both antibody based chimeric receptor and TCR-based allo-reactivity contribute to the antitumor response. (A) Preactivated allogeneic T cells (either transgenic T-bodies—Black-N29 or wild-type—Black) were incubated with Renca-Her2/neu-luciferase cells at the indicated ratios. Killing activity was quantified by measuring the bioluminescent signal after 24 hours (no killing was observed using Black cells at 1:1 and 5:1 ratios). (B) Preactivated allogeneic T cells (either transgenic T-bodies—Black-N29 or wild-type—Black) were incubated with Renca-Her2/neu cells, and thymidine incorporation was measured 24 hours later. (C) Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 6/group) were irradiated with 200 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 100 × 106 T cells. The T cell populations used were either: allogeneic mock transduced T cells (Black, empty black triangles, P = .0011 vs control), syngeneic T cells transduced with the N29 CAR (Balb-N29 Ts, filled purple squares), allogeneic T cells transduced with the N29 CAR (Black-N29 Ts, filled orange triangles, P = .0005 vs Black, P = .012 vs Balb-N29), syngeneic T cells from N29 transgenic Balb/c mice (Balb-N29 Tg, open pink squares, no significant difference was seen vs transduced cells), or allogeneic T cells from N29 transgenic C57BL/6 mice (C57BL-N29 Tg, open brown triangles, no significant difference was seen vs transduced cells).
Allo-reactivity modulates the migration and persistence of allogeneic cells
To understand the dynamics of the T-body response under the conditions described above, we transferred Luciferase+ T-bodies (obtained by crossing N29-Tg mice to Luciferase-Tg mice from the same background) to Renca-Her2/neu-bearing mice and monitored the labeled cells using the in vivo imaging system (IVIS). Varying the transferred cell number and radiation dose of the host did not significantly alter the migration or persistence of syngeneic T-bodies (Figure 4A), but had a striking effect on the allo–T-bodies (Figure 4A). At the lowest cell and radiation doses (200 rads and 30 × 106 cells) allogeneic T-bodies could not be detected, suggesting they were rapidly rejected (Figure 4A). At a higher cell dose (200 rads and 100 × 106 cells), 1 day after the second cell injection (3 days after the first cell batch), the whole-body bioluminescent (BLI) signal from allo–T-bodies was higher than that of syngeneic T-bodies (P = .005, Figure 4B). However, the allogeneic cell signal decayed until it became undetectable, 7 days after cell transfer, in contrast to the syngeneic signal which persisted for the duration of the observation period (20 days). These data demonstrate the partial dominance of the HVG response after irradiation with 200 rads. In contrast, inhibiting the HVG further by increasing the radiation dose to 400 rads (while keeping the cell dose constant at 30 × 106) changed the dynamics of the response to a bell-shaped one (Figure 4A-B). During the first week, the BLI signal from the allogeneic cells increased, followed by gradual decline reflecting HvG-mediated clearance (Figure 4A). Using this protocol the signal from the allogeneic cells was stronger than the syngeneic signal on day 7 (P = .005, Figure 4B) but weaker by day 14 (P = .002, Figure 4B). As expected, increased lymphodepletion (using a higher irradiation dose) allowed allogeneic T-bodies to persist longer in vivo (Figure 4A-B). The transient nature of the persistence of allo–T-bodies using either protocol explains the absence of serious GVHD related toxicity under these conditions. The dynamics of the allogeneic cells suggested that they underwent GVH-driven proliferation. To verify this, we repeated the experiment, but instead of using luciferase+ T cells, we labeled them with CFSE; staining with an anti-idiotypic antibody to the CAR allowed us to identify the transferred T-bodies. FACS analysis of recovered splenocytes on day 4 after transfer, demonstrated that a subset of the allogeneic T-bodies underwent extensive proliferation (Figure 4C). Syngeneic cells did not proliferate suggesting homeostatic proliferation did not occur. This is not surprising because any lymphopenia induced by the irradiation was reversed with the adoptive transfer of cells. Furthermore, the proliferation of allo–T-bodies was associated with loss of CD62L expression (P = .0001, Figure 4D) in splenic T cells, indicating that the T cells differentiated to effector memory cells.
Allo-reactivity modulates the migration and persistence of allo-T-bodies. (A) Comparative in vivo bioluminescence imaging of Renca-Her2/neu-bearing mice (n = 6/group) treated with either syngeneic (Balb-N29) or allogeneic (Black-N29) T-bodies according to different protocols, as indicated. Day 1 indicates 1 day after the transfer of the second cell batch. BLI was performed as described in “Methods.” Images shown are of 3 representative mice per group. 200R/30 = Irradiation with 200 rads and 30 × 106 T-bodies. 200R/100 = Irradiation with 200 rads and 100 × 106 T-bodies. 400R/30 = Irradiation with 400 rads and 30 × 106 T-bodies. (B) Whole body BLI signal intensities from sequential imaging depicted in panel A. Each line represents a single animal. Pairwise differences between groups were analyzed using the Mann-Whitney test. P = .005 for Black-N29 vs Balb-N29 using the 200R/100 protocol on day 1. P = .005 for Black-N29 vs Balb-N29 using the 400R/30 protocol on day 7. P = .002 for Black-N29 vs Balb-N29 using the 400R/30 protocol on day 14. (C) In vivo proliferative capacity of allogeneic and syngeneic T-bodies. CFSE-labeled T-bodies were systemically transferred to preconditioned (200R or 400R) tumor-bearing mice according to the protocols described. Splenocytes of mice were harvested 4 days later and stained with an anti-idiotypic antibody to identify donor T-bodies. CFSE staining is shown for the transferred T-bodies. (D) CD3+ splenocytes from mice treated with allo–T-bodies using the 400R/30 protocol analyzed for expression of CFSE vs CD62L. Progressive differentiation (loss of CD62L) was concomitant with proliferation (loss of CFSE staining), P = .0001 using the χ2 test.
Allo-reactivity modulates the migration and persistence of allo-T-bodies. (A) Comparative in vivo bioluminescence imaging of Renca-Her2/neu-bearing mice (n = 6/group) treated with either syngeneic (Balb-N29) or allogeneic (Black-N29) T-bodies according to different protocols, as indicated. Day 1 indicates 1 day after the transfer of the second cell batch. BLI was performed as described in “Methods.” Images shown are of 3 representative mice per group. 200R/30 = Irradiation with 200 rads and 30 × 106 T-bodies. 200R/100 = Irradiation with 200 rads and 100 × 106 T-bodies. 400R/30 = Irradiation with 400 rads and 30 × 106 T-bodies. (B) Whole body BLI signal intensities from sequential imaging depicted in panel A. Each line represents a single animal. Pairwise differences between groups were analyzed using the Mann-Whitney test. P = .005 for Black-N29 vs Balb-N29 using the 200R/100 protocol on day 1. P = .005 for Black-N29 vs Balb-N29 using the 400R/30 protocol on day 7. P = .002 for Black-N29 vs Balb-N29 using the 400R/30 protocol on day 14. (C) In vivo proliferative capacity of allogeneic and syngeneic T-bodies. CFSE-labeled T-bodies were systemically transferred to preconditioned (200R or 400R) tumor-bearing mice according to the protocols described. Splenocytes of mice were harvested 4 days later and stained with an anti-idiotypic antibody to identify donor T-bodies. CFSE staining is shown for the transferred T-bodies. (D) CD3+ splenocytes from mice treated with allo–T-bodies using the 400R/30 protocol analyzed for expression of CFSE vs CD62L. Progressive differentiation (loss of CD62L) was concomitant with proliferation (loss of CFSE staining), P = .0001 using the χ2 test.
FTY720 augments allogeneic but not syngeneic adoptive therapy
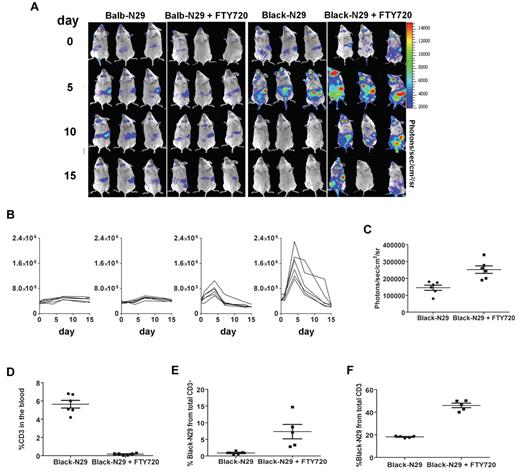
We next sought to determine whether modulating lymphocyte migration through the use of FTY720, that is known to block GVHD and HVG,16-18 could augment the therapeutic benefit of T-bodies. First we showed that adding FTY720 to the treatment protocol (0.3mg/kg intraperitoneally) for the first 10 days after irradiation did not affect the survival of tumor bearing mice. We then checked the effect of FTY720 on the adoptive transfer of both syngeneic and allogeneic T-bodies (400 rads and 30 × 106 T-bodies). While FTY720 did not have any notable effect on syngeneic T-body therapy (median survival of 95 days without FTY720 as opposed to 90 days with FTY720, Figure 5A), it had a profound effect on allo–T-body therapy, with 58% mice surviving long term (> 350 days) when treated with FTY720, as opposed to only 8% in its absence (P = .013, Figure 5A). At the end of the observation period (> 350 days), all mice were autopsied and found to be tumor free. To investigate the mechanism of action of FTY720 in this system, we tested its effect on luciferase+ T-bodies in tumor bearing mice. In agreement with the survival data, FTY720 did not significantly alter the migration or the persistence of syngeneic T-bodies (Figure 6A). However, FTY720 did have a significant impact on the allogeneic immune response. Without FTY720 the BLI signal from allo–T-bodies lasted for a week compared with up to 2 weeks in some mice with FTY720 (Figure 6A-C). As expected with FTY720 the BLI signal from spleen, lymph nodes and bone marrow substantially increased. Interestingly and in accordance with our survival data the mice treated with FTY720 also showed an increased BLI signal from the chest (mainly on day 5 after transfer) and the abdomen area (Figure 6A-C).
FTY720 augments allogeneic but not syngeneic adoptive cell therapy. (A) Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 12/group) were irradiated with 400 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 30 × 106 transgenic T-bodies. Some groups received FTY720 0.3mg/kg intraperitoneally for 10 days after transfer. T cells were either: syngeneic T-bodies (Balb-N29, pink squares), syngeneic T-bodies with FTY720 (Balb-N29, purple squares), allogeneic T-bodies (Black-N29, orange triangles), allogeneic T-bodies with FTY720 (Black-N29, brown triangles, Black-N29+FTY720 vs Black-N29, P = .013). P values were computed separately for each experiment using the log-rank test and combined using the Fisher method. (B) FTY720 suppresses GVHD but augments ACT of high dose of allo–T-bodies. As in panel A, Renca-Her2/Neu-bearing mice, (n = 6/group) were irradiated with 400 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 100 × 106 allogeneic T-bodies either with (brown triangles) or without FTY720 (orange triangles).
FTY720 augments allogeneic but not syngeneic adoptive cell therapy. (A) Kaplan-Meyer survival plots of Renca-Her2/Neu-bearing mice. Mice (n = 12/group) were irradiated with 400 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 30 × 106 transgenic T-bodies. Some groups received FTY720 0.3mg/kg intraperitoneally for 10 days after transfer. T cells were either: syngeneic T-bodies (Balb-N29, pink squares), syngeneic T-bodies with FTY720 (Balb-N29, purple squares), allogeneic T-bodies (Black-N29, orange triangles), allogeneic T-bodies with FTY720 (Black-N29, brown triangles, Black-N29+FTY720 vs Black-N29, P = .013). P values were computed separately for each experiment using the log-rank test and combined using the Fisher method. (B) FTY720 suppresses GVHD but augments ACT of high dose of allo–T-bodies. As in panel A, Renca-Her2/Neu-bearing mice, (n = 6/group) were irradiated with 400 rads, and 1 day later either left untreated as a control (blue diamond), or injected with 100 × 106 allogeneic T-bodies either with (brown triangles) or without FTY720 (orange triangles).
FTY720 reduces the number of circulating allo-T-bodies in the recipient mice and increases T-body number in the spleen. (A) Comparative in vivo BLI of Renca-Her2/neu-bearing mice (n = 6) irradiated with 400 rads, and treated with either 30 × 106 syngeneic (Balb-N29) or allogeneic (Black-N29) T-bodies. BLI was performed as in Figure 4A. (B) Whole body BLI signal intensities from sequential imaging depicted above, and measured every 3-4 days after T cell transfer from the same groups shown in panel A. Each line represents 1 animal. (C) A region of interest was defined around the spleens of mice from panel A. Shown are absolute photon counts from mice treated with allogeneic T-bodies either with or without FTY720. Means and SEM are shown. (D) Mononuclear cells were isolated 4 days after adoptive transfer from the blood of tumor-bearing mice treated with allogeneic T-bodies either with or without FTY720, and the number of T cells in the blood was quantified by staining for CD3. Means and SEM are shown. (E-F) Splenocytes of tumor-bearing mice treated with allogeneic T-bodies either with or without FTY720, were stained with anti-H2Kb 4 (E) or 7 (F) days after adoptive transfer to identify the allogeneic T-bodies. Means and SEM are shown. Each line represents 1 animal.
FTY720 reduces the number of circulating allo-T-bodies in the recipient mice and increases T-body number in the spleen. (A) Comparative in vivo BLI of Renca-Her2/neu-bearing mice (n = 6) irradiated with 400 rads, and treated with either 30 × 106 syngeneic (Balb-N29) or allogeneic (Black-N29) T-bodies. BLI was performed as in Figure 4A. (B) Whole body BLI signal intensities from sequential imaging depicted above, and measured every 3-4 days after T cell transfer from the same groups shown in panel A. Each line represents 1 animal. (C) A region of interest was defined around the spleens of mice from panel A. Shown are absolute photon counts from mice treated with allogeneic T-bodies either with or without FTY720. Means and SEM are shown. (D) Mononuclear cells were isolated 4 days after adoptive transfer from the blood of tumor-bearing mice treated with allogeneic T-bodies either with or without FTY720, and the number of T cells in the blood was quantified by staining for CD3. Means and SEM are shown. (E-F) Splenocytes of tumor-bearing mice treated with allogeneic T-bodies either with or without FTY720, were stained with anti-H2Kb 4 (E) or 7 (F) days after adoptive transfer to identify the allogeneic T-bodies. Means and SEM are shown. Each line represents 1 animal.
These data were confirmed by FACS analysis showing that FTY720 partially inhibited lymphocyte egress to the blood (Figure 6D), thereby resulting in increased accumulation in the lymphatic organs (Figure 6E). In addition, FACS analysis confirmed that FTY720 enhanced the persistence of allogeneic T cells in vivo, with higher numbers of allo–T-bodies detected in the spleen on day 7 (Figure 6F). This enhanced persistence of allogeneic T-bodies can explain the increased survival of tumor bearing mice treated with allogeneic T-bodies and FTY720.
When the effect of FTY720 was tested in a protocol which generally causes lethal GVHD (manifested by severe cachexia) in 100% of mice (400 rads and 108 allo–T-bodies, Figure 2D), only 16% of the mice died of GVHD (Figure 5B, P = .005). In addition, most of the remaining mice survived long term (> 350 days), demonstrating that FTY720 can concurrently inhibit GVHD while allowing the antitumor response to proceed.
Discussion
In this study, we demonstrate that the use of genetically redirected open repertoire allogeneic T cells can be a safe and effective alternative to syngeneic T cells for the treatment of disseminated tumors. Despite only transient persistence in sub-lethally irradiated mice, allo–T-bodies provided as much therapeutic benefit as syngeneic cells, when sufficient numbers of T-bodies were transferred and were not accompanied by lethal GVHD (Figure 2B-C). Allo–T-bodies were superior to nonredirected allogeneic T cells, highlighting the importance and the potency of the CAR (Figure 3). The GVH reactivity of the allogeneic T cells modulated the immune response causing these cells to proliferate extensively and to differentiate to effector cells (Figure 4). When we inhibited lymphocyte egress through the addition of FTY720 to the treatment protocol, allo–T-bodies provided long lasting therapeutic benefit, in contrast to syngeneic cells (Figure 5A).
The outcome of the T-body response depends on the dual specificities of the T-body, including those of the CAR and the endogenous TCR. The CAR conferred the T-bodies with antitumor specificity while the TCR conferred allo-reactivity to a subset of the T-bodies (Figure 4). The balance between antitumor activity and anti-host activity determines the success of this modality. When we tested the activity of nonredirected allogeneic T cells they could kill the tumor both in vitro, and under some conditions provide modest therapeutic benefit in vivo (Figure 3). However, the frequency of allo-reactive T cells has been estimated to 1%-10%7 in contrast to T-bodies express the CAR uniformly, such that there is an order of magnitude more tumor-specific T cells than allo-specific T cells per cell batch. Indeed allogeneic T-bodies were much more effective than nonredirected cells both in vitro and in vivo. In addition, the incorporation of the CD28 signaling moiety into the CAR has been shown to activate bcl-xL, thereby reducing CAR-associated AICD, compared with the endogenous TCR.21 Improvements to the CAR such as the incorporation of additional costimulatory moieties (for example 4-1BB5,26 ) may further increase the potency and persistence the allo–T-bodies, thus enhancing the graft-versus-tumor (GVT) response without increasing the risk of GVHD.
The risk of GVHD limits the number of allo–T-bodies which could be transferred safely, but the inherent GVH reactivity of these cells could also potentiate ACT. The GVH reactivity stimulated allo–T-bodies in vivo, and caused substantial proliferation (Figure 4) in the absence of vaccination or high dose IL-2. GVH-driven proliferation is one possible explanation why allo–T-bodies were able to provide similar therapeutic benefit to that of syngeneic ones (Figure 2B-C) despite limited persistence (Figure 4). However, in vivo stimulation may potentiate ACT through mechanisms other than enhanced proliferation. In a recent study, Pule et al showed that autologous GD2-specific T-bodies derived from anti-EBV CTL persisted for a longer time than T-bodies derived from polyclonal T cells in patients latently infected with EBV, demonstrating the contribution of in vivo antigenic stimulation to persistence.4
There have only been a small number of studies on the use of allogeneic cells without allogeneic HCT. One such study, which was conducted by Boni et al, compared the antitumor activity of either syngeneic or allogeneic haploidentical naive TCR transgenic T cells expressing the pmel TCR in a model of experimental melanoma.13 The authors showed that the pmel TCR did not posses allo-reactivity against the other mouse strains which were tested.13 Myeloablative irradiation of the mice at 900 rads delayed the rejection of allogeneic T cells, which were able to cause regression of established tumors. Nevertheless, allogeneic T cells were inferior to syngeneic T cells in causing tumor regression, presumably because the allogeneic ones were eventually rejected. Addition of allogeneic open repertoire T cells to the pmel T cells increased tumor regression, demonstrating that allo-reactivity can enhance the antitumor response, similarly to our results (Figure 3). However, the improvement seen in Boni's study came at the cost of lethal GVHD while in our study, using milder irradiation doses, no GVHD developed. This contribution of allo-reactivity to the antitumor response can also help explain why in our study allogeneic T cells, despite their ultimate rejection, provided comparable benefit to syngeneic T cells.
Our strategy for allogeneic ACT hinged on exploiting the GVH response to augment therapy. However, this approach can also benefit from reducing the frequency of allo-reactive T cells, which may allow transfer of a greater allogeneic T cell dose, or from increased irradiation before transfer, thus maintaining the GVH response at a safe level, while increasing the potency of the ACT protocol. One way to reduce the frequency of allo-reactive T cells is to use antigen-specific T cells, which have a restricted TCR repertoire, instead of open repertoire T cells, similarly to the cells used by Boni et al.13 In fact, these 2 approaches are not mutually exclusive, because antigen-specific T cells can be transduced with an additional specificity to express a tumor-specific CAR. While clinical experience with allogeneic tumor-specific T cells has been very limited, allogeneic virus-specific T cell lines have been frequently used after allogeneic HCT to treat viral reactivation, usually without causing serious GVHD.9,27,28 Interestingly it has recently been demonstrated that even though they generally do not cause GVHD, many virus-specific lines posses allo-reactivity.29-31 These findings suggest that combining T-bodies and antigen-specific T cells is a promising approach for allogeneic ACT. Translation of this approach to patients will require incorporation of additional safety measures to deal with the risk of GVHD; one possible approach is the expression of suicide genes by the allo–T-bodies.32,33
Addition of FTY720 to the treatment protocol increased the percentage of long term (> 350 days) surviving mice treated with allogeneic but not syngeneic T-bodies, such that allo–T-bodies provided superior therapeutic benefit over syngeneic ones (Figure 5A). The superiority of allo–T-bodies proves that their allo-reactivity can be exploited to potentiate ACT. As expected, FTY720 blocked egress of lymphocytes to the blood, thereby supporting increased accumulation in the lymphatic organs (Figure 6E-F). In agreement with the survival data the BLI signals from extra-lymphatic organs increased, including from the chest area (Figure 6A). Adoptive transfer of cells circumvents the process of lymphocyte egression from the lymphoid organs, and introduces lymphocytes directly into the blood. From the blood the allogeneic T cells can reach peripheral tissues where they can eliminate the cancer. FTY720 can potentiate this process in 2 ways: first it can inhibit egression of the host's T cells thus directly inhibiting the HVG reaction. In addition allogeneic T cells, which enter the lymphoid organs, also become trapped therein. In this way the GVH reaction is essentially redirected against the host's immune system rather than peripheral tissues, thereby indirectly weakening the HVG reaction. The result of these processes is that the survival of allogeneic lymphocytes is enhanced both outside the lymph nodes because of the inhibition of the HVG reaction and inside the lymph nodes because proliferating allogeneic T cells accumulate there in agreement with our imaging data. Importantly, when FTY720 was added to a protocol that causes lethal GVHD, it inhibited GVHD without ablating therapeutic benefit (Figure 5B); thus, addition of FTY720 was beneficial to allogeneic ACT whether or not the protocol caused GVHD. However, blocking egress can also potentially inhibit the efficacy of immunotherapy, especially when vaccination is used and effector cells are generated in the lymph node (LN). By virtue of their CAR, T-bodies can be activated by cells in peripheral tissues which do not express costimulatory molecules21 and are therefore less dependent on stimulation by APCs in the LN.
The lymph nodes are one of the most common metastatic sites for virtually all cancers (solid as well as hematologic) and transfer of allo–T-bodies is particularly promising for this indication. The GVH-driven proliferation of allo–T-bodies can potentially allow for extremely powerful responses against LN metastases. Administration of FTY720 in conjunction with allo–T-body transfers can further augment such responses by blocking lymphocyte egress and concentrating the antitumor response to the lymph nodes.
Translation of this approach to the clinic would require rigorous dose escalation clinical trials as well as additional safety measures. Suicide genes could be extremely useful in controlling GVHD development as has recently been demonstrated in early clinical trials.32,33 An alternative strategy would be to target the T-bodies with a depleting antibody to the CAR. However, the best safety feature of this approach is the HVG reaction, which ultimately clears the allogeneic T cells in stark contrast to what happens in allogeneic HCT where donor T cells persist indefinitely. In addition development of GVHD should be less variable in this scenario because the immune response in an MHC-mismatched setting is more predictable than in an MHC-matched setting, which is typical of allogeneic HCT. Finally, because the HVG response occurs independently of any tumor, overcoming it (as has been demonstrated here) and using the right CAR, one could probably get therapeutic benefit in other malignancies models including hematopoietic ones.
Taken together, our results provide a proof of principle for the application of allogeneic adoptive therapy, which is both safe and effective in our mouse model using fully mismatched allogeneic open repertoire T cells redirected by a tumor-specific CAR. The combination of MHC-mismatched allogeneic T cells with an MHC unrestricted chimeric antigen receptor yields ‘universal effector cells’ which could potentially be used as an ‘off-the-shelf’ cellular therapy for cancer. Inhibition of lymphocyte egress augmented allogeneic adoptive therapy, such that allogeneic T-bodies provided greater therapeutic benefit than syngeneic T-bodies despite their limited persistence. These results suggest that with further fine-tuning, allogeneic adoptive therapy may become the treatment of choice both because of its obvious logistical and economical advantages, and because of its greater efficacy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to S. Schwarzbaum for scientific editing and Dan Blat assistance with the graphics.
This study was supported by funds from the Israel Science Foundation, the EC HEATH FP6 ATTACK Consortium, and the Moross Cancer Center at The Weizmann Institute of Science.
Authorship
Contribution: A.M. designed and performed experiments, analyzed and interpreted data, and wrote the paper; T.W. designed experiments, and interpreted data; and Z.E. designed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.M. is Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, CA.
Correspondence: Zelig Eshhar, Dept of Immunology, The Weizmann Institute of Science, PO Box 26, Rehovot, Israel 76100; e-mail: zelig.eshhar@weizmann.ac.il.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal