Abstract
Interleukin (IL)–32 was originally identified in natural killer cells and IL-2–activated human T lymphocytes. As T cells are activated in allogeneic transplantation, we determined the role of IL-32 in human mixed lymphocyte cultures (MLCs) and GVHD. In allogeneic MLCs, IL-32 increased two-fold in responding T cells, accompanied by five-fold increases of TNFα, IL-6, and IL-8. After allogeneic hematopoietic cell transplantation, IL-32 mRNA levels in blood leukocytes were statistically significantly higher in patients with acute GVHD (n = 10) than in serial samples from patients who did not develop acute GVHD (n = 5; P = .02). No significant changes in IL-32 levels were present in patients with treated (n = 14) or untreated (n = 8) chronic GVHD, compared with healthy controls (n = 8; P = .5, and P = .74, respectively). As IL-32 is activated by proteinase-3 (PR3), we determined the effect of the serine protease inhibitor α-1 antitrypsin (AAT) on IL-32 levels and showed suppression of IL-32 and T-lymphocyte proliferation in MLCs. In an MHC-minor antigen disparate murine transplant model, preconditioning and postconditioning treatment with AAT resulted in attenuation or prevention of GVHD and superior survival compared with albumin-treated controls (80% vs 44%; P = .04). These findings suggest that AAT modulates immune and inflammatory functions and may represent a novel approach to prevent or treat GVHD.
Introduction
An extensive literature describes the role of proinflammatory cytokines in the manifestations of conditioning-related toxicity in patients undergoing hematopoietic cell transplantation (HCT) and the immunologic interactions of donor and recipient cells that follow HCT. We were interested in determining specifically the involvement of IL–32 in the “cytokine storm” that has been described in the peri-HCT and post-HCT period.1 For one, we and others have shown that tumor necrosis factor α (TNF), which is consistently up-regulated in transplant recipients, is a potent inducer of IL-32.2 Conversely, IL-32 has been shown to induce TNF,2-5 suggesting the possibility of an amplification loop between these two cytokines. Secondly, IL-32 was originally identified in IL-2–activated T lymphocytes and natural killer (NK) cells,6 supporting a potential role in T-cell activation and function after allogeneic HCT. Furthermore, IL-32 is present in supernatants of IL-12, IL-18, and IL-12 plus IL-18–stimulated human NK cells and in7 the supernatant of concanavalin A-stimulated human PBMCs.7 In addition, in patients with myelodysplastic syndrome who exhibit excessive apoptosis in hematopoietic cells, we reported, in agreement with others, that silencing of endogenous IL-32 significantly reduced apoptosis and the expression of other proinflammatory mediators.3,8
Based on these data, we postulated a role for IL-32 in alloactivation and in GVHD. As IL-32 is activated via partial cleavage by proteinase-3 (PR3),9 we hypothesized that the naturally occurring serine protease inhibitor α-1 antitrypsin (AAT) would prevent IL-32 activation via inhibition of PR3, thereby interfering with alloactivation. Although PR3 activity is not restricted to IL-32, and hence AAT will affect additional targets, an inhibitory effect of ATT on alloactivation might prove beneficial in the prevention or therapy of GVHD. As there is a long clinical track record of AAT for other indications, which shows excellent tolerability of the compound in the clinic,10,11 observations made in a preclinical model could be transferred quickly to the clinic where GVHD remains a major problem in patients undergoing allogeneic HCT.
Methods
Patients, sample collection, and follow-up
Patient characteristics, treatment regimens, and clinical outcome data were collected prospectively and stored in the Fred Hutchinson Cancer Research Center (FHCRC) database. Patients were transplanted for various hematologic malignancies; they were 12-65 (median 43) years of age at the time of HCT. Patients received cyclosporine or tacrolimus, combined with a short course of methotrexate or mycophenolate mofetil as GVHD prophylaxis. The source of stem cells was peripheral blood stem cells in 31 patients and bone marrow in 6 patients. All patients and controls had given informed consent to participate in research studies as required by the Institutional Review Board of the FHCRC and the Declaration of Helsinki.
Patients with acute GVHD.
White blood cells (WBCs) were collected from 15 patients at a median of 24 (range 18-38) days after HCT; among these, 10 developed acute GVHD and were studied before systemic therapy was started. Five of 10 acute GVHD patients had serially collected samples before onset of GVHD for a total of 15 samples. Eight samples (including 3 sequential ones) were collected from 5 patients who never developed GVHD during the first 100 days after HCT. Four of 15 patients were serologically CMV+, as were 4 of 15 transplantation donors.
Patients with chronic GVHD.
PBMCs were collected from 22 patients with active chronic GVHD at a median of 806 (range 349-5473) days after HCT; among these, 14 received immunosuppressive therapy and 8 did not. Among the 22 patients with chronic GVHD, 12 were CMV+, as were 7 of the donors.
Healthy controls.
Control samples were collected from 9 healthy individuals, 22-73 (median 37) years old.
Cell separation and reagents
WBCs were separated by Dextran sedimentation (early after HCT when blood cell counts were low) and PBMCs were separated by Ficoll-Hypaque density gradient centrifugation (in patients with chronic GVHD). RNA was extracted from WBCs and PBMCs using Trizol as previously described.12 cDNA synthesis was performed from 500 ng of total RNA using Invitrogen Superscript RT (Invitrogen). Goat polyclonal anti–human IL-32 antibody AF3040, was obtained from R&D Systems, rabbit policlonal anti-β actin antibody from Santa Cruz Biotechnology, and each used according to the manufacturers' recommended conditions. Concanavalin A was purchased from Sigma-Aldrich; Aralast NP (human α-1-antitrypsin), a serum serine-protease inhibitor that blocks the enzymatic activity of neutrophil elastase, cathespin G, PR3, thrombin, trypsin, and chymotrypsin, was purchased from Baxter.
RNA interference and transient transfection
Stealth siRNA oligonucleotides, specifically designed to silence the expression of all IL-32 isoforms, were obtained from Invitrogen. PBMCs from healthy donors (1 × 106) were electroporated with 500 ng of siRNA by nucleofection (Human Cell Nucleofector kit, Program V-024; Amaxa Biosystems). The siRNAs had the following sequences: scrambled -5′-: CGAAUCCUAAUGCUGCUCCCUACUU-3′ and 3′-AAGUAGGGAGGAGCAUUACCAUUCG-5′. IL-32 specific -5′-CUUUGGUGCCAACUCUGCCUCUCUU-3′, and 3′-AAGAGAGGCAGAGUUGGCACCAAAG-5.
Human cytokine protein array
After transfection with either scrambled or IL-32–specific siRNA, PBMCs were cultured for 96 hours in RPMI 1640 medium, containing 5% FBS, and penicillin/streptomycin (P/S; 50 U/mL and 50 μg/mL, respectively), and supernatants were collected. To determine the presence of various cytokines, we used the human proteome profiler array membrane kit Panel A (ARY005; R&D Systems) according to the manufacturer's instructions. Equal volumes (1 mL) of supernatant were collected from cultured PBMCs and added to the precoated membranes of the kit. The dot blot membranes (standardized for loading control) were analyzed using ImageQuant Version 2005 TL software (Molecular Dynamics).
Mixed leukocyte cultures
Mixed leukocyte cultures (MLCs) were used to assess alloreactivity as a simple in vitro model of GVHD. Human PBMCs were suspended in RPMI 1640 medium supplemented with 1% nonessential amino acids; 1% sodium pyruvate; 1% l-glutamine; and 10% heat-inactivated, pooled, normal human serum. Responder cells (1 × 105) and 1 × 105 irradiated (2200 cGy) stimulator cells per well were cocultured in triplicate in round-bottom 96-well plates for 6 days at 37°C in a humidified 5% carbon dioxide/air atmosphere. MLCs were carried out either in unmodified medium or with the addition of AAT (at concentrations of 0.1 to 0.5 mg/mL) or albumin. All final culture volumes were 200μL/well. Concanavalin A (Sigma-Aldrich) was added (4 μg/well) on day 3 to responder cells plated without stimulator cells to provide a control for cell proliferation. On day 6, cultures were pulsed with 1 μCi of 3H-thymidine for 18 hours before harvesting; 3H-thymidine uptake was measured as the mean counts per minute (cpm) from the 3 replicates and harvested onto filter paper strips using a β-scintillation counter (Packard BioScience Company). Results were expressed as stimulation index (SI) = (mean cpm of stimulated cells − mean cpm of nonstimulated cells: mean cpm of nonstimulated cells).
Markers of inflammation
Supernatants from MLCs were collected and analyzed for cytokines and inflammatory markers with potential relevance to GVHD, including TNF-α, IL-6, and IL-8, as determined by ELISA. The probes used included human BAF210 TNF-α, BAF206 IL-6, and BAF208 IL-8 (R&D Systems). When inflammatory marker concentrations were less than the assay detection limit, the sample was assigned the median value between 0 and the detection limit.
Analysis of human and murine cytokines by real-time PCR
RNA was extracted by standard techniques. Applied Biosystems Pre-Designed Gene Expression Assays containing both primers and fluorescent Taq-Man probes were used to determine human or mouse gene expression. β-actin and GUSB were used as “housekeeping” controls for normalization of quantitative RNA variation.
Human probes.
IL-32, all isoforms (Hs00170403_m1), IL-32 β and ϵ isoforms (Hs00997068_g1), IL-32 α and γ (Hs00992439_g1), β-actin, (Hs00607939), GUSB (Hs03929099_m1), TNFα (Hs00174128_m1), IL-1β (Hs01555410_m1), PR3 (Hs01597752_m1), and PAR2 (Hs00173741_m1).
Murine probes.
TNFα (Mm00443258_m1), IL-1β (Mm01336189_m1), IL-1Ra (Mm01337566_m1), and PR3 (Mm00478323_m1).
Each 20 μL reaction contained 2.0 μL 10× PCR Buffer without Mg2+, 2.8 μL 25mM MgCl2 (3.5mM final concentration), 0.4 μL ROX passive reference dye, 0.4 μL 10mM dNTPs, 1.0 μL ABI primer/probe, and 0.16 μL (0.8 U) Fast Start Taq Polymerase (Roche), 8.24 μL H2O, and 5 μL of the cDNA template. All reactions were carried out in triplicate in 384-well plates on an ABI7900HT (Applied Biosystems). For inclusion in the dataset, SD of the triplicates had to be < 0.15 CT (cycle threshold). In addition, we verified that the PCR efficiencies of the ABI assays were > 95% and that the slopes of the linear portion of the amplification curves varied by < 5%.
Effect of AAT on GVHD prevention in an MHC matched, minor antigen disparate murine transplant model
C57/BL6J mice (H-2b; The Jackson Laboratory), 10-14 weeks old with average body weight of 28g, received single-dose total body irradiation with 1000 cGy followed by intra-tail vein injection of T cell–depleted bone marrow (BM, 5 × 106 cells), and CD8+ splenic lymphocytes (0.2 × 106cells) from C3H.SW-H2b/SnJ donors (H-2bc; The Jackson Laboratory). BM was T cell–depleted using the T Cell Isolation Kit II (Milteny Biotec). CD8+ T cells were isolated from splenocytes by positive selection, using MACS CD8+ microbeads as directed by the manufacturer (Milteny Biotec).
Mice in the experimental group were given AAT intraperitoneally at 3 mg/dose, suspended in 125 μL before irradiation and donor cell infusion, and every 2 days after HCT for a total of 10 injections (see AAT treatment schedule). Mice in the control group were injected, also intraperitoneally, with 125 μL of human albumin on the same schedule. Each group consisted of 16 mice. GVHD was assessed by a standard scoring system.13 Body weights were obtained and recorded on day 0 and weekly thereafter. A weekly clinical index was generated by summation of 5 criteria scores: percentage of weight change, posture (hunching), activity, fur texture, and skin integrity (maximum score = 10). Animals that received a score of 6.5 or higher were killed using CO2 euthanasia. Blood samples were collected sequentially for cytokine assays. To determine the presence of various cytokines in the 2 groups of mice, we used the mouse proteome profiler array membrane kit Panel A (ARY006; R&D Systems) according to the manufacturer's instructions. Equal volumes (100 μL) of plasma were collected from individual animals and added to the precoated membranes of the kit. The dot blot membranes (standardized for loading control) were analyzed using ImageQuant Version 2005 TL software (Molecular Dynamics).The experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the FHCRC.
Chimerism analysis
Chimerism analyses were done on mouse PBMCs after separation of blood on Ficoll-Hypaque (density = 1.074).14 Cells at the interface were collected and washed in PBS by centrifugation. The contributions of recipient (C57/BL6J) and donor cells (C3H.SW-H2b/SnJ) to peripheral blood were quantified by fluorescent variable number of tandem repeat (VNTR) PCR analysis, as described.14
Histopathology
At autopsy skin, stomach, and small bowel samples were obtained from AAT and albumin-treated mice, fixed in 4% paraformaldehyde, and embedded in paraffin before sectioning. The sections were stained with H&E to assess for inflammatory lesions by light microscopy. The frequencies and severity of inflammatory lesions were estimated and compared between groups. At least 3 sections from each organ were scored.
Statistical analysis
For gene expression purposes, all values were expressed as the mean ± SEM. A Student t test was used to compare continuous variables between 2 groups; 1-way ANOVA was applied to compare continuous variables among 3 or more groups.
Results
IL-32 expression in MLCs
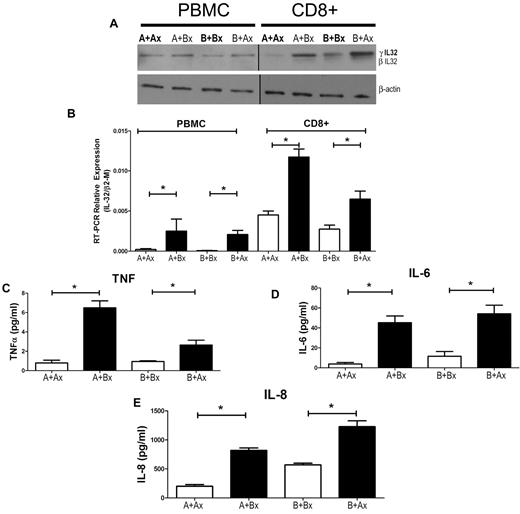
To determine a potential role of IL-32 in MLC reactivity, we processed responder cells from MLCs for Western blotting (Figure 1A) and RNA analysis (Figure 1 B). IL-32 was up-regulated both at the mRNA and protein levels in cells exposed to allogeneic stimulator cells in comparison to autologous controls. The supernatants of the same 7-day MLCs revealed high levels of TNF-α, IL-6, and IL-8 (Figure 1C-E).
Mixed leukocyte culture (MLC) and IL-32 expression. (A) PBMCs were cultured for 7 days, and Western blots were generated from either unsorted or sorted CD8+ responder cells; 1 blot is representative of 3 similar experiments. (B) IL-32 mRNA levels in allogeneic MLCs. Error bars represent mean ± SEM of 3 similar experiments. Cytokines from MLC supernatants were determined by ELISA, including TNF-α (C), IL-6 (D), and IL-8 (E). Solid columns represent results in allogeneic cultures, open columns represent results in autologous controls. The results are displayed as ± SEM from 3 similar experiments (*P < .01; Student t test).
Mixed leukocyte culture (MLC) and IL-32 expression. (A) PBMCs were cultured for 7 days, and Western blots were generated from either unsorted or sorted CD8+ responder cells; 1 blot is representative of 3 similar experiments. (B) IL-32 mRNA levels in allogeneic MLCs. Error bars represent mean ± SEM of 3 similar experiments. Cytokines from MLC supernatants were determined by ELISA, including TNF-α (C), IL-6 (D), and IL-8 (E). Solid columns represent results in allogeneic cultures, open columns represent results in autologous controls. The results are displayed as ± SEM from 3 similar experiments (*P < .01; Student t test).
Repression of IL-32 by siRNA or addition of AAT broadly inhibits inflammatory mediators
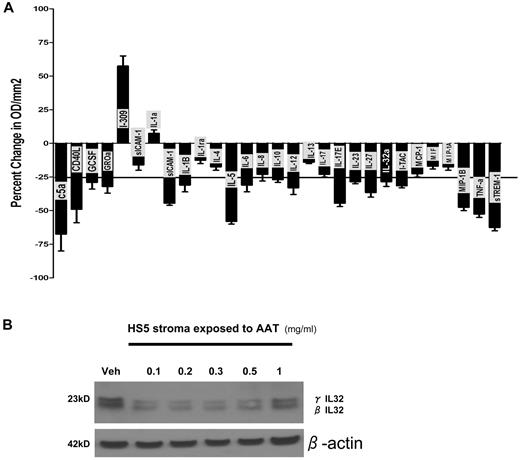
To study the role of endogenous IL-32 in PBMCs, we used IL-32–specific siRNA oligomeres, which target each of the IL-32 isoforms as confirmed by BLAST (basic local alignment search tool) alignment. As shown in Figure 2A, down-regulation of IL-32 by siRNA resulted in a global reduction of cytokine levels in the supernatants, as illustrated by an array of 36 cytokines. The only cytokine that was up-regulated (by 56% and 60% in 2 biologic duplicates) was I-309, a chemokine secreted by regulatory T cells.15
Effect of IL-32 specific siRNA and AAT on expression of inflammatory mediators. (A) Change in cytokine expression in PBMCs transfected with IL-32–specific or scrambled siRNA (control). Cytokine expression was assayed using profiler cytokine array (R&D Systems). Cytokine concentrations from siRNA transfected PBMC supernatants are expressed as percent change in comparison to control supernatants. Shown are changes (mean ± SEM) in 29 cytokines. The horizontal line indicates a decrease of 25% in comparison to controls transfected with scrambled sequence. Levels were determined after 72 hours of culture. The membrane contained probes for C5a, ICAM-1, IL-4, IL-13, IL-32α, MIP-1β, CD40 ligand, IFN-γ, IL-5, IL-16, IP-10, RANTES, G-CSF, IL-1α, IL-6, IL-17, I-TAC, SDF-1, GM-CSF, IL-1β, IL-8, IL-17E, MCP-1, Serpin-E1, GROα, IL-1ra, IL-10, IL-23, MIF, TNFα, I-309, IL-2, IL-12p70, IL-27 MIP-1α, and TREM-1. (B) Western blot of protein extract of the human stroma cell line HS5 exposed to vehicle only (veh) or various concentrations of AAT (in serum-free medium). Shown are levels of IL-32 β and γ isoforms at concentrations of ATT between 0.1 and 1 mg/mL. This blot is representative of 3 similar experiments.
Effect of IL-32 specific siRNA and AAT on expression of inflammatory mediators. (A) Change in cytokine expression in PBMCs transfected with IL-32–specific or scrambled siRNA (control). Cytokine expression was assayed using profiler cytokine array (R&D Systems). Cytokine concentrations from siRNA transfected PBMC supernatants are expressed as percent change in comparison to control supernatants. Shown are changes (mean ± SEM) in 29 cytokines. The horizontal line indicates a decrease of 25% in comparison to controls transfected with scrambled sequence. Levels were determined after 72 hours of culture. The membrane contained probes for C5a, ICAM-1, IL-4, IL-13, IL-32α, MIP-1β, CD40 ligand, IFN-γ, IL-5, IL-16, IP-10, RANTES, G-CSF, IL-1α, IL-6, IL-17, I-TAC, SDF-1, GM-CSF, IL-1β, IL-8, IL-17E, MCP-1, Serpin-E1, GROα, IL-1ra, IL-10, IL-23, MIF, TNFα, I-309, IL-2, IL-12p70, IL-27 MIP-1α, and TREM-1. (B) Western blot of protein extract of the human stroma cell line HS5 exposed to vehicle only (veh) or various concentrations of AAT (in serum-free medium). Shown are levels of IL-32 β and γ isoforms at concentrations of ATT between 0.1 and 1 mg/mL. This blot is representative of 3 similar experiments.
To determine the impact of AAT on IL-32 protein levels, we used the human stroma cell line HS5, which expresses and secretes IL-32 and can be grown in serum-free medium.3 As shown in Figure 2B, the addition of AAT (at 0.1-1.0 mg/mL) resulted in reductions of endogenous IL-32β and γ isoforms. The Western blot is representative of 3 similar experiments.
IL-32 and AAT effect on secreted cytokines in MLCs
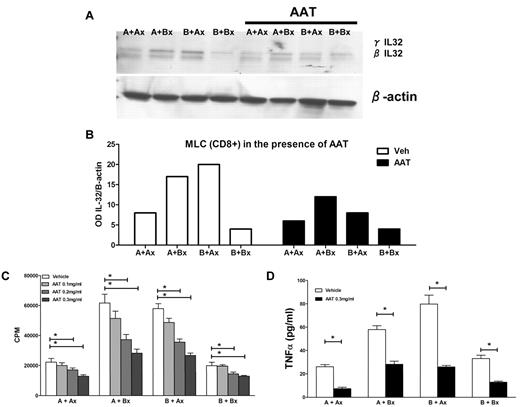
Next, we carried out MLCs to which AAT was added at concentrations ranging from 0.1 to 0.5 mg/mL. CD8+ cells sorted from MLCs showed levels of IL-32β and γ isoforms at least two-fold lower than in the absence of AAT (Figure 3A-B). Concurrently, there was significant dose-dependent suppression of the proliferative capacity as determined by 3H thymidine uptake (Figure 3C), and a 2-fold reduction in TNFα levels (Figure 3D). These data demonstrate that AAT had a profound effect on reducing alloreactivity in parallel with inhibition of IL-32 and TNFα production.
Inhibition of proliferation and TNFα secretion in MLCs by AAT. (A) Western blot of IL-32β and γ levels in CD8+ cells from 7-day MLCs under control conditions and in the presence of AAT (0.3 mg/mL). IL-32 β and γ isoforms in the presence of AAT. The Western blot is representative of 3 similar experiments. (B) Expression changes in IL-32 protein levels in allogeneic MLCs and autologous controls as determined by densitometry (OD) of the same biologic experiment. Open columns reflect results in the absence of AAT; solid columns in the presence of AAT. (C) Proliferation in MLCs (as measured by 3H thymidine uptake; CPM, mean ± SEM). (D) TNF-α ELISA. Secretion of TNF α in the presence and absence of AAT (*P < .05; Student t test).
Inhibition of proliferation and TNFα secretion in MLCs by AAT. (A) Western blot of IL-32β and γ levels in CD8+ cells from 7-day MLCs under control conditions and in the presence of AAT (0.3 mg/mL). IL-32 β and γ isoforms in the presence of AAT. The Western blot is representative of 3 similar experiments. (B) Expression changes in IL-32 protein levels in allogeneic MLCs and autologous controls as determined by densitometry (OD) of the same biologic experiment. Open columns reflect results in the absence of AAT; solid columns in the presence of AAT. (C) Proliferation in MLCs (as measured by 3H thymidine uptake; CPM, mean ± SEM). (D) TNF-α ELISA. Secretion of TNF α in the presence and absence of AAT (*P < .05; Student t test).
IL32 gene expression in blood cells of patients with clinical GVHD
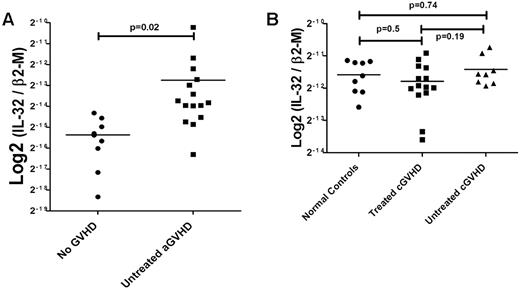
We next evaluated IL32 as a possible biomarker for GVHD by examining expression in WBCs and PBMCs from patients at various time intervals after HCT. Expression of IL32 in WBCs was 2-fold higher in patients with acute GVHD than in patients who did not show clinical evidence of GVHD (P < .02; Figure 4A); IL32 expression levels in PBMCs of patients with chronic GVHD, untreated (n = 8) or treated with steroids, cyclosporine, or both (n = 14) did not differ from IL32 expression levels in PBMCs of healthy controls (n = 9; P = .74 and .50, respectively; Figure 4B).
IL32 mRNA levels in WBCs and PBMCs from patients with or without clinical GVHD after hematopoietic cell transplantation. (A) Acute GVHD. IL-32 expression was determined with probe Hs00170403_m1 covering all isoforms (see “Analysis of human and murine cytokines by real-time PCR”) and validated using 4 different taqman probes for IL-32 (data not shown). Expression of IL-32 in WBCs from patients with acute GVHD (10 patients, 15 samples) was twice as high as in patients who did not develop clinical signs of GVHD (5 patients, 8 samples; P < .02). (B) Chronic GVHD. Steady-state levels of IL-32 mRNA in PBMCs from patients with chronic GVHD. No significant changes in IL-32 expression were seen in PBMCs of patients with chronic GVHD either untreated (n = 8, P = .74) or treated (n = 14, P = .5) compared with healthy controls. Similarly, there was no difference between treated and untreated patients with chronic GVHD (P = .19; Student t test for comparison of continuous variables between 2 groups; 1-way ANOVA for 3 or more groups).
IL32 mRNA levels in WBCs and PBMCs from patients with or without clinical GVHD after hematopoietic cell transplantation. (A) Acute GVHD. IL-32 expression was determined with probe Hs00170403_m1 covering all isoforms (see “Analysis of human and murine cytokines by real-time PCR”) and validated using 4 different taqman probes for IL-32 (data not shown). Expression of IL-32 in WBCs from patients with acute GVHD (10 patients, 15 samples) was twice as high as in patients who did not develop clinical signs of GVHD (5 patients, 8 samples; P < .02). (B) Chronic GVHD. Steady-state levels of IL-32 mRNA in PBMCs from patients with chronic GVHD. No significant changes in IL-32 expression were seen in PBMCs of patients with chronic GVHD either untreated (n = 8, P = .74) or treated (n = 14, P = .5) compared with healthy controls. Similarly, there was no difference between treated and untreated patients with chronic GVHD (P = .19; Student t test for comparison of continuous variables between 2 groups; 1-way ANOVA for 3 or more groups).
AAT abrogates GVHD and reduces mortality in an MHC matched, minor antigen disparate murine transplant model
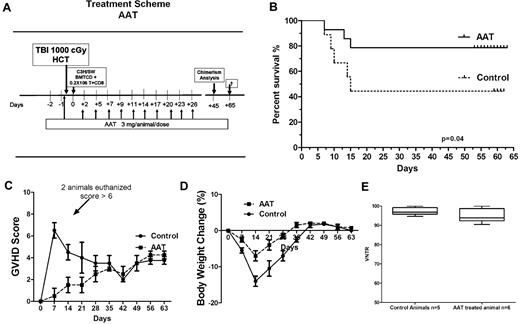
Lethally irradiated (1000 cGy) C57/BL6J (H-2b) mice were injected IV with 5 × 106 T cell–depleted BM cells and 0.2 × 106 CD8+ splenic T cells from C3H.SW-H2b/SnJ mice (H-2bc). Recipient mice were given 3 mg of AAT (125 μL) on day −1 and again on day +2 and every 72 hours for a total of 10 injections (Figure 5A). Albumin controls received the same volume of human serum albumin on the same schedule. As shown in Figure 5B, by day 65 after transplantation survival was 80% in AAT-treated mice versus 40% in albumin treated controls (n = 15; P = .04, log rank). In both albumin controls and AAT-treated mice C3H.SW-H2b/SnJ donor cells accounted for > 95% of cells in peripheral blood (P = .25; Figure 5C).
Effect of AAT on GVHD severity and mortality. (A) AAT treatment scheme. (B) Survival. Survival of AAT-treated mice versus albumin-treated controls (n = 15 each group, P = .04). (C) Severity of GVHD. GVHD was scored based on percentage of weight loss, skin integrity, posture, mobility, and fur texture. Clinical signs were graded on a scale of 0 to 2, where 0 was absent, 1 was moderate, 2 was severe, and the individual scores were added. Shown are GVHD clinical scores for 30 days after transplantation (mean ± SEM per time point; D) Change in body weight of transplanted mice over time after transplantation (mean ± SEM; n = 15). (E) Donor chimerism. Proportion of donor cells among PBMCs in AAT-treated (n = 6) versus albumin-treated (n = 5) mice at day 45 (P = .25).
Effect of AAT on GVHD severity and mortality. (A) AAT treatment scheme. (B) Survival. Survival of AAT-treated mice versus albumin-treated controls (n = 15 each group, P = .04). (C) Severity of GVHD. GVHD was scored based on percentage of weight loss, skin integrity, posture, mobility, and fur texture. Clinical signs were graded on a scale of 0 to 2, where 0 was absent, 1 was moderate, 2 was severe, and the individual scores were added. Shown are GVHD clinical scores for 30 days after transplantation (mean ± SEM per time point; D) Change in body weight of transplanted mice over time after transplantation (mean ± SEM; n = 15). (E) Donor chimerism. Proportion of donor cells among PBMCs in AAT-treated (n = 6) versus albumin-treated (n = 5) mice at day 45 (P = .25).
Albumin controls experienced significantly greater weight loss and showed higher GVHD scores than AAT treated mice (Figure 5D-E). Two AAT-treated mice that developed signs of gut-GVHD by day 45 (ie, after discontinuation of AAT) showed complete resolution of GVHD on reinstitution of AAT therapy, given every 72 hours, for 4-5 doses.
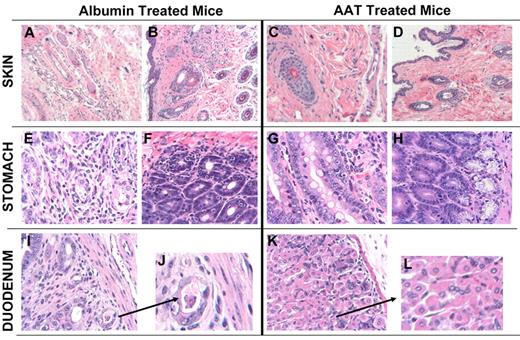
Histologic examination of albumin-treated mice showed patchy epithelial damage in the hair follicles and edema (Figure 6A-B). The forestomach and duodenum showed patchy lymphocytic infiltration of epithelium and damage to the glands as evidenced by exocytosis and apoptosis (Figure 6E-F). Mice treated with AAT, in contrast, had normal skin and only rare areas of infiltration in stomach and duodenum (Figure 6G-H). These results indicate that AAT significantly attenuated clinical and histologic manifestations of GVHD and reduced GVHD-related morbidity and mortality.
Skin, stomach, and duodenal histology in AAT-treated and albumin-treated C57/BL6J mice transplanted from C3H.SW-H2b/SnJ donors. Mice were sacrificed on day 21 after transplantation (3 mice per group). Tissues were placed in 10% formalin, embedded in paraffin, sectioned, stained with H&E, and analyzed. Slides were imaged on an Axio Observer inverted microscope (Carl Zeiss MicroImaging) and captured with an Axiocam MRm camera (Carl Zeiss MicroImaging). (A) Skin section of albumin-treated mouse showing edema with interstitial inflammation (10×). (B) Skin section of albumin-treated mouse showing interstitial fibrosis and inflammation invading and damaging hair follicles, characteristic of GVHD (10× lens). (C-D) Skin of 2 AAT-treated mice showing normal histology (10× and 4×, respectively). (E) Stomach of albumin-treated mouse showing interstitial gastritis and evidence of GVHD. (F) Stomach of albumin-treated mouse showing interstitial gastritis and evidence of GVHD with tissue edema and lymphocytic infiltration. (G) Forestomach of AAT-treated mouse showing minimal evidence of GVHD (10×). (H) Stomach of AAT-treated mouse showing normal histology. (I-J) Glandular forestomach/duodenum of albumin-treated mouse showing interstitial inflammation, crypt loss, and apoptotic bodies with 1 small crypt abscess, typical for GVHD (4× and 10×). (K-L) Duodenum of AAT-treated mouse with minimal focal evidence of GVHD (4× and 10×).
Skin, stomach, and duodenal histology in AAT-treated and albumin-treated C57/BL6J mice transplanted from C3H.SW-H2b/SnJ donors. Mice were sacrificed on day 21 after transplantation (3 mice per group). Tissues were placed in 10% formalin, embedded in paraffin, sectioned, stained with H&E, and analyzed. Slides were imaged on an Axio Observer inverted microscope (Carl Zeiss MicroImaging) and captured with an Axiocam MRm camera (Carl Zeiss MicroImaging). (A) Skin section of albumin-treated mouse showing edema with interstitial inflammation (10×). (B) Skin section of albumin-treated mouse showing interstitial fibrosis and inflammation invading and damaging hair follicles, characteristic of GVHD (10× lens). (C-D) Skin of 2 AAT-treated mice showing normal histology (10× and 4×, respectively). (E) Stomach of albumin-treated mouse showing interstitial gastritis and evidence of GVHD. (F) Stomach of albumin-treated mouse showing interstitial gastritis and evidence of GVHD with tissue edema and lymphocytic infiltration. (G) Forestomach of AAT-treated mouse showing minimal evidence of GVHD (10×). (H) Stomach of AAT-treated mouse showing normal histology. (I-J) Glandular forestomach/duodenum of albumin-treated mouse showing interstitial inflammation, crypt loss, and apoptotic bodies with 1 small crypt abscess, typical for GVHD (4× and 10×). (K-L) Duodenum of AAT-treated mouse with minimal focal evidence of GVHD (4× and 10×).
AAT suppresses proinflammatory signals and up-regulates IL-1Ra in MHC matched, minor antigen disparate murine transplant recipients
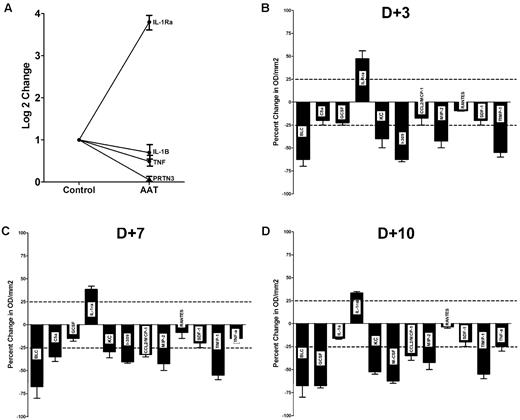
Steady-state levels of IL-1β, TNF-α, and PR3 mRNA on day 21 was significantly lower in AAT-treated compared with albumin-treated animals (Figure 7A). Moreover, in a panel of 40 cytokines as illustrated in Figure 7B through D, there was a global suppression of cytokine levels except for IL-1Ra in the plasma of AAT-treated mice. Also suppressed were, among others, factors such as CXCL13/BLC/BCA-1, a B cell–attracting chemokine 1(BCA-1), and CXCL2/MIP-2, known as macrophage inflammatory protein 2-α (MIP2-α), a chemokine chemotactic for polymorphonuclear leukocytes.
Effect of AAT on cytokine RNA and protein expression in PBMCs and plasma after transplantation. (A) IL-1Ra, IL-1β, TNF-α, and PR3 RNA levels, determined by RT-PCR, in PBMCs. Levels in AAT-treated mice (n = 6) are expressed relative to levels in albumin treated controls; mean ± SEM (n = 6; log2). (B-D) Mean ± SEM cytokine plasma levels at 3, 7, and 10 days after transplantation. Shown is a panel selected from a mouse array of 40 cytokines, showing significant changes. Changes in cytokine concentration are expressed as percent change compared with albumin control. The horizontal dotted line indicates an increase/decrease of 25%.
Effect of AAT on cytokine RNA and protein expression in PBMCs and plasma after transplantation. (A) IL-1Ra, IL-1β, TNF-α, and PR3 RNA levels, determined by RT-PCR, in PBMCs. Levels in AAT-treated mice (n = 6) are expressed relative to levels in albumin treated controls; mean ± SEM (n = 6; log2). (B-D) Mean ± SEM cytokine plasma levels at 3, 7, and 10 days after transplantation. Shown is a panel selected from a mouse array of 40 cytokines, showing significant changes. Changes in cytokine concentration are expressed as percent change compared with albumin control. The horizontal dotted line indicates an increase/decrease of 25%.
Discussion
We have shown in this report that suppression of endogenous IL-32 in a variety of models consistently correlated with a reduction in TNF-α levels and other cytokines.2,3,7,16,17 Gene expression studies in human marrow stroma cells showed that IL-32 was induced by TNF-α,2,3 a cytokine that is centrally involved in GVHD.18-24 Other studies show that, conversely, IL-32 induces up-regulation of TNF-α2,3 suggesting an amplification loop between these 2 cytokines.19-24 IL-32 expression increases on differentiation of T cells25 and Th22 cells.26 Such a pattern would be consistent with the present findings, which showed a correlation of IL-32 expression with responses in MLCs and with the manifestations of acute GVHD. As IL-32 has proapoptotic activity and can up-regulate TNF-α, its expression may contribute to target organ damage.27,28 Although IL-32 is produced locally7,29-31 the cytokine was readily detected in the systemic circulation, and IL32 mRNA concentrations in PBMCs discriminated between patients with and without acute GVHD.27,28 This observation raised the possibility that inhibition of IL-32 activation would interfere with alloactivation and possibly prevent or attenuate the development and manifestations of GVHD.
There is considerable evidence that endogenous proteinases induce cell proliferation,32 whereas proteinase inhibitors generally inhibit proliferation.33-35 Based on the observations that IL-32 is activated by PR39 and AAT inhibits PR3,36,37 we determined the impact of AAT on the expression of IL-32 (and other proinflammatory cytokines) and alloactivation. AAT, a member of the serine protease inhibitor family, is one of the major cytoprotective proteins in the circulation. AAT affects numerous processes, including reactive oxygen species toxicity, cell-mediated immunity, the development of immune tolerance, neutrophil and endotoxin-mediated inflammation, endothelial function, and apoptosis.38-43 The present data show that AAT strongly suppressed CD8+ cell proliferation in allogeneic MLCs, and inhibition of proliferation was associated with suppression of IL-32, as well as other proinflammatory proteins, such as TNF-α, IL-8, and IL-6. It is of note in this context that others have shown that AAT does not suppress IL-2 activity,44 which is required for the generation of regulatory T cells (Tregs), an essential cell compartment involved in establishing and maintaining tolerance.45 Therefore, ATT, although suppressing multiple proinflammatory cytokines but not IL-2, may favor the development of Tregs.
That concept is further supported by in vivo results which show suppression of presumably radiation-related cytokine release and activation of allogeneic T lymphocytes, which in turn was associated with attenuation or prevention of GVHD, entirely consistent with preferential development of Tregs. The fact that AAT administration suppressed GVHD manifestations even in mice that showed flares of GVHD after discontinuation of prophylactic ATT, suggests that this compound was also effective in inhibiting “downstream” events after activation of allogeneic T lymphocytes.44 Alternatively, the benefit of AAT in that setting may be related to inhibition of IL-32 activation in other tissues, (eg, epithelial cells). The efficacy of AAT in GVHD prevention or treatment is consistent with observations of the immunosuppressive or immunomodulatory effect of AAT in other models. Subramanian et al showed that C57/BL6J mice that received therapy with AAT for experimental autoimmune encephalomyelitis (EAE) contained significantly higher numbers of CD4+FoxP3+ Treg cells in spleen, lymph nodes, and blood than control mice.46 In tune with those findings, over-expression of AAT by gene delivery using recombinant adeno-associated virus significantly reduced insulinitis and prevented the development of overt hyperglycemia in NOD mice.47,48
The premise of our investigation was that IL-32 played a central role in alloreactivity and GVHD. Results support that hypothesis and show that administration of AAT profoundly affected expression of IL-32. However, as shown by the present study and other reports, AAT has rather broad effects. The lack of inhibition of IL-1Ra in the context of GVHD prevention is consistent with the report that IL-1Ra inhibited mouse islet allograft rejection49 and with the data by Lewis and collaborators,40 which show elevated IL-1Ra levels in long-lasting islet allografts explanted from AAT treated animals. Complementary to those data are results which suggest that IL-1β can break tolerance50 and facilitate islet antigen presentation.51 Thus, although our data are consistent with a role of IL-32 in allogeneic interactions, numerous pathways, including IL-1/IL-1Ra interactions, contribute. More importantly, our results support the hypothesis that AAT interferes with the development of GVHD, be it via increased IL-1Ra production, decreased IL-1 secretion, direct effects on IL-32 activation or additional mechanisms.
The clinical usefulness and safety of AAT as an immunossupressive and immunomodulatory protein is supported by strong clinical data. Long-term safety studies, extending over several years of weekly AAT infusions to patients with various degrees of AAT deficiency10,11 show no evidence of compromised host defense. We suggest, therefore, that AAT should be considered as a therapeutic tool in the setting of bone marrow transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Helen Crawford and Bonnie Larson for help with manuscript preparation and the staff of the vivarium for excellent care of the experimental animals.
The work is supported in part by National Institutes of Health grants NIDDK K08 DK085156-01, HL036444, HL095999, and AI-15614, AI33484, and HL094260 (C.A.D.).
National Institutes of Health
Authorship
Contribution: A.M.M. conceived the study, conducted most of the experiments, and wrote the paper; X.L. and M.B. carried out experiments; L.T. cared for the human sample repository, selected patients for the study, and extracted RNA for analysis; J.K. carried out murine transplantation experiments; G.E.S. carried out analysis of tissue histology and provided critique to the manuscript; J.A.H. contributed patient samples and provided critique; C.A.D. provided critique and cowrote the paper; and H.J.D. conceived the study, provided critique, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Mario Marcondes, Fred Hutchinson Cancer Research Center, D1-100, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mmarcond@fhcrc.org.