Abstract
Abstract 3298
Thrombotic thrombocytopenic purpura (TTP) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and microvascular thrombosis. TTP is usually due to acquired, autoimmune deficiency of ADAMTS13, a metalloprotease that cleaves von Willebrand factor (VWF) and inhibits the growth of platelet thrombi. Most patients respond to treatment with plasma exchange, but inhibitory autoantibodies and persistent ADAMTS13 deficiency are associated with an increased risk of refractory or relapsing disease. Therefore, ADAMTS13 activity and inhibitor assays can be useful for diagnosis, prognosis, and monitoring the response to therapy. ADAMTS13 assays currently use the fluorogenic substrate FRETS-VWF73, which absorbs/emits at 340 nm/430 nm. These spectral properties make FRETS-VWF73 subject to interference from plasma proteins, hemoglobin and bilirubin. To avoid this problem, plasma is diluted at least 1:20, which reduces assay sensitivity to 5% of normal ADAMTS13 levels and prevents the detection of some clinically relevant inhibitors. We have addressed these limitations by developing FRETS-rVWF71, a recombinant fluorogenic substrate with chromophores that absorb/emit in the near infrared.
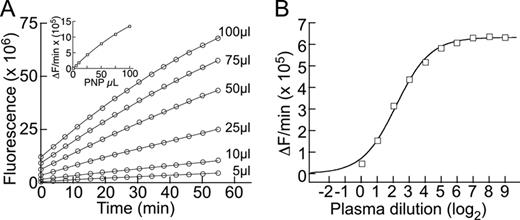
The substrate polypeptide corresponds to VWF residues Gln1599-Arg1668, with mutation N1610C to introduce a reactive thiol and K1617R to remove an amino group that competes with the N-terminus for chemical modification. This peptide was expressed in E. coli as a thioredoxin-(His6)-fusion protein, purified by Ni2+-NTA chromatography, and digested with TEV protease to remove the thioredoxin. After modification at Cys1610 with DyLight 633-maleimide (abs 638 nm, em 658 nm) and at the N-terminus with IRDye QC-1 N-hydroxysuccinimidyl ester (abs 500–800 nm), the substrate FRETS-rVWF71 was purified by C18-HPLC. Assays were performed with 1 μM FRETS-rVWF71 under physiological buffer conditions (50 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM CaCl2) to facilitate the assay of samples containing up to 95% plasma (Figure, panel A). Inhibitor assays were performed by preincubating equal volumes of pooled normal plasma and serially diluted patient plasma, followed by addition of an equal volume of buffer containing FRETS-rVWF71. Product generation was monitored in a fluorescence microplate reader with 635 nm excitation and 660 nm emission filters.
Serum and matched samples of plasma anticoagulated with citrate or heparin had equivalent ADAMTS13 activity that was stable indefinitely at −20°C. Bilirubin (>20 mg/dL) did not inhibit ADAMTS13 activity. As reported, hemoglobin was a weak inhibitor (EC50 approximately 1g/dL). Neither bilirubin nor hemoglobin interfered with product detection. Healthy donors (Li+-heparin plasma, n = 96) had a mean ADAMTS13 activity of 107.1 ± 18% (SD). Intra-assay and inter-assay coefficients of variation (CV) were <2%. No significant differences were observed by gender (male 104.9 ± 16%, n = 51; female 109.6 ± 16%, n = 45) or ethnicity (African American 102.7 ± 24%, n = 22; Caucasian 108.2 ± 17%, n = 48; Hispanic 108.4 ± 15%, n = 26). Results with FRETS-rVWF71 and FRETS-VWF73 correlated well with an inter-assay CV of 3.8%. For patients with idiopathic TTP, assays with FRETS-rVWF71 allowed accurate measurement of ADAMTS13 activity levels with a limit of detection of <0.5%. Inhibitor assays with FRETS-rVWF71 in minimally diluted plasma gave inhibitor titers approximately 3-fold higher than assays with FRETS-VWF73 at the 1:20 dilution required for that substrate. For example, a patient with an inhibitor titer of 4.8 U/ml in the FRETS-rVWF71 assay (Figure, Panel B) had an inhibitor titer of 1.8 U/ml in a FRETS-VWF73-based assay.
The use of chromophores that absorb/emit in the near infrared avoids interference from blood proteins, bilirubin or hemoglobin. The combination of brighter chromophores and compatibility with undiluted plasma makes ADAMTS13 activity assays with FRETS-rVWF71 substantially more sensitive than with FRETS-VWF73. Higher sensitivity allows discrimination between very low levels of activity that may influence the risk of relapse in congenital or acquired TTP. Optimized detection of ADAMTS13 inhibitors will facilitate the monitoring of antibody responses to therapy and should help to determine why some patients with acquired TTP relapse and others do not.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal