Abstract
Abstract 1029
Red blood cells (RBCs) continuously transport large amount of oxygen over their life time and require precise mechanism to protect themselves from oxidative stress. RBCs cannot respond to rapid oxygen changes by synthesizing enzymes and other proteins. Chronic hypoxia enhances erythropoiesis with ensuing polycythemia. With return to normoxia, red cell mass is reduced by neocytolysis, characterized by selective hemolysis of the young RBCs. Neocytolysis was described in astronauts, in those descending from high-altitude, and in newborn babies leaving hypoxic environment of uterus. While it has been suggested that neocytolysis is caused by very low erythropoietin levels, its molecular basis remains obscure. However, we argue against this postulate since RBCs lack pathway for erythropoietin signaling. We hypothesize that rapid changes of hypoxia-regulated hypoxia-inducible transcription factors (HIFs) regulated genes (other than erythropoietin) may be responsible, one such a gene (BNIP3L/NIX) regulates mitochondrial autophagy. Upon normoxic return young RBCs generated in hypoxia cannot cope because of decreased levels of oxidant protecting defenses regulated by HIF-dependent FOXO3a transcription factor.
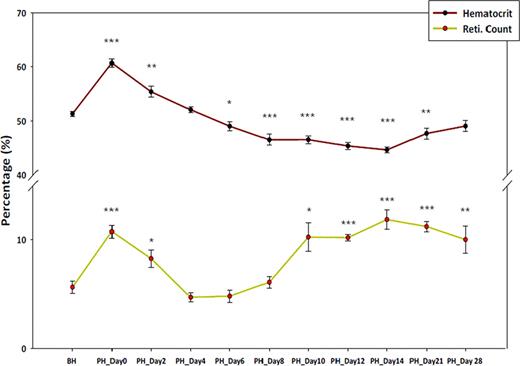
Hematocrit level and reticulocyte count before hypoxia and post hypoxia
Legend: BH: Before Hypoxia, PH: Post Hypoxia *; P value ≤ 0.05, **; P value ≤0.01, ***; P value ≤0.001, P value calculated using student T test comparing values before hypoxia.
Epo levels increased 1.6 fold during hypoxia and then reduced up to undetectable level at PH day 4. Then Epo gradually increased to ∼3 fold during PH day 10∼28. During PH day 10∼21, the mice became anemic, even though Epo and reticulocytes remained high. These results suggest that neocytolysis occurs after several days of exposure to normoxia and it is not caused by Epo mechanism.
To investigate the molecular basis of the observed neocytolysis in this mouse model, we measured the mitochondrial content in reticulocytes, anti-oxidative enzyme activities (glutathione peroxidase and reductase, catalase, and superoxide dismutase) that scavenge reactive oxygen species in RBCs, possibly coexistent with up-regulation of mitochondrial content upon normoxic return. Reticulocytes at returning normoxia generated more mitochondria several days after normoxic return, In contrast catalase activity was reduced during hypoxia and at PH day 4, but by PH day 10 its activity increased, and the catalase activity decrease coincided with a decrease in hematocrit.
To investigate whether hypoxia drives neocytolysis under our conditions, we tested 2 known HIF target genes, Bnip3L (also called Nix), a pro-apoptotic protein that causes mitochondrial autophagy. Bnip3L mRNA was induced 9x during hypoxia and reduced 2x at PH day 6, compared to before hypoxia. We also analyzed Foxo3a, a transcription factor, in sorted reticulocytes (CD71+/TER119+/Mitochondria+) which regulates cellular stress responses such as catalase and superoxide dismutase (SOD). Foxo3a was slightly increased during hypoxia and reduced 4x at PH day 6 from levels before hypoxia.
In conclusion, we developed mouse model to study neocytolysis. Our data suggest that increased mitochondria retained by Bnip3L repression leads to an accumulation of reactive oxygen species (whether in reticulocytes, platelets or leukocytes), and that young RBCs formed in hypoxia with insufficient antioxidant enzyme activity cannot survive because of excessive reactive oxygen species, with ensuing hemolysis. Studies of the role of other blood cells, as well as human studies of mountain climbers upon their return to sea level, are in progress.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal