Abstract
Abstract 1518
Treatment with TKIs has greatly improved the outcome of patients with Ph+ ALL. However, many patients treated with TKI-based therapy eventually have a relapse. The response to salvage therapy and long-term outcomes of these patients are unknown.
Describe the outcomes of patients with Ph+ ALL with resistance to or relapse after frontline TKI-based chemotherapy.
We analyzed the outcome of patients who were treated in clinical trials at our institution between February 2001 and July 2008 with TKI-based chemotherapy for newly diagnosed Ph+ ALL who had refractory or relapsed disease.
One hundred thirteen patients were treated with frontline hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone (HCVAD) plus imatinib (HCVAD+I; n=54) or HCVAD plus dasatinib (HCVAD+D; n=59). Of these, 35 (31%) experienced primary resistance (n=1) or relapse (n=34). The median age was 51 years [range (r): 20–85]; 12 patients (34%) were older than 60 years. Median follow-up was 21.1 mo (r: 4.2–56.7). Median white blood cell and platelet counts at diagnosis were 14.4 × 109/L (r: 1.2–292.9) and 48 × 109/L (r: 4–425), respectively. White blood cell count was >30 × 109/L in 13 patients (37%). Median peripheral and bone marrow blast percentages were 53% (r: 0–97%) and 80% (r: 1–98%), respectively. Twenty-two patients (63%) had received HCVAD+I and 13 (37%) HCVAD+D. Twenty-three patients (66%) had experienced first complete remission (CR1) with 1 cycle of induction. Median CR1 duration was 12 mo (r: 1.9–42). Four patients underwent allogeneic stem cell transplantation (ASCT) in CR1. ABL kinase domain mutations were investigated in 28 patients (80%) at relapse; 16 (57%) had mutations, including 5 (14%) with T315I (all had received HCVAD+D).
Upon relapse, 31 patients received first salvage therapy (S1), 24 with chemotherapy [HCVAD+D, n=8; HCVAD+I, n=3; HCVAD+nilotinib (N), n=1; HCVAD+asparaginase (Asp), n=1; methotrexate, vincristine, Asp, and dexamethasone (MOAD), n=2; others, n=9]; 6 with a TKI only (I, n=2; D, n=1; N, n=1; others, n=2); and 1 with ASCT. Three patients were unfit for treatment. Median cycles of S1 were 2 (r: 1–8). Thirteen patients (42%) had second complete remission (CR2) (HCVAD+D, n=6; HCVAD+I, n=2; HCVAD+N, n=1; HCVAD+Asp, n=1; others, n=3). Median time to CR2 was 1.5 mo (r: 0.7–8.8). Five patients underwent ASCT in CR2. Median CR2 duration was 7.3 mo (r: 1.4–36.2). Complete cytogenetic response was seen in 11 patients (35%); major molecular response (BCR-ABL/ABL ratio <0.05%) in 9 (29%); and complete molecular response in 7 (22%); and complete hematologic response in 15 (48%). Times to complete cytogenetic response and complete molecular response were 1.3 mo (r: 0.7–10.6) and 3 mo (r: 1.5–8.7), respectively. Seven patients had second relapse. Fifteen patients (7 relapse, 8 refractory) received second salvage therapy (S2) with systemic chemotherapy (MOAD, n=2; phase I/single-agent TKI, n=8; others, n=5); 1 patient had solitary central nervous system (CNS) relapse treated with intrathecal cytarabine and methotrexate. CR3 was obtained in 1 patient, the patient with sole CNS relapse.
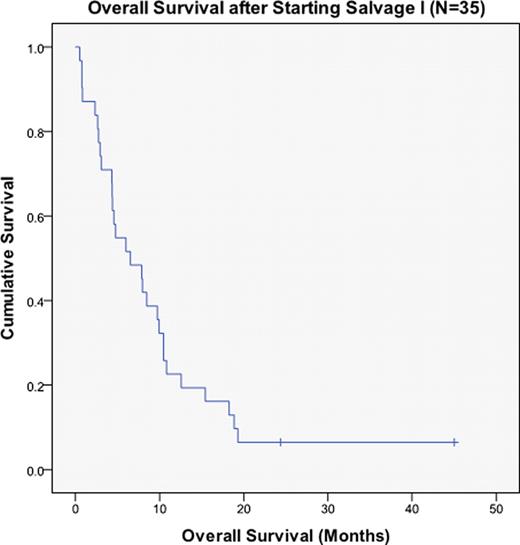
Median disease-free survival (DFS) and overall survival (OS) after S1 were 6.5 mo (r: 0.5–45) and 7.3 mo (r: 1.4–36.2), respectively. At last follow-up, 2 patients (6%) were alive and 33 had died, 11 (33%) of infectious complications, 5 (15%) of organ failure, 3 (9%) of bleeding complications, 2 (6%) of graft-versus-host disease complications, 2 (6%) of CNS relapse, and 10 (30%) of other or unknown causes. Median OS after S2 was 2.1 mo (r: 1.4–2.6). In univariate analysis, age >60 years was associated with worse OS after S1 [4.2 vs. 12.7 mo; 95% confidence interval (CI) 1.8 to 6.7 vs. 7.5 to 17.9 (P=0.006)]. Complete hematologic response was associated with improved OS after S1 [15.4 vs. 4.3 mo; 95% CI 9.1 to 21.8 vs. 2.5 to 6.0 (P<0.001)]. Major molecular response was associated with improved OS after S1 [18.1 vs. 5.7 mo; 95% CI 9.3 to 26.8 vs. 3.6 to 7.8 (P=0.003)]. Choice of prior TKI (HCVAD+I vs. HCVAD+D) did not significantly influence CR and OS after relapse.
Patients with refractory or relapsed Ph+ ALL after TKI-based therapy have poor outcome, particularly those who are older or have persistent BCR/ABL transcripts. New agents are needed to improve the outcome in this population.
Kantarjian:BMS: Research Funding. Ravandi:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria. Cortes:Chemgenex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal