Abstract
Recent studies indicate that the plasma contact system plays an important role in thrombosis, despite being dispensable for hemostasis. For example, mice deficient in coagulation factor XII (fXII) are protected from arterial thrombosis and cerebral ischemia-reperfusion injury. We demonstrate that selective reduction of prekallikrein (PKK), another member of the contact system, using antisense oligonucleotide (ASO) technology results in an antithrombotic phenotype in mice. The effects of PKK deficiency were compared with those of fXII deficiency produced by specific ASO-mediated reduction of fXII. Mice with reduced PKK had ∼ 3-fold higher plasma levels of fXII, and reduced levels of fXIIa-serpin complexes, consistent with fXII being a substrate for activated PKK in vivo. PKK or fXII deficiency reduced thrombus formation in both arterial and venous thrombosis models, without an apparent effect on hemostasis. The amount of reduction of PKK and fXII required to produce an antithrombotic effect differed between venous and arterial models, suggesting that these factors may regulate thrombus formation by distinct mechanisms. Our results support the concept that fXII and PKK play important and perhaps nonredundant roles in pathogenic thrombus propagation, and highlight a novel, specific and safe pharmaceutical approach to target these contact system proteases.
Introduction
The blood coagulation system responds to vascular injury with local production of a clot formed of fibrin mesh and activated platelets. While this process is essential for hemostasis, dysregulated coagulation can lead to blood vessel occlusion (thrombosis), precipitating life-threatening events such as myocardial infarction, stroke and venous thromboembolism. In the classic view of blood coagulation, thrombin generation and fibrin formation can be initiated by 2 distinct mechanisms referred to as the extrinsic and intrinsic pathways.1,2 The extrinsic pathway involves binding of plasma factor VIIa (fVIIa) to extravascular tissue factor (TF) at a site of vessel injury.3 The first step in the intrinsic pathway requires the surface-dependent activation of plasma factor XII (fXII) to fXIIa in a process called contact activation.4,5 Contact activation involves 2 other proteins, prekallikrein (PKK) and high molecular weight kininogen (HK). HK serves as a docking molecule for PKK on the contact surface. PKK is cleaved by fXIIa to form the protease α-kallikrein, which in turn cleaves fXII to generate additional fXIIa. Collectively, fXII, PKK and HK comprise the plasma contact system. FXIIa generated by contact activation can activate factor XI (fXI) to fXIa, triggering a series of proteolytic cleavage events that culminates in thrombin generation and fibrin clot formation.
While the contact system can clearly trigger coagulation in vitro, it is not required for hemostasis. Humans and other animals deficient in a contact activation protein are largely asymptomatic.4,6-8 However, the contact system may play an important role in thrombotic disease, as pharmacologic inhibition of fXIIa or ablation of the fXII or HK genes can protect mice from experimentally induced thrombosis in a variety of models.9-13 Interestingly, fXII deficiency confers somewhat greater protection from thrombosis than fXI deficiency in some models,11 implying that fXII may contribute to thrombus formation through additional pathways distinct from fXI-dependent intrinsic pathway activation. These data have generated interest in developing strategies to therapeutically inhibit fXIIa and contact activation to treat or prevent thromboembolic disorders.
Based on the clear importance of fXII to thrombus formation in mice,11,13 and the critical role of PKK in optimal activation of fXII during contact activation in human plasma,14,15 we hypothesized that PKK deficiency may have an antithrombotic effect in vivo. Here we show that antisense oligonucleotide (ASO) mediated selective knockdown of PKK or fXII in mice can inhibit thrombus formation in venous and arterial thrombosis models, without affecting hemostasis. We also provide evidence that PKK deficiency reduces fXII activation in vivo, consistent with PKK contributing to thrombus formation through activation of fXII. This is the first report describing antithrombotic affects of PKK depletion in vivo, as well the first demonstration of kallikrein-dependent fXII activation in vivo.
Methods
Oligonucleotides
All oligonucleotides for PKK and fXII mRNA knockdown in vivo were 20 nucleotides in length and chemically modified with phosphorothioate in the backbone and 2′-O-methoxyethyl (MOE) on the wings with a central deoxy gap (“5-10-5” design). Oligonucleotides were synthesized and purified as previously described.16 To identify potent PKK and fXII ASOs, ASOs were designed and tested in primary mouse hepatocytes for their ability to suppress mRNA levels of the respective target. From these experiments optimized ASOs were selected for evaluation in mice.
Animals and oligonucleotide dosing
Eight-week-old male BALB/c, C57BL/6 and Swiss Webster mice (Charles River Laboratories) were treated according to the indicated schedules. The animals were housed in micro-isolator cages on a constant 12 hour light-dark cycle with controlled temperature and humidity and were given access to food and water ad libitum. All animal husbandry and procedures performed at Isis were approved by the Institutional Animal Care and Use Committee.
Measurement of hepatic PKK and fXII mRNA
Mouse liver was homogenized in RLT buffer (QIAGEN). Total mRNA was prepared using PureLink Pro 96 RNA total RNA isolation kit (Invitrogen, Life Technologies). The sequences of primers and probe for RT-PCR measurement were as follows: fXII: GGGCCACCACGCATTTT (forward), TGTCGCCACTCCAGACGAA (reverse), CCGGAACCCAGATAATGACACACGTCC (probe), PKK: ACAAGTGCATTTTACAGACCAGAGTAC (forward), GGTTGTCCGCTGACTTTATGCT (reverse), AAGCACAGTGCAAGCGGAACACCC (probe). The amount of specific mRNA was analyzed using a StepOne Real-Time PCR System (Applied Biosystems, Life Technologies).
FXII and PKK immunoblot analysis
Plasma anticoagulated with sodium citrate was fractionated on 4%-12% gradient SDS-polyacrylamide gels (Invitrogen Life Technologies) followed by immunoblotting with human fXII (Accurate Chemicals), mouse PKK (R&D Systems) or mouse α2-antiplasmin (A2AP; R&D Systems) antibodies. Blots were incubated with secondary fluorophore-labeled antibodies (LI-COR) and imaged on Odyssey Imager (LI-COR). PKK and fXII relative plasma protein levels were determined by densitometry analysis (ImageJ 1.43).
Plasma fXIIa-antithrombin complex ELISA
fXIIa-antithrombin plasma complex levels were measured by sandwich ELISA. Briefly, assay plates were coated with anti–human fXII antibody (Accurate Chemicals) and blocked with 2% BSA before incubation with diluted mouse platelet poor plasma. After extensive washing, fXIIa-antithrombin complex was detected by incubation with HRP-conjugated antithrombin antibody (Enzygnost human TAT Micro ELISA kit, Siemens). Relative levels of fXIIa-antithrombin complex were calculated using serial dilutions of control mouse plasma as a standard.
Ferric chloride-induced inferior vena cava thrombosis
Antithrombotic activity was studied using a well-established ferric chloride (FeCl3) induced inferior vena cava (IVC) thrombosis model.17,18 Total mRNA was purified from vena cava tissue samples and analyzed by RT-PCR for Platelet Factor 4 (PF4) mRNA levels. PF4 mRNA levels were used to determine the effect of treatment on platelet deposition as a measure of thrombus formation.19 PF4 mRNA levels in the IVC tissue exposed to FeCl3 was normalized to nonexposed vena cava tissue.
Stenosis-induced IVC thrombosis
The St Tomas model which uses a combination of reduced blood flow and endothelial damage, was used to study stenosis-induced IVC thrombosis.18 Briefly, the IVC of male BALB/c mice anaesthetized with 2.5% inhalant isoflurane was exposed via a midline abdominal incision below the left renal vein, and separated from the abdominal aorta. A 6-0 silk tie (Ethicon) was placed behind the vessel and a metal 4-0 suture (Ethicon) was placed longitudinally over the IVC and tied over the top. Finally the metal 4-0 suture was removed. Next, 2 neurovascular surgical clips (Braun Medical) were applied at 2 separate positions below the ligation for 20 seconds each. The bowel was placed back into the abdominal cavity and the abdomen was closed. Twenty-four hours later IVC thrombi were collected and fixed in 10% formalin for 24 hours, photographed, and weighed.
FeCl3-induced mesenteric arterial thrombosis
Swiss Webster mice were anesthetized with ketamine/xylazine (75 mg/kg and 25 mg/kg) by subcutaneous injection. Mice were given subcutaneous injections of Rhodamine-6-G (5 mg/kg) and intravenous injection of Alexa-647 labeled anti-fibrinogen antibody (1 mg/kg). The abdomen was opened though a middle incision and the visceral mesentery was spread on a glass cover slip. Mesenteric arterioles (70-120 um in diameter) were injured by application of cotton threads soaked in 6% FeCl3 for 3 minutes. After injury platelet and fibrin accumulation was recorded for 60 minutes with appropriate filters on an Olympus FluoView 1000 confocal laser scanning microscope.
Tail vein bleeding assay
A mouse tail bleeding assay was performed as described.17
Statistical analysis
Statistical comparisons were made by ANOVA. Student t test was used for comparison of single pairs. Correction for multiple comparisons between individual groups was made using Fisher's LSD posthoc test. Data are expressed as mean ± SEM. P values < .05 were considered significant. Data were analyzed using SPSS software package for Windows Version 14.0 (SPSS Inc). Graphics were constructed using GraphPad Prism Version 5 for Windows (GraphPad Software Inc).
Results
Systemic delivery of ASO results in suppression of fXII and PKK levels in mice
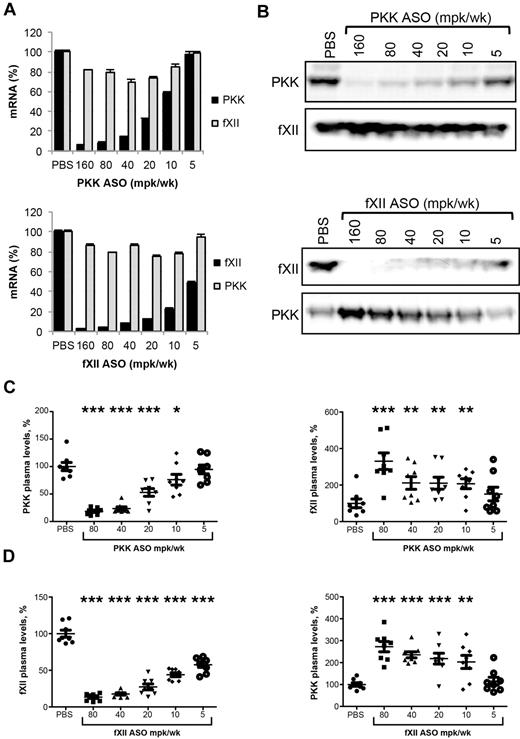
The role of the intrinsic pathway and contact system in thrombosis in mice has been studied using several approaches, including knockout strategies and specific inhibitors.9-11,13,20,21 It is clear that fXII is involved in thrombus formation in these models. In vitro, PKK is required for optimal fXII activation, and we postulated that PKK may have a pro-thrombotic function in vivo. As PKK knockout mice are not currently available, antisense technology was used to explore the importance of PKK in thrombosis. The effect of PKK and fXII ASO treatment on expression of PKK and fXII mRNA in liver, and levels of PKK and fXII protein in plasma, are shown in Figure 1. Systemic delivery (subcutaneous injections twice per week for 3 weeks) of ASO resulted in a dose-dependent reduction of target mRNA levels (92% and 96% reduction for PKK and fXII expression, respectively) at the highest tested ASO dose (Figure 1A). ASO treatment produced dose-dependent reduction of the target plasma protein, with maximum reductions of 83% and 85% for PKK and fXII, respectively (Figure 1B-D). Both PKK and fXII ASOs were highly specific for their targets, as demonstrated by unchanged liver mRNA expression of several nontargeted coagulation factor mRNAs. Specifically, fXII ASO did not change mRNA expression of coagulation factors II (prothrombin), V, VII, VIII, IX, X, XI, as well as TAFI and PKK. PKK ASOs did not change mRNA expression of coagulation factors II (prothrombin), VII, XI and XII (Figure 1A and data not shown). Both PKK and fXII mRNA levels correlated with plasma protein levels of the respective factors (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It is important to note that the potencies of the ASOs were slightly different, with ED50 of 12 mg/kg/wk for PKK ASO and 5 mg/kg/wk for fXII ASO for liver reduction in mRNA levels, and 18 mg/kg/wk and 7 mg/kg/wk for plasma protein expression, respectively.
Effect of PKK and fXII ASO treatment on PKK and fXII hepatic mRNA and plasma protein levels. Male BALB/c mice were treated subcutaneously with PKK or fXII ASO for 3 weeks at indicated doses (n = 8 per treatment group). Two days after final dosing mice were collected for quantification of PKK and fXII hepatic mRNA levels (A) and PKK and fXII immunoblot analysis on pooled (B) and individual (C-D) samples. PKK and fXII relative plasma protein levels were quantified by densitometry. *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Effect of PKK and fXII ASO treatment on PKK and fXII hepatic mRNA and plasma protein levels. Male BALB/c mice were treated subcutaneously with PKK or fXII ASO for 3 weeks at indicated doses (n = 8 per treatment group). Two days after final dosing mice were collected for quantification of PKK and fXII hepatic mRNA levels (A) and PKK and fXII immunoblot analysis on pooled (B) and individual (C-D) samples. PKK and fXII relative plasma protein levels were quantified by densitometry. *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
FXII or PKK ASO-mediated knockdown stabilizes PKK or fXII proteins, respectively
Plasma protein levels of PKK on fXII depletion, and fXII on PKK depletion, were significantly increased over basal levels (Figure 1B-D). In both cases the effects correlated well with ASO dose and degree of target knockdown. While the increase in PKK protein followed fXII ASO-mediated knockdown in an almost linear fashion, fXII protein levels showed a bi-phasic response with PKK depletion, with a sudden increase of fXII protein levels with PKK protein reduction < 20% (supplemental Figure 1B-D). Since liver mRNA expression of PKK in animals receiving fXII ASOs, and fXII mRNA in mice receiving PKK ASOs, were unchanged from baseline (Figure 1A), it is likely the changes in plasma fXII and PKK levels were due mainly to a reduction in activation-dependent consumption.
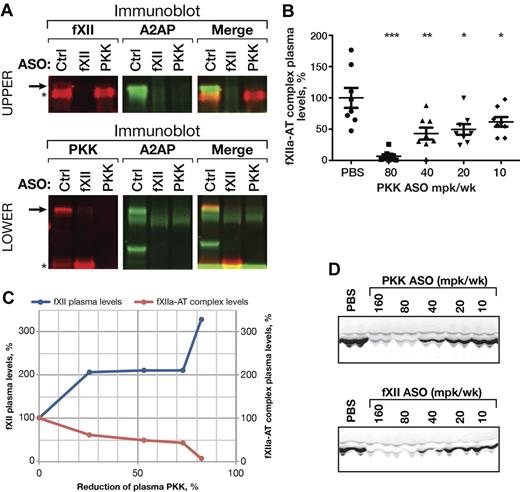
To determine whether fXII activation is affected by reduction of PKK in vivo, we measured fXIIa in complex with serpins in the plasma of PKK ASO treated mice (Figure 2). With PKK ASO-mediated knockdown we detected a significant decrease in levels of fXIIa-α2-antiplasmin (Figure 2A top row) and fXIIa-antithrombin complexes (Figure 2B), suggesting basal activation of fXII was reduced. Interestingly, when > 80% PKK knockdown was achieved, we saw almost complete inhibition of fXII activation (93%). This correlated well with the increase in plasma fXII protein level (Figure 2C). Consistent with its role in PKK activation, ASO-mediated knockdown of fXII reduced plasma α-kallikrein generation, as reflected by a reduction in complexes with α2-antiplasmin (Figure 2A bottom row) and lower levels of kallikrein heavy chain in plasma (Figure 2D).
Effect of PKK and fXII ASOs on PKK and fXII activation in vivo. Pooled plasma samples from PKK, fXII or Control (Ctrl) ASO treated male C57BL/6 mice (3 weeks at 80 mg/kg/wk dose, n = 8 per treatment group) were subjected to 2-color immunoblot analysis of PKK (A, bottom row, shown in red) and fXII (A, top row, shown in red) complexes with α2-antiplasmin (A2AP, shown in green). PKK (A, bottom row) and fXII (A, top row) proteins are marked by asterisks. Complexes of PKK (A, bottom row) or fXII (A, top row) with A2AP are stained by both antibodies and are marked by arrows. Pooled plasma samples from PKK or fXII ASO treated male C57BL/6 mice (3 weeks at indicated dose, n = 8 per treatment group) were subjected to immunoblot analysis of kallikrein heavy chain levels (D). fXIIa complexes with antithrombin (AT) were analyzed in plasma samples from PKK and fXII ASO treated male C57BL/6 mice (3 weeks at indicated dose, n = 8 per treatment group) by ELISA (B). Mean values of fXIIa-AT complex levels and fXII plasma protein levels were plotted against PKK protein inhibition for the direct comparison (C). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Effect of PKK and fXII ASOs on PKK and fXII activation in vivo. Pooled plasma samples from PKK, fXII or Control (Ctrl) ASO treated male C57BL/6 mice (3 weeks at 80 mg/kg/wk dose, n = 8 per treatment group) were subjected to 2-color immunoblot analysis of PKK (A, bottom row, shown in red) and fXII (A, top row, shown in red) complexes with α2-antiplasmin (A2AP, shown in green). PKK (A, bottom row) and fXII (A, top row) proteins are marked by asterisks. Complexes of PKK (A, bottom row) or fXII (A, top row) with A2AP are stained by both antibodies and are marked by arrows. Pooled plasma samples from PKK or fXII ASO treated male C57BL/6 mice (3 weeks at indicated dose, n = 8 per treatment group) were subjected to immunoblot analysis of kallikrein heavy chain levels (D). fXIIa complexes with antithrombin (AT) were analyzed in plasma samples from PKK and fXII ASO treated male C57BL/6 mice (3 weeks at indicated dose, n = 8 per treatment group) by ELISA (B). Mean values of fXIIa-AT complex levels and fXII plasma protein levels were plotted against PKK protein inhibition for the direct comparison (C). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
The effects of fXII and PKK ASO treatment on FeCl3-induced IVC thrombosis in mice
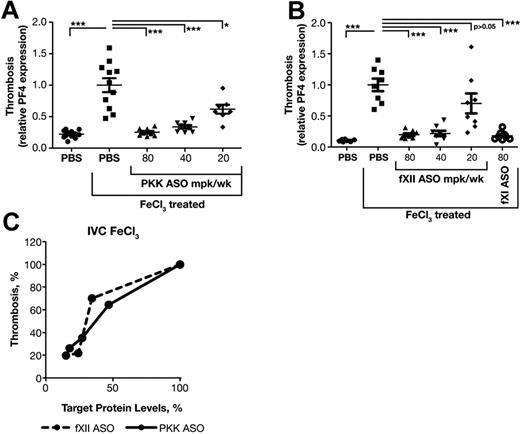
fXII contributes to thrombosis in several models; however, a role for PKK is not established. In addition, prior studies used homozygous fXII null (knockout) mice, thus the level of fXII required to support thrombosis has not been determined. Furthermore, we wanted to compare the activity of ASOs targeting the contact system to fXI inhibition, which we previously showed possesses remarkable anti-thrombotic activity in vivo.18 The antithrombotic activity of fXII and PKK ASOs were compared in a widely used FeCl3-induced inferior vena cava (IVC) thrombosis model. Application of FeCl3 to the exterior of blood vessels causes endothelial damage and exposure of subendothelial material that activates platelets and triggers the intrinsic and extrinsic pathways of coagulation, leading to thrombus formation. In this model, we assessed platelet accumulation into thrombi by measuring platelet factor 4 (PF4) mRNA levels at the injury site. PF4 mRNA level is proportional to the amount of platelets trapped in the clot.18,19 As FeCl3-induced clots are platelet rich, PF4 mRNA gives a good estimation of relative clot sizes.
Mice were treated for 3 weeks with PKK or fXII ASO at indicated doses. Two days after the final dose, thrombosis was induced with 10% FeCl3 and clot size determined. FXI ASO was used as a positive control.18 PKK and fXII ASO therapies produced significant reductions in thrombus formation starting at doses of 40 mg/kg/wk, which produces ∼ 75% reduction of target protein (Figures 1C-D,3A-B). Interestingly, fXII ASO produced a steep dose response with a moderate antithrombotic effect (30% reduction in clot size) at 20 mg/kg/wk dose, which was associated with fXII protein levels > 35% of normal (Figures 1D,3B-C). PKK ASO produced a more linear dose-response relationship between antithrombotic activity and reduction in PKK levels (Figure 1C, 3A-C), where greater activity was observed with reduction in PKK levels of ∼ 50%.
Effect of PKK and fXII ASOs on FeCl3-induced inferior vena cava thrombosis. Male BALB/c mice were treated with either PKK (A) or fXII (B) ASO at indicated doses administered subcutaneously for 3 weeks (n = 8 per treatment group). FXI ASO treatment was used as a reference antithrombotic drug (B). Thrombosis was induced by 3 minute exposure of the inferior vena cava to a 10% FeCl3 solution and assessed by RT-PCR measurement of platelet factor 4 (PF4) mRNA levels at the site of injury. Comparison of effects of PKK and fXII protein inhibition in FeCl3-induced vena cava (IVC) thrombosis (C). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Effect of PKK and fXII ASOs on FeCl3-induced inferior vena cava thrombosis. Male BALB/c mice were treated with either PKK (A) or fXII (B) ASO at indicated doses administered subcutaneously for 3 weeks (n = 8 per treatment group). FXI ASO treatment was used as a reference antithrombotic drug (B). Thrombosis was induced by 3 minute exposure of the inferior vena cava to a 10% FeCl3 solution and assessed by RT-PCR measurement of platelet factor 4 (PF4) mRNA levels at the site of injury. Comparison of effects of PKK and fXII protein inhibition in FeCl3-induced vena cava (IVC) thrombosis (C). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Stenosis-induced IVC thrombosis
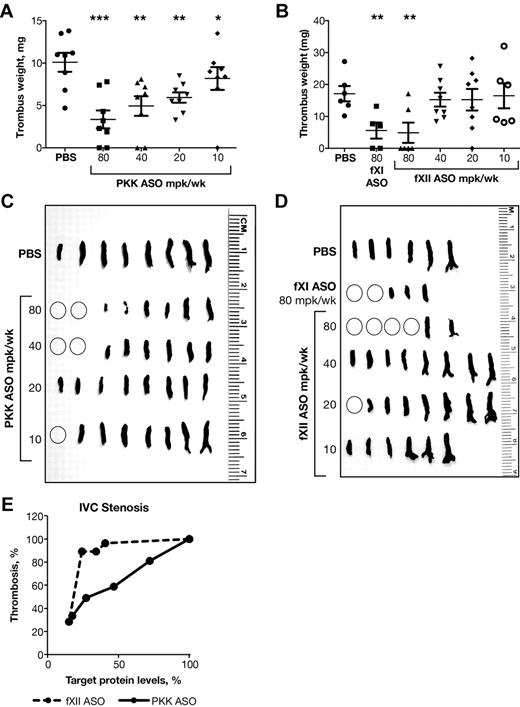
The antithrombotic activity associated with fXII and PKK ASO-mediated knockdown was evaluated in a stenosis model of thrombosis (Figure 4). Here, partial IVC ligation is used to reduce blood flow and produce minor endothelial damage, leading to thrombosis. We have previously shown that inhibition of the intrinsic pathway (fXI ASO), but not the extrinsic pathway (fVII ASO) produces an antithrombotic effect in this model, suggesting that contact activation drives thrombosis (D. Gao, unpublished observations, September 2009). PKK and fXII ASO treatments resulted in dramatic reductions in thrombus weight (Figure 4A-B) and size (Figure 4C-D), with results comparable to those previously reported with fXI ASO.18 As in the FeCl3-induced IVC thrombosis model, we observed a steep dose response with fXII ASO, with reductions of fXII levels > 75% required to produce antithrombotic activity (Figures 1D,4B,D,E), while PKK inhibition generated a more linear dose-response effect on thrombus weight, with significant (40%) inhibition in thrombus growth observed with reductions of ∼ 50% in plasma PKK levels (Figures 1C,4A,C,E). Interestingly, in the stenosis model, fXI ASO treatment resulted in antithrombotic activity similar to that of fXII ASO, suggesting that fXI activation could be the primary mechanism by which fXII stimulates thrombosis.18
Effect of PKK and fXII ASOs on stenosis-induced inferior vena cava thrombosis. Male BALB/c mice were treated subcutaneously with PKK or fXII ASO for 3 weeks at indicated doses (n = 6-8 per treatment group). Vena cava thrombosis was induced by combination of reduced blood flow with minor endothelial damage. Twenty-four hours after thrombosis induction thrombi were collected, fixed in formalin, weighed (A-B) and photographed (C-D). Thrombus weight relative to PBS control mice was calculated (A-B). FXI ASO was used as a reference antithrombotic drug (B,D). Comparison of effects of PKK and fXII protein inhibition in stenosis-induced vena cava (IVC) thrombosis (E). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Effect of PKK and fXII ASOs on stenosis-induced inferior vena cava thrombosis. Male BALB/c mice were treated subcutaneously with PKK or fXII ASO for 3 weeks at indicated doses (n = 6-8 per treatment group). Vena cava thrombosis was induced by combination of reduced blood flow with minor endothelial damage. Twenty-four hours after thrombosis induction thrombi were collected, fixed in formalin, weighed (A-B) and photographed (C-D). Thrombus weight relative to PBS control mice was calculated (A-B). FXI ASO was used as a reference antithrombotic drug (B,D). Comparison of effects of PKK and fXII protein inhibition in stenosis-induced vena cava (IVC) thrombosis (E). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
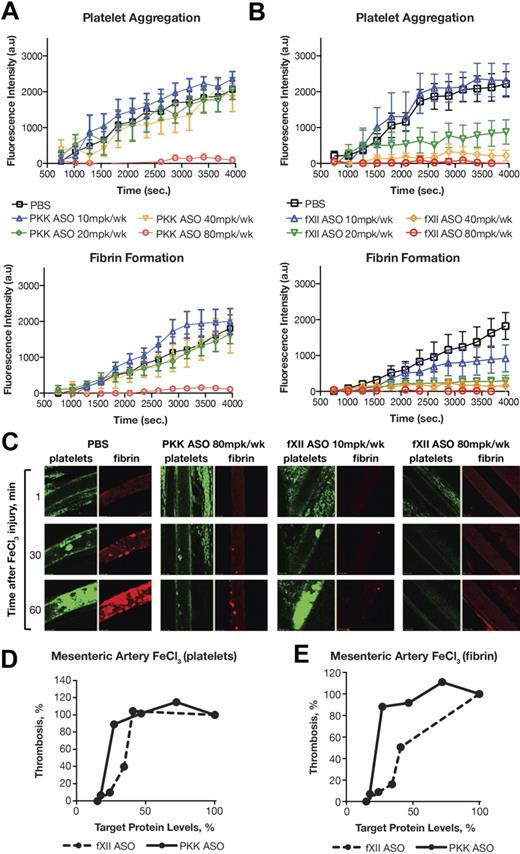
FeCl3-induced mesenteric arterial thrombosis
The venous and arterial systems are fundamentally different with respect to physiologic parameters such as shear stress, as well as mechanisms that produce thrombosis. We assessed the effects of PKK and fXII ASO-mediated knockdown in a FeCl3-induced mesenteric arterial thrombosis model using confocal intravital microscopy. This technique allowed us to simultaneously detect platelet aggregation and fibrin generation at the injury site (Figure 5). Mice treated with vehicle (PBS) typically developed arterial occlusion ∼ 40 minutes after FeCl3 application (determined by platelet accumulation). Surprisingly, fibrin formation did not show saturation kinetics in this time frame, but constantly accumulated at the site of injury in an almost linear fashion (Figure 5A-B black line).
Antithrombotic effect of PKK and fXII ASO treatments in mouse model of FeCl3-induced mesenteric artery thrombosis. Male Swiss Webster mice were treated with either PKK (A) or fXII (B) ASO at indicated doses administered subcutaneously for 3 weeks (n = 6-8 per treatment group). Mesenteric artery thrombosis was induced by a 3 minute exposure of the mesenteric vein to a 6% FeCl3 solution. Platelets were labeled through subcutaneous injection of Rhodamine-6G and fibrin was labeled through intravenous injection of Alexa 647–conjugated anti-fibrinogen antibody. Continuous recording of venous thrombus formation by confocal intravital microscopy (confocal-IVM) was done for a total period of 70 minutes. Normalized integral intensity for each of 2 channels (fibrin and platelet detection) was plotted against time. Intensities for control group are shown in black, 10 mg/kg/wk dose of PKK and fXII ASOs are shown in blue, 20 mg/kg/wk dose in green, 40 mg/kg/wk dose in yellow, and 80 mg/kg/wk dose in red. Platelet and fibrin content of formed thrombi in the vena cava in different treatment groups at different time points is shown in panel C. Comparison of effects of PKK and fXII protein inhibition in FeCl3-induced platelet aggregation (D) and fibrin accumulation (E) in mesenteric artery. *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Antithrombotic effect of PKK and fXII ASO treatments in mouse model of FeCl3-induced mesenteric artery thrombosis. Male Swiss Webster mice were treated with either PKK (A) or fXII (B) ASO at indicated doses administered subcutaneously for 3 weeks (n = 6-8 per treatment group). Mesenteric artery thrombosis was induced by a 3 minute exposure of the mesenteric vein to a 6% FeCl3 solution. Platelets were labeled through subcutaneous injection of Rhodamine-6G and fibrin was labeled through intravenous injection of Alexa 647–conjugated anti-fibrinogen antibody. Continuous recording of venous thrombus formation by confocal intravital microscopy (confocal-IVM) was done for a total period of 70 minutes. Normalized integral intensity for each of 2 channels (fibrin and platelet detection) was plotted against time. Intensities for control group are shown in black, 10 mg/kg/wk dose of PKK and fXII ASOs are shown in blue, 20 mg/kg/wk dose in green, 40 mg/kg/wk dose in yellow, and 80 mg/kg/wk dose in red. Platelet and fibrin content of formed thrombi in the vena cava in different treatment groups at different time points is shown in panel C. Comparison of effects of PKK and fXII protein inhibition in FeCl3-induced platelet aggregation (D) and fibrin accumulation (E) in mesenteric artery. *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Both PKK and fXII ASO treatments at the highest dose (80 mg/kg/wk) prevented fibrin formation, platelet aggregation and arterial occlusion over the time course of the study (70 minutes after FeCl3 injury). In contrast to the venous thrombosis models, at lower doses of ASO, smaller reductions in PKK levels had a significantly smaller antithrombotic effect compared with similar reductions in fXII levels (Figure 5D-E). Indeed, all antithrombotic activity was lost for the PKK ASO at lower doses (40 mg/kg/wk or less; Figure 5A), while fXII ASO still effectively inhibited both fibrin generation and platelet aggregation (Figure 5B). Furthermore, fXII ASO had a more prominent inhibitory effect on fibrin generation than platelet activation (Figure 5C-E).
In a video recording of arterial thrombus formation, we were able to see that small thrombi formed on the surface of the injured vessel after PKK or fXII ASO-mediated knockdown, but the development of thrombi was markedly delayed and their growth was severely compromised. Continuous shedding of platelet aggregates from the site of the growing thrombi was detected, which prevented vessel occlusion. We have observed similar inhibition of thrombus growth in animals treated with fXI ASO,18 suggesting that a significant component of fXII or PKK-initiated thrombosis involves fXI activation and subsequent thrombin generation. The similar sensitivity of fibrin formation to fXI or fXII ASO-mediated knockdown (supplemental Figure 2G) supports the idea that activation of fXI is the primary mechanism by which fXII drives thrombus formation.
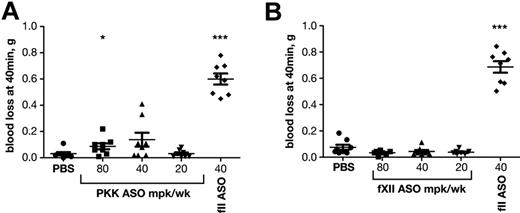
The effects of fXII and PKK ASO treatment on hemostasis
We have previously shown that inhibition of the intrinsic pathway by ASO-mediated knockdown of fXI results in a potent antithrombotic effect without compromising hemostasis, while antithrombotic treatment with low molecular weight heparin (Lovenox) or coumadin (Warfarin) caused pronounced bleeding.18 We were interested to see whether inhibition of the contact system proteases would also be safe with respect to bleeding. The effects of PKK or fXII ASO-mediated knockdown on bleeding were compared with that of prothrombin (fII) depletion in a tail vein bleeding assay (Figure 6). As expected, reductions in fII levels resulted in significant blood loss. In contrast, PKK or fXII inhibition did not induce excessive bleeding even at the highest doses, consistent with the premise that contact proteases are not required for hemostasis.
Effect of PKK and fXII ASO treatments on bleeding. Effect of PKK (A) and fXII (B) antisense treatment at indicated doses on tail bleeding was evaluated in male BALB/c mice (n = 10 per treatment group). FII ASO treatment at indicated dose was shown to cause increased bleeding and was used as reference drug (A-B). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Effect of PKK and fXII ASO treatments on bleeding. Effect of PKK (A) and fXII (B) antisense treatment at indicated doses on tail bleeding was evaluated in male BALB/c mice (n = 10 per treatment group). FII ASO treatment at indicated dose was shown to cause increased bleeding and was used as reference drug (A-B). *P ≤ .05, **P ≤ .01 and ***P ≤ .001 compared with untreated control.
Discussion
Exposure of blood to artificial surfaces triggers reciprocal activation of the protease zymogens fXII and PKK.5,6 In vivo, substances such as collagen in basement membranes22 and polyphosphates23 released from platelet granules may serve as suitable surfaces to support contact activation. α-FXIIa and α-kallikrein, the proteases generated during contact activation, and their active degradation products β-fXIIa and β-kallikrein, may contribute to several host defense (inflammation, innate immunity, complement activation) and homeostatic (kinin generation, activation of the renin-angiotensin system) processes.5,24,25 It is interesting to note that during vertebrate evolution the genes for fXII and PKK make their first appearances together in amphibians (they are not found in fish).26 The fXII gene is a product of a duplication event involving the gene for hepatocyte growth factor activator (HGFA). Similarly, the apple domains that are a prominent feature of PKK are homologs of domains from the HGFA substrate hepatocyte growth factor.26,27 Thus, from an evolutionary perspective, there are strong links between fXII and PKK. Nevertheless, the functional relationships between these proteins in vivo are not well understood, and mechanisms for PKK and fXII activation distinct from contact activation have been described.28
In blood from most mammals, but not other vertebrate groups, induction of contact activation readily leads to thrombin generation. This is because fXIIa can activate fXI, a coagulation protease zymogen that is unique to placental and marsupial mammals.26 Contact activation-mediated fXI activation is required for clot formation in the activated partial thromboplastin time (aPTT) assay, a test widely used to assess hemostasis and monitor anticoagulant therapy in clinical practice. Despite the importance of fXII and PKK to the aPTT, it is clear that these proteins are not required for hemostasis.29 However, the absence of bleeding symptoms with fXII and PKK deficiency does not establish that these proteases are not active at a site of injury. Furthermore, recent evidence suggests that fXII makes important contributions to pathologic coagulation. Disruption of the fXII gene or specific fXIIa inhibitors protect rodents from thrombosis in a variety of experimental models,9-11,13,21 raising the possibility that targeted inhibition of fXIIa may have a role in treating thromboembolism.
We reasoned that if contact activation was involved in fXII activation in vivo, then PKK should contribute to thrombus formation. Mice with targeted deletion of the PKK gene are not available, so we used ASO technology to create mice with low plasma PKK or fXII levels. At maximal doses, ASOs for murine PKK or fXII mRNA produced specific reductions of the appropriate mRNA in liver (95%), without affecting mRNA levels of other liver-derived coagulation factors. While PKK ASO did not affect fXII mRNA levels, and fXII ASO did not affect PKK mRNA levels, depletion of either protein resulted in an increase in the plasma concentration of the other. At the highest level of fXII or PKK knockdown, plasma PKK and fXII protein levels, respectively, were ∼ 3-fold greater than control. The decreased levels of fXIIa- or α-kallikrein-serpin complexes in plasmas of PKK and fXII ASO-treated mice, respectively, are consistent with reduced rates of fXII and PKK activation resulting in zymogen stabilization. Work from several groups suggests that the contact system in mice is active in normal health.28,30-32 Our data are consistent with this notion that the contact system “idles,“ resulting in basal rates of fXII and PKK consumption, and provide evidence that these proteins are major substrates for each other in vivo.
Both fXII and PKK ASO treatment produced impressive antithrombotic effects in mice, however, there were interesting differences in efficacy, depending on the model used. In FeCl3-induced and stenosis-induced venous thrombosis, PKK ASO produced a dose-dependent inhibition of thrombus growth that correlated linearly with reduction in plasma PKK. FXII, on the other hand, required a more significant reduction in plasma level to affect thrombus growth, particularly in the stenosis-based model, where fXII mRNA knockdown of > 90% was required to protect mice from thrombus formation. This suggests that PKK may contribute to thrombus formation through one or more fXII-independent mechanisms, in addition to fXII-dependent pathways. However, this does not necessarily have to be the case. In contact activation in vitro, it is fXIIa that promotes clot formation through fXI activation, and it is probably the amount of fXIIa generated (and not the absolute amount of fXII) that is most important. In the venous thrombosis models, a reduction in fXII may not have as potent an effect on fXIIa generation (until the fXII level is < 20% of normal) as loss of PKK-mediated activation of fXII, even though thrombus formation is ultimately mediated through fXIIa.
In contrast, in the FeCl3-induced arterial thrombosis model, a dose-response was observed with fXII ASO treatment, while PKK ASO produced an effect only at the highest concentration tested. This indicates relatively low PKK levels are required to support arterial thrombus formation. It is well established that reagents used to trigger contact activation in vitro differ in their sensitivities to PKK concentration.32,33 For example, aPTT assays that use micronized silica or ellagic acid to induce contact activation only detect PKK deficiency when the level drops to a few percent of normal.33 A feature of PKK-deficient plasma that distinguishes it from plasmas deficient in other clotting factors is that the prolonged aPTT can be shortened by increased incubation time with the contact reagent.29 This allows fXII to undergo autoactivation for a longer period of time, compensating for the loss of reciprocal fXII activation by α-kallikrein. The trigger for contact activation in the arterial thrombosis model may be a more efficient inducer of fXII autoactivation than the trigger in the venous thrombosis models, decreasing the amount of reciprocal activation by α-kallikrein required for thrombus formation. The fact that plasma fXII concentration rises with PKK ASO-mediated knockdown may also contribute to enhanced autoactivation.
In a recent study, Adams et al reported that inhibition of plasma kallikrein activity in vivo by soybean trypsin inhibitor (SBTI), Pro-Phe-Arg-chloromethylketone (PFR-CK) or the plasma kallikrein inhibitor PKSI-527 shortened the time to arterial occlusion in a Rose Bengal-laser injury thrombosis model.24 While none of these compounds are specific for α-kallikrein (they also inhibit fXIIa and/or fXIa), the authors felt that their activities were sufficiently specific to justify the conclusion that inhibiting α-kallikrein is prothrombotic. These results are at odds with reports describing an antithrombotic effect of PFR-CK in mouse arterial thrombosis and cerebral ischemia models.13,21 The reasons for the discrepancies are not clear. It is possible that there are fundamental differences between thrombosis models based on FeCl3 or stenosis/stasis and the Rose Bengal-laser injury model that could account for the markedly different results. However, our results with highly specific antisense oligonucleotides raise the distinct possibility that inhibitors such as SBTI and PFR-CK affect murine thrombosis models through mechanisms not directly related to α-kallikrein inhibition.
It is interesting to compare the effects of the antisense drugs targeting PKK and fXII with those previously reported for fXI ASO (supplemental Figure 2).18 Results with the fXII ASO were roughly similar to those of the fXI ASO for both arterial and venous thrombosis models, perhaps with the fXI ASO showing somewhat greater potency. This is consistent with fXII contributing to thrombus formation through activation of fXI. These results are somewhat different from those obtained with fXII and fXI knockout mice, where fXII deficiency conferred a greater antithrombotic effect than fXI deficiency.11,13 The discrepancy could be related to residual fXI and fXII protein and activity in the ASO-treated mice. Using intravitral microscopy, we noted that thrombus growth in animals treated with fXII or PKK ASOs, like those treated with fXI ASO, was severely compromised, exhibiting continuous disintegration under flow. The results are strikingly similar to those reported for arterial thrombi in fXII and fXI knockout mice,13 and in baboons treated with anti-fXI antibodies.34,35 The underlying mechanism responsible for inhibition of thrombus growth has not been firmly established, but could include components of decreased platelet stimulation and fibrin formation as a result of reduced thrombin generation.
It is important to consider the relevance of our findings for development of antithrombotic strategies for human patients. A substantial amount of data indicates that fXI contributes to arterial and venous thromboembolism in humans,4 but available information on the roles of fXII and PKK in these processes are not as clear. Indeed, for many years fXII deficiency was considered a prothrombotic state, dating back to the death of the index case for severe fXII deficiency from a pulmonary embolism.36 Subsequent evaluations suggested other thrombotic risk factors are present in most fXII deficient patients with thrombosis,37 and 2 case-controlled studies did not find a correlation between fXII deficiency and adverse outcome.38,39 However, the Study of Myocardial Infarction, Leiden (SMILE) project identified an inverse association between fXII levels and myocardial infarction risk, with an odds ratio for individuals in the highest quintile of fXII level of 0.4 compared with the lowest quintile.40 This study examined fXII levels within the broad normal range and did not assess severe deficiency, which would be more relevant for predicting effects of therapeutic fXII inhibition. In a recent analysis of > 8500 individuals in Austria, death from cardiovascular disease again was increased as fXII level decreased within the normal range.41 However, mortality for those with severe fXII deficiency (1%-10% of normal level) was similar to that for the population median, suggesting a fundamental difference between severe and moderate deficiency. Elevated levels of fXIIa, on the other hand, have been associated with severity of atherosclerosis, increased risk for coronary heart disease, and increased recurrence of coronary events.42-46 While this is consistent with the premise that fXIIa is prothrombotic in humans, it is not clear at this point if elevated fXIIa levels contribute to thrombotic events or are caused by them. When considering clinical data on fXII, it should be noted that antibodies to fXII and reduced fXII levels have been associated with antiphospholipid antibodies (APA) and the prothrombotic APA syndrome.47,48 APAs are relatively common in the general population, and it is possible they are more prevalent in patients with low to low-normal fXII levels, giving the false impression of an inherited deficiency. There is scant data on a role for PKK in human thromboembolism. Only ∼ 75 cases for congenital PKK deficiency have been published,7,8,29 precluding meaningful epidemiologic analysis, and an association between elevated PKK levels and thrombosis has not been established.49
Small molecule inhibitors, antibodies and ASOs targeting fXI or fXIa are currently under development for therapeutic use. Strategies based on these agents should be associated with a considerably lower bleeding risk than with currently used anticoagulant drugs. As fXI deficiency in humans is associated with a mild-to moderate hemorrhagic diathesis, fXII or PKK may be even more attractive targets because their inhibition may not cause any increase in risk of bleeding. Accordingly, the potent antithrombotic effects of fXII or PKK ASO-mediated knockdown in mice were not associated with increased bleeding in a tail-bleeding model. In comparison, prothrombin ASO-mediated knockdown, which is more comparable with anticoagulation with the vitamin K antagonist warfarin, was associated with significant bleeding.
However, there are several considerations remain to be carefully addressed if this approach progresses toward clinical evaluation. As we have mentioned, the contact system contributes to the aspects of host defense process (complement activation, generation of bradykinin and antimicrobial peptides) as well as to the regulation of blood pressure through bradykinin.5,24,25 Existing data and our current report suggest that the contact system “idles” in normal healthy subjects.28,30-32 Theoretically, basal levels of bradykinin generation may contribute to “normal” systemic blood pressure and thus, inhibition of the contact system could lead to hypertension. In preliminary experiments we have not detected significant differences in systemic blood pressure between normal and PKK ASO-treated mice (data not shown), however, a more rigorous evaluation of these possible effects should be carefully considered. Similarly, although no clinical data exists suggesting that fXII or PKK deficiency results in susceptibility to infectious diseases, these must also be carefully assessed during formal preclinical toxicology studies and throughout clinical evaluation.
In conclusion, the results of the present study support the premise that the contribution of fXII to thromboembolism involves a process similar or identical to contact activation, and indicate that PKK can be considered as an attractive target for treating or preventing thromboembolic disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to acknowledge the important contributions of Andrew Watt and Sue Freier for their research support and Tracy Reigle for the assistance with manuscript preparation
Authorship
Contribution: A.R.M., J.R.C., and D. Gailani conceived and implemented the project strategy; A.S.R., D. Gao, J.R.C., and G.B. designed and performed in vitro studies and animal studies; C.Z. and C.M. assisted with animal studies; A.S.R. analyzed the data and wrote the first draft of the manuscript; A.R.M. and D. Gailani edited and finalized the manuscript; and B.P.M. and A.R.M. supervised the research.
Conflict-of-interest disclosure: A.S.R., D. Gao, J.R.C., G.B., C.Z., C.M., B.P.M., and A.R.M. are employees of Isis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Robert MacLeod, Isis Pharmaceuticals Inc, Department of Antisense Drug Discovery, 1896 Rutherford Rd, Carlsbad, CA 92008; e-mail: rmacleod@isisph.com.
References
Author notes
A.S.R. and D.G. contributed equally to this study