Abstract
Recent population-based studies demonstrate an increased fracture risk with monoclonal gammopathy of undetermined significance (MGUS). The etiology of this increased risk remains unclear, however, because areal bone mineral density (aBMD) measurements by dual-energy x-ray absorptiometry cannot assess bone microstructural properties critical to determining bone quality and strength. To better define the skeletal effects of MGUS, we performed aBMD and high-resolution peripheral quantitative computed tomography volumetric bone mineral density (vBMD) measurements in 50 MGUS patients (20 females, 30 males; mean ± SEM age, 70.5 ± 1.4 years) and 100 matched control subjects. Relative to controls, MGUS patients had decreased aBMD at the femoral neck (P = .05) and total femur (P < .05) but no differences at other sites. In contrast, high-resolution peripheral quantitative computed tomography showed markedly diminished cortical thickness (P < .05) and increased endocortical area (P < .01). Average vBMD (P < .01), cortical vBMD (P < .001), and trabecular thickness (P < .01) were all significantly decreased in MGUS patients, suggestive of impaired bone formation. Serum levels of the Wnt pathway inhibitor Dickkopf-related protein 1 (P < .001) and osteoclast-activating factor MIP-1α (P < .05) also were significantly elevated in MGUS patients. Our data provide the first evidence of altered bone microstructure in MGUS and suggest that cytokines elevated in osteolytic myeloma also may be associated with bone loss in MGUS.
Introduction
Multiple myeloma (MM) results from the clonal expansion of malignant plasma cells within the bone marrow. Bone disease is nearly universal in MM. Roughly 80% of patients develop a pathologic fracture at some point during their disease, and nearly 90% have radiographic evidence of skeletal lesions.1 At the other end of the monoclonal gammopathy spectrum, monoclonal gammopathy of undetermined significance (MGUS) is a premalignant condition, with an ∼ 1% annual risk of progression to an MM-related malignancy.2 MGUS is a common finding in clinical practice, with a prevalence of ∼ 3.2% in white persons 50 years of age and older.3 This increases with age, such that in persons older than 85 years of age, the prevalence of MGUS is ∼ 7.5%.
By definition, MGUS patients lack lytic bone lesions.3 Nonetheless, population-based studies show that MGUS is associated with a significantly increased risk of fracture,4,5 suggesting that alterations in bone quantity, quality, or both are present even before disease progression to MM.6 However, little is known about the skeletal phenotype of MGUS and whether abnormalities exist to explain this increased fracture risk. Indeed, even whether bone loss is increased in MGUS is a subject of debate. Thus, some studies7,8 but not others9,10 have reported that biochemical markers of bone resorption are increased in MGUS. Furthermore, although several studies have reported that fractures are increased in MGUS,4,10-12 some of these same studies have provided conflicting results as to whether MGUS subjects have decreased bone mass using standard areal bone mineral density (aBMD) measurements by dual-energy x-ray absorptiometry (DXA).10,12
Conventional assessment of skeletal status has relied on DXA, but DXA imaging has several limitations, including the include the following: (1) the extrapolation of a 2-dimensional (areal) measurement of bone mineral density (BMD) to derive a 3-dimensional structure; (2) the inability to accurately differentiate between cortical and trabecular bone compartments; and (3) the inability to assess bone microstructure. High-resolution peripheral quantitative computed tomography (HRpQCT) is a recently developed technology that can be used at peripheral skeletal sites such as the wrist and tibia.13 HRpQCT allows for the accurate and reproducible noninvasive measurement of important components of bone quality in humans, including separate determinations of trabecular and cortical volumetric BMD (vBMD), as well as detailed visualization of trabecular and cortical bone microstructure, measurements not possible with DXA.
As well-recognized, bone loss in myeloma results from both inhibition of osteoblast activation and increased osteoclast activation. Multiple factors have now been identified that correlate with the extent of altered bone cell activity. For osteoblast inhibition, these factors include the secreted Wnt pathway inhibitors Dickkopf-related protein 1 (DKK1) and sclerostin. For osteoclast activation, these factors include the cytokine macrophage inflammatory protein-1α (MIP-1α)/chemokine (C-C motif) ligand 3 (CCL3).14 Whether similar factors might play a role in MGUS, leading to increased skeletal fragility and increased fracture risk, is unknown.
Because of the increased fracture risk in MGUS patients, it is important to better understand the effects of MGUS on bone health. The purpose of our study was to determine (1) whether MGUS is associated with altered skeletal microstructure that is not evident by standard DXA imaging; and (2) whether changes in serum markers of bone turnover, changes in circulating levels of cytokines previously found to be associated with myeloma bone disease, or both also occur in subjects with MGUS.
Methods
Subjects
Fifty patients meeting diagnostic criteria for MGUS according to standard diagnostic criteria as defined by the International Myeloma Working Group15 were recruited. One hundred control subjects were recruited from an age-stratified random sample of Rochester, Minnesota residents selected using the medical records linkage system of the Rochester Epidemiology Project.16 Control subjects were age-, sex-, and body mass index–matched in a 2:1 ratio to the MGUS patients. Potential subjects who had received prior bisphosphonate therapy were excluded. All patients provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the Mayo Clinic Institutional Review Board.
Areal BMD measurements
Lumbar spine, femoral neck, total femur, total body, and nondominant distal radius aBMD measurements were made by DXA (Lunar Prodigy System; GE Healthcare). DXA spine scans were evaluated according to International Society of Clinical Densitometry criteria (www.iscd.org/visitors/positions/OPReferences.cfm). Thus, vertebrae with deformities were deleted, and the mean L1-L4 aBMD value was recalculated from the remaining vertebrae.
HRpQCT imaging
Imaging of the nondominant ultradistal radius (or nonfractured side in case of a prior wrist fracture) was performed with the XtremeCT device (Scanco Medical AG). From a digital image (scout view) of the lower forearm, a reference point was set electronically at the intersection of the joint space with the radio-ulnar junction (Figure 1A). An automated program was then used to select a scanning site 9.5 to 18.5 mm proximal to this site to maintain interpatient scanning site uniformity. An in vivo measurement protocol was used to acquire a 3-dimensional stack of 116 high-resolution quantitative computed tomography slices (Figure 1B) at the distal end of the radius with an isotropic voxel size and slice thickness of 82 μm, using an effective energy of 40 keV; field of view of 125.9 mm; and image matrix of 1536 × 1536 pixels, from which total (coefficient of variation [CV], 0.3%), trabecular (CV, 0.4%) and cortical (CV, 0.4%) vBMD values were obtained. A representative 3-dimensional reconstructed imaged is shown in Figure 1C. Radiation exposure to the subjects was minimal, with a local absorbed dose of 0.065 Gy and total radiation exposure of less than 0.01 mSv.
HRpQCT imaging at the radius. (A) Site of imaging in the ultradistal radius. (B) Representative cross-sectional images from proximal (top left) to distal (bottom right), (C) Representative 3-dimensional reconstructed image.
HRpQCT imaging at the radius. (A) Site of imaging in the ultradistal radius. (B) Representative cross-sectional images from proximal (top left) to distal (bottom right), (C) Representative 3-dimensional reconstructed image.
Trabecular parameters.
Bone volume/total volume (BV/TV, %) was derived from trabecular vBMD, assuming mineral density of fully mineralized bone of 1.2 g hydroxyapatite/cm3. Recognizing that individual trabeculae would not be resolved at their correct thickness (∼ 100 μm) because of partial volume effects, a thickness-independent structure extraction was used to identify 3-dimensional ridges (center points of the trabeculae)17 ; trabecular number (Tb.N, mm−1) was then taken as the inverse of the mean spacing of the ridges.18 Analogous with standard histomorphometry,19 trabecular thickness (Tb.Th, μm) was calculated using the formula Tb.Th = BV/TV ÷ Tb.N, and trabecular spacing (Tb.Sp, μm) was calculated as Tb.Sp = (1 − BV/TV) ÷ Tb.N. Tb.Sp.SD, the standard deviation of Tb.Sp, is a measure of trabecular variation.20 Validation studies show excellent correlation (R ≥ 0.96) for these parameters compared with the standard ex vivo microCT (μCT) technique.21
Cortical parameters.
The cortex was segmented from the gray scale image with a Gaussian filter and threshold.18 Cortical vBMD and area were measured directly, and periosteal circumference was calculated from the contour. Cortical thickness (Ct.Th, μm) was then derived using the formula Ct.Th = area ÷ circumference. Again, excellent correlation (R = 0.98) has been shown for Ct.Th measurements with HRpQCT versus μCT.22 Endocortical circumference was calculated assuming that the trabecular compartment was circular.
Serum biochemical studies
Bone resorption markers.
Serum C-terminal telopeptide of type I collagen (CTX) was measured by ELISA (interassay CV, < 8%; Immunodiagnostic Systems). Serum tartrate-resistant acid phosphatase isoform type 5b (TRAP5b) also was measured by ELISA (interassay CV, < 14%; Immunodiagnostic Systems).
Bone formation marker.
Serum amino-terminal propeptide of type I collagen (PINP) was measured by radioimmunoassay (interassay CV, < 10%; IDS).
Cytokine measurements.
Serum levels of DKK1 were measured by ELISA (interassay CV, < 9%; ALPCO Immunoassays), as were serum levels of MIP-1α/CCL3 (interassay CV, < 6%; R&D Systems) and osteoprotegerin (OPG; interassay CV, < 8%; ALPCO Immunoassays). Serum sclerostin concentrations was assessed by ELISA obtained from ALPCO Immunoassays (developed by Biomedica; interassay CV, < 4% and lower limit of detection, 86 pg/mL). All assays were performed according to manufacturers' instructions.
M-protein levels.
Serum monoclonal protein (M-protein) level was obtained from review of the medical record of MGUS patients.
Statistical analyses
Statistical analyses were completed using the SAS and JMP Statistical Discovery software (SAS Institute). All anthropometric, imaging, and serum biochemical data are reported as mean ± SEM. We used the 2-sample t test to compare the MGUS measurements with those from an age- and sex-matched control population. Correlations comparing M-protein levels and individual DXA and HRpQCT imaging parameters were obtained using the Pearson product-moment correlation coefficient. Results were considered significant at P < .05.
Results
As seen in Table 1, MGUS patients and controls were well matched for age, sex, height, weight, and body mass index. Ages were 70.5 ± 1.3 years for MGUS patients and 70.3 ± 1.0 years for control subjects. Sixty percent of all subjects were men.
Anthropometric variables in MGUS patients and control subjects
| . | MGUS . | Control . | P . |
|---|---|---|---|
| Total, n | 50 | 100 | |
| Age, y | 70.5 ± 1.4 | 70.3 ± 1.0 | .927 |
| Sex, n | |||
| Male | 30 | 60 | |
| Female | 20 | 40 | |
| Height, cm | |||
| Male | 177 ± 1.4 | 176 ± 0.8 | .422 |
| Female | 161 ± 1.4 | 162 ± 0.9 | .743 |
| Weight, kg | |||
| Male | 89.0 ± 2.4 | 89.8 ± 1.9 | .813 |
| Female | 72.1 ± 3.6 | 72.9 ± 1.6 | .827 |
| Body mass index, kg/m2 | |||
| Male | 28.4 ± 0.7 | 29.0 ± 0.5 | .533 |
| Female | 27.8 ± 1.4 | 27.9 ± 0.6 | .913 |
| . | MGUS . | Control . | P . |
|---|---|---|---|
| Total, n | 50 | 100 | |
| Age, y | 70.5 ± 1.4 | 70.3 ± 1.0 | .927 |
| Sex, n | |||
| Male | 30 | 60 | |
| Female | 20 | 40 | |
| Height, cm | |||
| Male | 177 ± 1.4 | 176 ± 0.8 | .422 |
| Female | 161 ± 1.4 | 162 ± 0.9 | .743 |
| Weight, kg | |||
| Male | 89.0 ± 2.4 | 89.8 ± 1.9 | .813 |
| Female | 72.1 ± 3.6 | 72.9 ± 1.6 | .827 |
| Body mass index, kg/m2 | |||
| Male | 28.4 ± 0.7 | 29.0 ± 0.5 | .533 |
| Female | 27.8 ± 1.4 | 27.9 ± 0.6 | .913 |
Standard assessments by DXA demonstrated significantly lower total femur aBMD in MGUS compared with control subjects, with differences in aBMD at the femur neck and total radius approaching statistical significance (Table 2). At the lumbar spine, the site at which fracture incidence is most notably increased in MGUS subjects,4,5 bone density was reduced but did not differ statistically from control subjects. Whole body aBMD also was not different. Likewise, there were no differences in DXA-determined areas at any skeletal site measured, with the exception of the ultradistal radius were MGUS patients had a statistically significantly greater area (Table 2).
Regional and total and aBMD measurements by DXA in MGUS patients and control subjects
| . | MGUS . | Control . | Pr > t* . |
|---|---|---|---|
| Total femur BMD, g/cm2 | 0.95 ± 0.02 | 1.00 ± 0.02 | .044 |
| Total femur area, cm2 | 35.8 ± 0.6 | 36.0 ± 0.4 | .667 |
| Femur neck BMD, g/cm2 | 0.86 ± 0.02 | 0.91 ± 0.01 | .052 |
| Femur neck area, cm2 | 5.46 ± 0.10 | 5.38 ± 0.05 | .260 |
| Spine L2-L4 BMD, g/cm2 | 1.21 ± 0.03 | 1.26 ± 0.02 | .117 |
| Spine L2-L4 area, cm2 | 47.2 ± 0.9 | 46.9 ± 0.6 | .729 |
| Total radius BMD, g/cm2 | 0.67 ± 0.02 | 0.70 ± 0.01 | .077 |
| Total radius area, cm2 | 16.7 ± 0.4 | 16.1 ± 0.2 | .026 |
| Total body BMD, g/cm2 | 1.18 ± 0.02 | 1.20 ± 0.01 | .335 |
| Total body area, cm2 | 2317 ± 44 | 2364 ± 27 | .186 |
| . | MGUS . | Control . | Pr > t* . |
|---|---|---|---|
| Total femur BMD, g/cm2 | 0.95 ± 0.02 | 1.00 ± 0.02 | .044 |
| Total femur area, cm2 | 35.8 ± 0.6 | 36.0 ± 0.4 | .667 |
| Femur neck BMD, g/cm2 | 0.86 ± 0.02 | 0.91 ± 0.01 | .052 |
| Femur neck area, cm2 | 5.46 ± 0.10 | 5.38 ± 0.05 | .260 |
| Spine L2-L4 BMD, g/cm2 | 1.21 ± 0.03 | 1.26 ± 0.02 | .117 |
| Spine L2-L4 area, cm2 | 47.2 ± 0.9 | 46.9 ± 0.6 | .729 |
| Total radius BMD, g/cm2 | 0.67 ± 0.02 | 0.70 ± 0.01 | .077 |
| Total radius area, cm2 | 16.7 ± 0.4 | 16.1 ± 0.2 | .026 |
| Total body BMD, g/cm2 | 1.18 ± 0.02 | 1.20 ± 0.01 | .335 |
| Total body area, cm2 | 2317 ± 44 | 2364 ± 27 | .186 |
P values are adjusted for age and sex.
As shown in Table 3, HRpQCT imaging at the ultradistal radius confirmed that MGUS patients had a significantly greater total bone area relative to controls. This was accompanied by a marked increase in endocortical area. In contrast to results at the radius by DXA, in which aBMD did not differ between MGUS and control subjects, HRpQCT demonstrated a significantly lower average vBMD as well as cortical vBMD, with a trend toward lower trabecular vBMD in the MGUS patients (Table 3).
vBMD and bone structure parameters by HRpQCT at the nondominant ultradistal radius in MGUS patients and control subjects
| . | MGUS . | Control . | Pr > t* . |
|---|---|---|---|
| Bone area, mm2 | 319.9 ± 12.5 | 294.1 ± 7.4 | .004 |
| Cortical area, mm2 | 60.9 ± 2.7 | 64.9 ± 2.0 | .120 |
| Endocortical area, mm2 | 252.2 ± 11.4 | 224.0 ± 6.6 | .003 |
| Averge vBMD, mg HA/cm3 | 300.1 ± 10.1 | 334.9.0 ± 7.2 | .005 |
| Trabecular vBMD, mg HA/cm3 | 149.9 ± 5.8 | 160.9 ± 4.0 | .080 |
| Cortical vBMD, mg HA/cm3 | 822.2 ± 11.8 | 862.4 ± 6.9 | .001 |
| Cortical thickness, mm | 0.80 ± 0.03 | 0.88 ± 0.02 | .029 |
| Trabecular bone volume/tissue volume | 0.125 ± 0.005 | 0.134 ± 0.003 | .080 |
| No. of trabeculae, 1/mm | 1.82 ± 0.05 | 1.81 ± 0.03 | .895 |
| Trabecular thickness, mm | 0.068 ± 0.002 | 0.074 ± 0.001 | .004 |
| Trabecular separation, mm | 0.518 ± 0.030 | 0.501 ± 0.015 | .547 |
| SD of trabecular separation, mm | 0.258 ± 0.025 | 0.231 ± 0.012 | .273 |
| . | MGUS . | Control . | Pr > t* . |
|---|---|---|---|
| Bone area, mm2 | 319.9 ± 12.5 | 294.1 ± 7.4 | .004 |
| Cortical area, mm2 | 60.9 ± 2.7 | 64.9 ± 2.0 | .120 |
| Endocortical area, mm2 | 252.2 ± 11.4 | 224.0 ± 6.6 | .003 |
| Averge vBMD, mg HA/cm3 | 300.1 ± 10.1 | 334.9.0 ± 7.2 | .005 |
| Trabecular vBMD, mg HA/cm3 | 149.9 ± 5.8 | 160.9 ± 4.0 | .080 |
| Cortical vBMD, mg HA/cm3 | 822.2 ± 11.8 | 862.4 ± 6.9 | .001 |
| Cortical thickness, mm | 0.80 ± 0.03 | 0.88 ± 0.02 | .029 |
| Trabecular bone volume/tissue volume | 0.125 ± 0.005 | 0.134 ± 0.003 | .080 |
| No. of trabeculae, 1/mm | 1.82 ± 0.05 | 1.81 ± 0.03 | .895 |
| Trabecular thickness, mm | 0.068 ± 0.002 | 0.074 ± 0.001 | .004 |
| Trabecular separation, mm | 0.518 ± 0.030 | 0.501 ± 0.015 | .547 |
| SD of trabecular separation, mm | 0.258 ± 0.025 | 0.231 ± 0.012 | .273 |
HA indicates hydroxyapatite.
P values are adjusted for age and sex.
HRpQCT imaging also provides detail on bone microstructure. As seen in Table 3, cortical thickness was significantly lower in MGUS patients, whereas the decrease in trabecular bone volume over total tissue volume approached statistical significance. Importantly, although trabecular number and separation did not differ between MGUS and control subjects, MGUS patients had a significantly lower trabecular thickness while maintaining a relatively normal pattern of trabecular homogeneity.
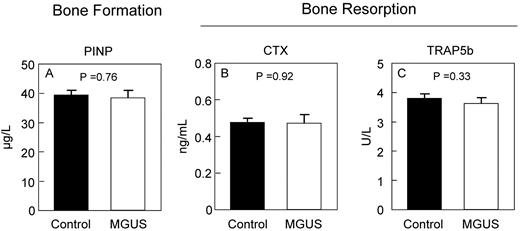
To determine whether the changes in bone microstructure by HRpQCT were associated with changes in bone turnover, we measured standard biochemical markers of bone formation and resorption. As shown in Figure 2, serum levels of the bone formation marker, PINP, and the bone resorption markers CTX and TRAP5b did not differ between MGUS and control subjects. Interestingly, however, serum levels of OPG (Figure 3A) were elevated in MGUS patients and approached statistical significance. The mean M-protein level was 1.4 g/L in the MGUS patients but was not assessed in control subjects.
Biochemical markers of bone turnover. Serum markers of bone formation (PINP; A) and bone resorption (CTX; B) and TRAP5b (C) are unchanged in MGUS.
Biochemical markers of bone turnover. Serum markers of bone formation (PINP; A) and bone resorption (CTX; B) and TRAP5b (C) are unchanged in MGUS.
Serum levels of osteoprotegerin and sclerostin. Circulating levels of OPG (A) and sclerostin (B) are not different in MGUS.
Serum levels of osteoprotegerin and sclerostin. Circulating levels of OPG (A) and sclerostin (B) are not different in MGUS.
Although sclerostin levels were no different between the groups (Figure 3B), DKK1 levels were nearly 2-fold higher in MGUS compared with control subjects (Figure 4A), suggesting that some of the decrease in cortical and trabecular parameters measured by HRpQCT in MGUS subjects may reflect impaired bone formation because of DKK1-mediated Wnt pathway inhibition.
Comparison of circulating DKK1 and MIP-1α levels. Serum levels of DKK1 (A) and MIP-1α (B) are increased in MGUS.
Comparison of circulating DKK1 and MIP-1α levels. Serum levels of DKK1 (A) and MIP-1α (B) are increased in MGUS.
As shown in Figure 4B, MIP-1α levels were nearly 6-fold higher in MGUS compared with control subjects, again suggesting that some of the decreases in bone parameters determined to occur in MGUS by HRpQCT imaging may reflect increased osteoclast activation. Neither DKK1 (r = −0.027; P = .853) nor MIP-1α (r = −0.166; P = .251; data not shown) levels were associated with M-protein level in the MGUS patients.
Discussion
Although DXA was able to reveal differences in aBMD between MGUS patients and matched control subjects at only the total femur, HRpQCT imaging readily revealed significant differences in vBMD between the groups at the distal radius due its superior ability to differentiate cortical from trabecular bone compartments. In addition to confirming an increase in total area of the distal radius, HRpQCT further determined that MGUS patients had substantially diminished cortical thickness because of a concomitant increase in endocortical area. These results suggest that MGUS leads to increased endocortical bone resorption with a likely compensatory increase in periosteal bone apposition, although cortical thickness was not increased. Both average vBMD and cortical vBMD also were lower in MGUS patients. Importantly, trabecular number and separation did not differ between the groups, but those with MGUS did have significantly lower trabecular thickness, suggesting that MGUS patients may have impaired bone formation perhaps secondary to osteoblast inhibition. Interestingly, the M-protein levels were not correlated with any HRpQCT parameter assessed (data not shown), consistent with a recent report in which M-protein level at diagnosis was not shown to correlate with fracture risk in an analysis of more than 5000 Swedish MGUS subjects.23
In a cross-sectional study of postmenopausal women with MGUS, Pepe et al showed that lumbar spine, femoral neck, and total aBMD were all lower in subjects who had at least 1 vertebral fracture compared with those without a vertebral fracture.10 The study did not, however, include a matched control group without MGUS for comparison. Based on receiver operating characteristics, Pepe et al suggested that lumbar spine aBMD was the best predictor of vertebral fractures in postmenopausal women with MGUS.10 In our study, the lack of difference in spine aBMD between MGUS patients and controls, despite a well-recognized increased incidence of vertebral fractures in MGUS subjects,4,5,11 strongly suggests that DXA measurements are not able to accurately reflect the complex skeletal changes that occur in MGUS. Rather, the microstructural changes observed by HRpQCT suggest that bone remodeling is altered in MGUS. Thus, our results are consistent with both increased bone resorption at the endocortical surface, in addition to reduced bone formation at the trabecular surfaces in MGUS patients. Furthermore, MGUS subjects also had an overall increase in bone size, probably because of increased periosteal bone apposition, perhaps as a compensatory biomechanical adaptation to improve bone strength.24 Importantly, because of technical aspects underlying the DXA methodology, this increase in bone size introduces an artifactual increase in aBMD when measured by DXA,25 an artifact which may in part account for the inability of DXA to detect the concomitant cortical and trabecular bone loss seen by HRpQCT in MGUS subjects.
In our study, we were unable to detect any differences in serum biochemical markers of bone formation (PINP) or resorption (CTX or TRAP5b) between MGUS patients and matched controls. Of note, these serum measurements reflect whole body bone turnover and therefore provide little information about local changes in skeletal homeostasis.26 Previous studies examining bone turnover markers in MGUS have shown mixed results. Thus, whereas the bone resorption marker N-telopeptide of type 1 collagen was found to be increased in MGUS subjects,27 other investigators found no differences in serum markers of either bone formation (bone-specific alkaline phosphatase) or resorption (deoxypyridinoline) between MGUS and control subjects.28 Interestingly, quantitative bone histomorphometry demonstrates that at least some MGUS subjects have histomorphometric evidence for increased bone resorption.29 Our finding that serum OPG levels are increased in MGUS subjects is consistent with this increase in bone resorption and with previous data demonstrating increased OPG levels in conditions of increased bone loss, perhaps as a compensatory mechanism.30
DKK1 is a secreted inhibitor of the Wnt signaling pathway that has been shown to be an important regulator of MM osteolytic bone disease31 and disease progression both through inhibition of Wnt-regulated osteoblastic differentiation and indirectly by increasing osteoclastic activity by increasing the RANKL/OPG ratio.32 Previous work by Kaiser et al demonstrated that DKK1 levels were ∼ 6-fold lower in subjects with MGUS compared with those with MM but that significant overlap existed in serum DKK1 levels between MGUS subjects and subjects with early stage (Durie-Salmon stage I) MM.33 Importantly, however, that study did not include an unaffected control group, so comparison between DKK1 levels in MGUS patients and normative values could not be made. In another study that included 18 MGUS and 22 control subjects, Politou et al noted a trend toward increased DKK1 levels in the MGUS patients, although this trend did not reach statistical significance, perhaps because of small sample size.6 Thus, our finding that circulating DKK1 levels are significantly higher in a larger and well-defined MGUS cohort than in a matched control group strongly suggests that the inhibition of osteoblast function known to be important in myeloma bone disease also may extend to the premalignant condition MGUS and perhaps relate to the increased fracture risk seen in these patients.
We also found a statistically significant increase in serum levels of the osteoclast-activating factor MIP-1α in the MGUS patients. In a previous study, MIP-1α levels were decreased in MGUS relative to MM patients, but they were not different between MGUS and matched control subjects.27 We do not have an explanation for such differences, although we note that only 12 subjects were included in the control group in the described study, and limited statistical power may be a contributing factor to these earlier nonstatistically significant results. Although receptor activator of nuclear factors κ-B ligand (receptor activator of nuclear factor κ-B ligand [RANKL]) is also a well-recognized factor in regulating osteoclast formation and activity in MM, we have previously been unable to measure detectable free RANKL levels in the majority of our control subjects. As such, we did not attempt to measure RANKL levels in the MGUS patients, because we would have lacked a valid comparator group. Notably, we did not find a correlation between M-protein levels and either DKK1 or MIP-1α levels in our MGUS cohort.
Our study has significant strengths, including the inclusion of novel methodology (HRpQCT) not previously used in any study of patients with any monoclonal gammopathy. Because of the high resolution possible with HRpQCT (voxel size, 82 μm), our data represent the present limit of feasibility for in vivo assessment of skeletal microstructure in humans through use of a technology that has been validated for both cortical and trabecular bone parameters against the current standard of μCT that because of high radiation doses can only be used on ex vivo specimens.22 Nevertheless, we recognize that even with this high level of resolution, the parameters assessed by HRpQCT are probably estimates of the true bone microarchitectural measures that can only be obtained through direct tissue analysis. The major limitation of HRpQCT, however, is that we are at present only able to image peripheral skeletal sites such as the distal radius and tibia because of limitations on radiation exposure. A future ability to include high-resolution central quantitative computed tomography imaging may allow us to explain why the increased fracture risk in MGUS seems to predominantly involve the axial skeleton. One hypothesis would be that vertebral anatomy is unfavorable to changes in bone geometry,24 such that an increase in compensatory periosteal apposition is unable to mitigate ongoing trabecular bone loss and cortical thinning, thereby leading to reduced bone strength and increased vertebral fracture risk.
It is important to recognize that although bone loss and fracture incidence increases in all adults with age, so too does the risk for developing MGUS. Unlike patients with MM, however, patients with MGUS are more likely to die from a cause other than a plasma cell disorder.34 Therefore, avoiding events associated with significant morbidity and mortality—such as fractures—is of paramount importance for patients with MGUS. In 2 studies of short duration, both alendronate35 and zoledronic acid36 were shown to increase aBMD in MGUS patients after 18 and 13 months, respectively. Although both bisphosphonates would be expected to decrease fracture risk based on this documented increase in aBMD, this has not yet been demonstrated in randomized trials. Thus, at present, bisphosphonates are not recommended for routine use in patients with MGUS.37
In summary, these data represent the first assessment of in vivo bone microstructure in subjects with MGUS or any other related plasma cell disorder, and they show that bone alterations are present even in the early stages of myelomagenesis. These changes are probably important for understanding the increased fracture risk seen in this population. Furthermore, our findings demonstrate that (1) the increased incidence of fractures previously found to occur in patients with MGUS may not be reflected in aBMD measurements by DXA that were relatively insensitive compared with HRpQCT for measuring bone loss in MGUS; (2) patients with MGUS have skeletal microstructural changes in both cortical and trabecular bone compartments; and (3) MGUS is associated with increased serum levels of both DKK1 and MIP-1α, both of which have been shown to correlate with MM bone disease. Because this is a recently studied cohort of MGUS patients, we cannot say whether alterations in cytokine levels (particularly of DKK1 and MIP-1α) are associated with an increased risk for either fracture or progression to MM, although we plan to follow this cohort longitudinally. Together with the well-documented increased fracture risk in subjects with MGUS, however, our findings have implications for the development of future clinical studies to more closely evaluate skeletal health in this population to limit further bone loss and to provide insight into the true continuum of monoclonal gammopathy-associated bone disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James M. Peterson for help with preparation of the figures and tables and Kelley A. Hoey for processing of the samples.
This work was supported by grants from the Mayo Hematologic Malignancies Program, a Mayo Career Development Award (to M.T.D.), K08-AR059138 (to M.T.D), AR027065 and AG004875 (to S.K.), CA 107476 (to S.V.R.), and UL1-RR24150 (Center for Translational Science Activities).
National Institutes of Health
Authorship
Contribution: A.C.N., N.C., S.K., S.V.R., and M.T.D. designed the study; A.C.N., N.C., M.F.H., L.K.M., and M.T.D. performed the work; S.J.A. and M.T.D. performed all statistical analyses; all authors were involved in interpretation of the results; A.C.N., S.V.R., and M.T.D. wrote the report; all authors read, provided comments, and approved the final version of the manuscript; and A.C.N., S.V.R., and M.T.D. had full access to the data in the study and take responsibility for accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew T. Drake, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: drake.matthew@mayo.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal