Abstract
Abstract 2988
Plerixafor (AMD3100, Mozobil) with filgrastim (G-CSF, Neupogen) is approved for hematopoietic stem cell (HSC) mobilization in patients with non-Hodgkin Lymphoma and multiple myeloma (MM). Plerixafor pharmacokinetics (PK) and pharmacodynamics (PD) are well described, with linear, dose-dependent PK following subcutaneous (SC) administration, peak concentrations 30–60 mins post-injection and an elimination half-life (t1/2) of 5.3 hr. In pharmacodynamic studies of plerixafor in conjunction with filgrastim in healthy volunteers, peak CD34+ cell counts occur 10–14 hours following administration, however, data is limited in the 14–24 hr timeframe. Plerixafor labeling requires SC dosing approximately 11 hours prior to apheresis, which translates into dosing 10 :00 PM the night before apheresis, and 54% of MM patients collect ≥ 6 × 106 CD34+ cells/kg following a single apheresis procedure. The current regimen is inconvenient for patients and requires additional health care resources. Based on PK and PD, we hypothesized that plerixafor given at 3 :00 PM (17 hr prior to apheresis) would yield equivalent CD34+ HSC yield to 10 :00 PM dosing in MM patients.
In a Simon's two-stage design, we enrolled MM patients undergoing cytokine-only HSC mobilization. All subjects received filgrastim 7.5 mcg/kg SC BID for 4 days followed by plerixafor (0.24 mg/kg SC daily) for up to 4 days beginning at 3 :00 PM the day prior to the first day of a 24-liter apheresis procedure at 8 :00 AM. Target CD34+ HSC collection for stem cell transplant (SCT) was ≥ 10 × 106 CD34+ cells/kg. Blood samples for CD34+ fluorescence-activated cell sorting analysis were collected prior to the first plerixafor dose and at 1, 3, and 17 ± 1 hr, then daily prior to apheresis as needed.
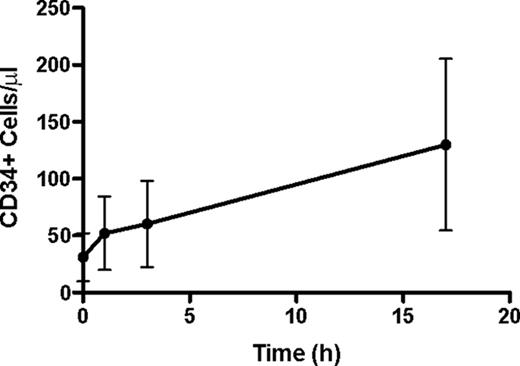
Thirty patients (17 female, median age 59 years [range 44–70]) were evaluable; 27 received 1 pre-mobilization regimen (RVD n=20, VTD n=2, VD n=2, V/PLD/D n=1, VT n=1, RD n=1) for a median of 4 (1–6) cycles. Three received 2 regimens [CMF × 6 (breast cancer), then VTD × 5; RD × 4, then RVD × 4; and V/PLD × 1 with maintenance R]. Six patients received prior radiation. Mean (± SD) CD34+ cell counts in peripheral blood pre-plerixafor and 1, 3, and 17 hr post-first dose increased through the dosing interval (Figure). Twenty-two (73%) patients collected target cell numbers in 1 day of apheresis, 7 (23%) in 2 days, and 1 (3%) in 3 days. Twenty-seven (90%) patients collected ≥ 6 × 106 CD34+ cells/kg in 1 day. Institutional data with filgrastim 7.5 mcg/kg SC BID for 4 days alone in MM in 22 subjects showed a day 1 collection of ≥ 10 × 106 CD34+ cells/kg in 18% of patients (Renfroe H, et al. Transfusion Feb 2011). Adverse events were generally mild and consistent with known side effects of the combination [gastrointestinal disorders (diarrhea, nausea) and injection site reactions]. To date, 16 (53%) patients have proceeded to autologous SCT with melphalan conditioning and all patients have engrafted, with median time to an ANC ≥ 500/mm3 of 13 (range 11–15) days and platelets ≥ 20, 000/mm3 of 16 (range 11–21) days.
This is the first prospective trial demonstrating the safety and efficacy of plerixafor given 17 hr prior to apheresis. Pharmacodynamic data showed the peripheral blood CD34+ cell population increased throughout the dosing interval, with a 4.6-fold increase over pre-plerixafor counts at 17 hr. Comparison with historical institutional controls and published data suggests this regimen yields at least equivalent, if not superior, collection rates with one apheresis procedure.
Flowers:Genentech/Roche (unpaid): Consultancy; Celgene: Consultancy; Millennium/Takeda: Research Funding; Wyeth: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal