Abstract
Abstract 429
While standard clinical prognostic factors such as those included in the International Prognostic Score (IPS) predict outcome in cHL patients, predicting the outcome of patients might be further refined using biological factors. We therefore tested whether pretreatment serum cytokines, that measure the biological activity of the tumor as well as the host response, could provide additional prognostic information in cHL patients.
Newly diagnosed cHL patients were prospectively enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER). Serum cytokines were measured from pretreatment blood draws using a multiplex ELISA (Invitrogen, Camarillo, CA). Thirty cytokines, including pro-inflammatory, Th1 and Th2 associated cytokines, were analyzed using the Luminex-100 system Version 1.7 (Luminex, Austin, TX). Data analysis was performed using the MasterPlex QT 1.0 system (MiraiBio, Alameda, CA). The assay was also performed on research serum draws from 50 non-lymphoma epidemiology controls enrolled on a corresponding lymphoma etiology case-control study. The upper limit of normal for each cytokine was defined as the 95th percentile from the control distribution. Clinical data were abstracted using a standard protocol and all patients were followed systematically for event-free (progression, retreatment, or death due to any cause) and overall survival (EFS and OS, respectively). Cox proportional hazards models were used to evaluate the association between cytokines and outcome.
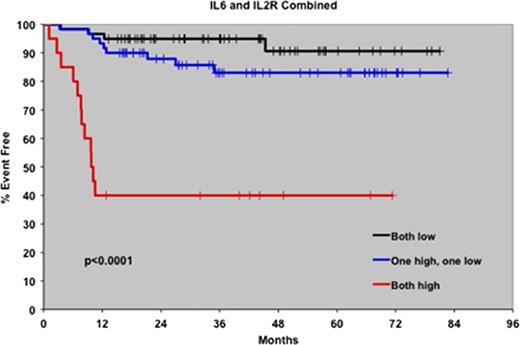
140 HL patients with cHL diagnosed between September 2002 and February 2008 with available pretreatment serum were included. The median age was 40 years (range 18–89) and 53% were male. Seventy-seven patients (55%) had limited stage disease and 63 (45%) had advanced disease. One hundred and twenty one patients (86%) were treated with ABVD chemotherapy, while the remainder received the Stanford V regimen (9%), BEACOPP (4%) or MOPP chemotherapy (2%). At a median follow-up of 47 months (range 0–87), 25 patients (18%) had an event and 16 patients had died (11%). The serum levels of 12 cytokines (EGF, FGFb, GCSF, HGF, IL-6, IL-8, IL-12, IL-2R, IP-10, MIG, TNFa and VEGF) were significantly higher in cHL patients than controls. When correlated with clinical factors, IL-2R, IP-10, MIG, IL-6 and HGF were associated with an increased sedimentation rate, B-symptoms, advanced stage and splenic involvement. Elevated IL-8 levels were associated with bulky disease. Elevated levels of HGF, IL-6, IL-8, IL-2R, IP-10 and MIG were all associated with a poorer EFS, however only IL-2R (p=0.002) and IL-6 (p<0.001) were independently prognostic for both EFS and OS. Furthermore, using IL-6 and IL-2R in a 2-cytokine model (see Figure), patients with elevation of both cytokines had a dramatically greater likelihood of early relapse and death than those with normal levels or elevations of only one of the cytokines (1-year EFS 40% compared to 97% and 93% respectively, p<0.0001; 2-year OS 49% compared to 98% and 95%, p<0.0001). While elevated IL-6 and IL-2R levels correlated with the IPS (p=0.002), this 2-cytokine model remained independently predictive of prognosis. In fact, when only good risk patients (IPS 0–3) were considered, the 2-cytokine model remained predictive of patients at risk for early relapse (1-year EFS 54% versus 95% and 97%, p<0.0001).
Elevated pretreatment serum cytokines are associated with an increased likelihood of disease relapse and an inferior survival in patients with cHL. The pretreatment cytokine profile, particularly serum levels of IL-6 and IL-2R, may identify cHL patients at high risk for early disease relapse who may benefit from novel therapeutic approaches.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal