Abstract
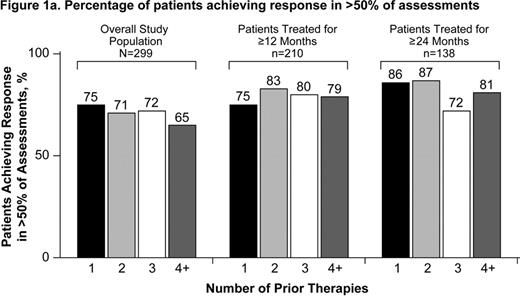
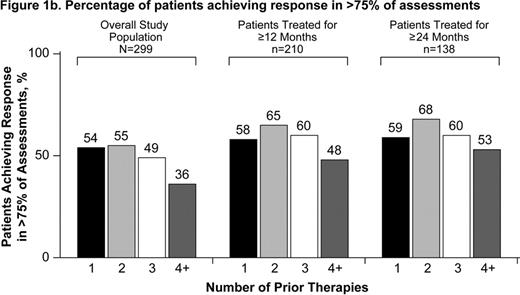
Patients with chronic immune thrombocytopenia (ITP) have an increased risk of bleeding, ranging from minor to life-threatening. The goal of treatment is to increase and maintain platelets in a safe range to prevent bleeding. Guidelines state that achieving platelet counts of 30,000/μL to 50,000/μL in patients without other risk factors avoids the most serious complications of ITP, namely intra-cerebral or gastrointestinal hemorrhage (George 1996; Provan 2010). Many patients are refractory or relapse after multiple treatments. Eltrombopag is an oral, nonpeptide thrombopoietin receptor agonist approved for the treatment of chronic ITP. In 6-week, and 6-month, placebo-controlled trials in patients with heavily pre-treated chronic ITP, eltrombopag increased platelets and reduced bleeding and the need for concomitant ITP therapy (Bussel 2007; Bussel 2009; Cheng 2011). Long-term treatment with eltrombopag is being evaluated in EXTEND, an extension study in chronic ITP patients who completed a previous eltrombopag study (Saleh 2010). Aims: To analyze in EXTEND the ability of eltrombopag to increase platelet counts to ≥50,000/μL in >50% and >75% of assessments and to determine whether the number of prior ITP therapies influences this ability. Methods: Patients in EXTEND received eltrombopag or placebo in 1 of the following prior studies of eltrombopag in chronic ITP: a 6-week phase 2 (TRA100773A; Bussel 2007) or phase 3 (TRA100773B; Bussel 2009) study, a 6-month phase 3 study (RAISE; Cheng 2011), or a phase 3 study of intermittent treatment (REPEAT; Psaila 2008). Dosing in EXTEND is individualized in order to maintain platelet counts ≥50,000/μL and <200,000/μL while minimizing the use of concomitant ITP medications. For the purpose of this analysis, response is defined as a platelet count ≥50,000/μL. Results: Among the 299 patients enrolled in EXTEND between June 2006 and February 2010, 67 (22%), 73 (24%), 47 (16%), and 112 (37%) patients had received 1, 2, 3, and ≥4 prior therapies (excluding eltrombopag). The most commonly used prior therapies were corticosteroids (81%), IVIg (45%), splenectomy (38%), and rituximab (23%). Of the 299 patients enrolled, 70% achieved response in >50% of study assessments and 46% achieved response in >75% of assessments. Among 210 patients treated ≥12 months, 79% achieved response in >50% of assessments and 56% in >75% of assessments. Among 138 patients treated for ≥24 months, 82% achieved response in >50% and 59% in >75% of assessments. Response in >50% and >75% of assessments by the number of prior therapies was similar between the groups (Figure 1). The proportion of patients who achieved a response in >50% of assessments was similar between splenectomized and non-splenectomized patients (65% and 73%, respectively). Conclusion: The majority of patients treated with eltrombopag for ≥12 months achieved a platelet count of ≥50,000/μL in >50% of study assessments. This response was observed even among patients previously treated with 4 or more ITP therapies, suggesting that eltrombopag may be a viable treatment option even for more refractory chronic ITP patients.
Bussel:Portola: Consultancy; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Cangene: Research Funding; Genzyme: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sysmex: Research Funding. Bailey:GlaxoSmithKline: Employment, Equity Ownership. Brainsky:GlaxoSmithKline: Employment, Equity Ownership.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal