Abstract
Focal adhesion (FA) proteins have been associated with transformation, migration, metastasis, and poor outcome in many neoplasias. We previously showed that these proteins were inhibited by E7123, a new celecoxib derivative with antitumor activity, in acute myeloid leukemia. However, little is known about FAs in diffuse large B cell lymphoma (DLBCL). This paper aimed to determine whether E7123 was effective against DLBCL and whether FAs were involved in its action. We evaluated the cytotoxicity and mechanism of action of E7123 and celecoxib in DLBCL cell lines. We also assessed the E7123 in vivo activity in a DLBCL xenograft model and studied FA signaling in primary DLBCL patient samples. We found that E7123 showed higher antitumor effect than celecoxib against DLBCL cells. Its mechanism of action involved deregulation of FA, AKT, and Mcl-1 proteins, a pathway that is activated in some patient samples, apoptosis-inducing factor release and induction of caspase-independent cell death. Moreover, E7123 showed suppression of in vivo tumor growth. These findings indicate that E7123 is effective against DLBCL in vitro and in vivo, with a mechanism of action that differs from that of most current therapies for this malignancy. Our results support further preclinical evaluation of E7123.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent non-Hodgkin lymphoma subtype. Cure rates have increased in recent years with the addition of rituximab to the combination chemotherapy: cyclophosphamide, doxorubicin, vincristine, and prednisone.1 However, a significant percentage of patients still relapse because lymphoma cells acquire resistance to therapy, which is associated with defective caspase-dependent apoptotic pathways.2-4 Paradoxically, almost all cytotoxic drugs currently in clinical use for DLBCL induce caspase-dependent apoptosis.5 Thus, the development of novel compounds with mechanisms of action able to induce caspase-independent cell death probably improves the therapeutic outcome in DLBCL, particularly in patients who relapse after front-line therapy.
Focal adhesion (FA) complexes are structures that link the actin filaments of the cytoskeleton with the extracellular matrix. They are formed by transmembrane receptors (integrins) that activate various protein kinases, including the focal adhesion kinase (FAK) and Src family kinases.6 Crk-associated substrate (CAS) family proteins act as docking proteins that associate with FAK and Src to generate specific cellular responses.7 FA proteins have been related to transformation,8 migration,9 metastasis,10 and poor outcome11 in many solid tumors and in some hematologic malignancies.12,13 However, there are limited data regarding the role of FA proteins in DLBCL. To our knowledge, only FAK and the Src-family protein LYN have been studied in DLBCL. FAK is expressed in 70% of DLBCLs14 and LYN is constitutively phosphorylated and required for DLBCL growth, survival, and proliferation.15,16 Other FA proteins, such as HEF1 or p130Cas from the CAS family, or PYK2 from the FAK family, play crucial roles in normal B-cell development,17-19 but their involvement in DLBCL has not been assessed.
We have previously found that celecoxib, a COX-2 inhibitor with antitumor activity,20 induced apoptosis in human colon carcinoma cells through deregulation of FA proteins.21 We have also shown that E7123, a celecoxib derivative that does not inhibit COX-2, was more potent than celecoxib in inducing cell death by FA signaling inhibition in acute myeloid leukemia cells.22 Our aim in this study was to evaluate whether E7123 was effective in DLBCL and determine its mechanism of action. We compared the action of celecoxib and E7123 in DLBCL cell lines and evaluated the E7123 in vivo activity in a xenograft model of DLBCL. We also studied FA expression and activation in primary DLBCL patient samples.
Methods
Cell lines and culture conditions
HT, KARPAS-422, DB, WSU-DLCL-2, OCI-LY-19, and NU-DHL-1 (DMSZ Cell Line Bank) and TOLEDO (ATCC) are all human DLBCL cell lines. They were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% glutamine, and 100 U/mL penicillin/streptomycin (Invitrogen) and incubated at 37°C in a humidified atmosphere containing 5% CO2.
The chemical structure of the compound E7123 is 4-(5-(2,5-dimethylphenyl)-3-(trifluoromethyl)-4,5-dihydro-1H-pyrazol-1-l) benzenesulfonamide. Celecoxib and E7123 were synthesized by the HSCSP Pharmacy Department and the Parc Científic de Barcelona. The stock solution of both compounds was reconstituted in DMSO and diluted in culture media before use.
Cytotoxicity assays
Antitumor activity was evaluated measuring cell metabolic capacity (viability), using the Cell Proliferation Kit II (Roche Diagnostics), as previously described.22 Cells were seeded into 96-well plates (4 × 104 cells/well) and exposed to vehicle or drug with or without pretreatment with the pancaspase inhibitor ZVAD-FMK (ZVAD; BD Biosciences PharMingen) or the mitochondrial permeability transition (MPT) inhibitor cyclosporine A (CsA; Fluka). The growth inhibitory activity was expressed as percentage of cell growth inhibition compared with untreated controls. Values are averages of at least 6 independent experiments.
Apoptotic detection and DNA fragmentation
To assess the induction of apoptotic nuclei, we performed nuclear staining with the Hoechst 3342 dye (Sigma-Aldrich) in cells exposed to vehicle or drug with or without pretreatment with ZVAD or CsA. During the exposure, we added 2 μL/mL of Hoechst dye, centrifuged, and rinsed cells with PBS at pH 7.4 (Ca2+ and Mg2+ free) and fixed them (3.7% p-formaldehyde in PBS, pH 7.4) for 10 minutes at −20°C. After rinsing 3 times with PBS, cells were permeabilized (0.5% Triton X-100 in PBS, pH 7.4) for 10-30 minutes in the dark at room temperature. Cells were then rinsed with distilled water and resuspended in 10 μL PBS. Finally, they were mounted on a slide and observed under fluorescence microscope. For pulse-field gel electrophoresis, DNA was prepared from agarose plugs, which were treated with 1 mg/mL proteinase K in lysis buffer (Tris 50mM, pH 7.8, 0.5M EDTA, and 1 mg lauroylsarcosine) at 55°C for 2 hours and electrophoresed in a CHEF-DR III apparatus (Bio-Rad, 1% agarose, 0.5× Tris-borate/EDTA, 6 V/cm, 20 hours, 120° angle, 5-25-second pulse, 14°C).
Signal transduction analysis by Western blot
Western blot was performed as previously described.23 Dilutions in TBS-T, containing 0.1% BSA for primary anti–human antibodies were as follows: mouse anti-p130Cas, anti-PYK2, anti-LYN, anti-AKT, anti-poly(ADP-ribose) polymerase (PARP), anti–Bcl-2, anti-Bax, and anti–Mcl-1 (BD Biosciences PharMingen, 1:1000), mouse anti-FAK, anti-FAK Tyr397, and rabbit anti–caspase-8 (BD Biosciences PharMingen, 1:4,000), rabbit anti-PYK2 Tyr402, anti-AKT Thr308, anti–caspase-9, anti-Omi/HtrA2, anti-FLIP, and mouse anti-Lyn Tyr396 (Cell Signaling 1:1,000), mouse anti-HEF1 (ABR-Affinity BioReagents; 1:1,000), mouse anti-AIF (Santa Cruz Biotechnology; 1:1,000), rabbit anti-EndoG (Millipore; 1:1000), mouse anti-Omi/HtrA2, mouse anti-COXII (Invitrogen; 1:1000), and mouse anti-GAPDH (Chemicon International; 1:10 000). Secondary antibodies were anti–mouse-IgG, antirabbit IgG, and anti–goat IgG (Jackson ImmunoResearch Laboratories; 1:10 000).
Protein extraction of the cytosolic fraction
Cells were seeded in 100-mm plates (2.4 × 106/plate) and exposed to vehicle or drug with or without pretreatment with the ZVAD or CsA. Then, cells were collected by centrifugation and washed with extraction buffer (20mM HEPES, pH 7.5, 10mM KCl, 1.5mM MgCl2, 1mM EDTA, 1mM EGTA, 250mM sucrose, 1mM DTT, 0.1mM PMSF, 5 μg/mL pepstatin A, 10 μg/mL leupeptin, and 2 μg/mL aprotinin). Next, cells were resuspended with the same extraction buffer and incubated on ice for 30 minutes. Finally, cells were homogenized with a glass piston (pestle B) and centrifuged twice at 20 000g for 30 minutes. Supernatants were kept at −80°C until immunoblotting was performed.
Immunofluorescence analysis
Cells were seeded onto 60-mm plates (1.2 × 106 cells/plate) and exposed to vehicle or compound. Then, cells were collected by centrifugation, washed with PBS, and fixed with cold methanol for 1 minute. They were washed twice with PBS and left to dry on a slide. After this, cells were blocked for 1 hour in 1% BSA on PBS at room temperature, washed with PBS, and incubated with p130Cas (BD Biosciences PharMingen; 1:100) or AIF (Santa Cruz Technology; 1:100) antibodies for 1 hour. They were then rinsed with PBS and incubated with the secondary antibody (anti–mouse tetramethylrhodamine isothiocyanate; 1:100) and Hoechst dye (50 ng/mL) in the dark. After this, cells were rinsed with PBS and coverslips were mounted using Fluoprep (Biomérieux). Samples were observed in an Axiovert 200M fluorescence microscope (Carl Zeiss) at 630× (oil), using a rhodamine or 4,6-diamidino-2-phenylindole filter. Images were obtained with a digital camera (Coolsnap, Photometrics) and MetaMorph Version 5.01 software.
Plasmid constructs and lentiviral vector preparation
Plasmids containing full-length p130Cas (pSIN-DUAL-CASFL-GFP) or myristoylated constitutively active FAK (pSIN-DUAL-MyrFAK-GFP) were constructed by subcloning CASFL or MyrFAK and replacing the GFP1 gene of the plasmid pSIN-DUAL-GFP1-GFP2 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CASFL cDNA was obtained from the plasmid pcDNA3-CASFL, and the MyrFAK gene was obtained from the vector pCEFLMyrFAK, which were kindly provided by Drs Amy Bouton24 and Silvio Gutking,25 respectively. To produce lentivirus, 3 × 106 293T cells were seeded onto a 60-mm Petri dish and transfected with 2.25 μg of the pSIN-DUAL plasmid containing CASFL or MyrFAK, 1.5 μg of pCMVDR8.91 gag-pol expression vector, and 0.75 μg of pMDG (vesicular stomatitis virus envelope protein expression vector) using Lipofectamine 2000 (Invitrogen). Lentiviral vectors were kindly provided by Dr Mary Collins (College Medical School, Cleveland, OH). Lentivirus were harvested 48 hours after transfection, passed through a 0.45-μm filter, and stored at −80°C. For virus titration, 1.5 × 105 293T cells were infected. The percentage of infection was determined 48 hours later by flow cytometry (FACS) and expressed as transduction units (tu) per milliliter. We infected the DB cell line and performed a cell sorting with the BD FACSAria cell sorter selecting the cells expressing GFP to obtain a homogeneous population expressing the vector (supplemental Figure 1B).
Patient samples
We analyzed 29 DLBCL patient samples cryopreserved in optimal cutting temperature (OCT). Patients were diagnosed at our institution between 1999 and 2005 according to the criteria of the World Health Organization classification. Patients with a follicular lymphoma or other indolent lymphomas with subsequent transformation into a large-cell lymphoma were excluded. Approval was obtained from the Ethics Research Committee at Hospital de la Santa Creu i Sant Pau. Patients gave informed consent in accordance with the Declaration of Helsinki.
Animal experiments
Swiss-nude mice were injected with 20 × 106 HT cells per flank and monitored 3 times a week for the appearance of tumors. When tumors reached a volume range of 200 to 400 mm3, they were randomized in 2 groups. Experimental group mice were orally administered E7123 100 mg/kg every day for 30 days, whereas control mice were administered with vehicle (PEG-400:FBS 3:1) with the same frequency. Tumor volume was measured 3 times a week with a calliper and calculated using the following formula: tumor volume (mm3) = length (mm) × width (mm2) × 0.5. Mice were killed when all tumors in the control group exceeded 1000 mm3. Mouse weight was measured daily from the first day of E7123 administration to assess drug toxicity. These procedures were approved by the Hospital Sant Pau Animal Ethics Committee according to established guidelines.
Statistical analysis
Data on cell viability and tumor volumes were compared using the Mann-Whitney U test. Differences were considered statistically significant at P < .05. Statistical analysis was performed using the SPSS software (SPSS Statistics 18).
Results
E7123 inhibits cell viability in human DLBCL cell lines more potently than celecoxib
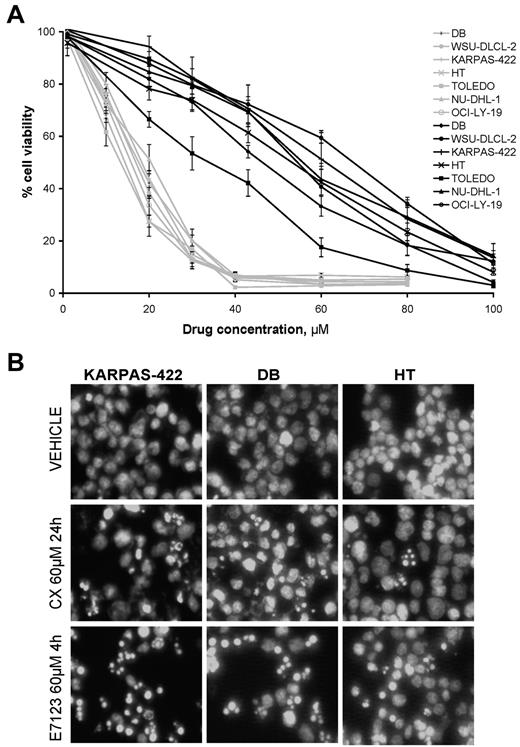
We found that the viability of all DLBCL cell lines was inhibited in a concentration-dependent manner after 4 hours of exposure to celecoxib or E7123 (Figure 1A). When comparing IC50 values for both compounds in each cell line, E7123 always showed a significantly higher antitumor activity (IC50 range, 13.08-17.66μM) than celecoxib (IC50 range, 30.97-63.55μM; Mann-Whitney, P < .01; Table 1).
Cytotoxic activity and induction of apoptosis by celecoxib and E7123 in DLBCL lines. (A) Viability/concentration curves for celecoxib (black) or E7123 (gray) on DLBCL cell lines after 4-hour exposure. Error bars represent SE. (B) Apoptotic induction was evaluated by Hoechst staining after 24-hour exposure to 60μM celecoxib (CX) and 4-hour exposure to 60μM E7123 (original magnification ×400). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software.
Cytotoxic activity and induction of apoptosis by celecoxib and E7123 in DLBCL lines. (A) Viability/concentration curves for celecoxib (black) or E7123 (gray) on DLBCL cell lines after 4-hour exposure. Error bars represent SE. (B) Apoptotic induction was evaluated by Hoechst staining after 24-hour exposure to 60μM celecoxib (CX) and 4-hour exposure to 60μM E7123 (original magnification ×400). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software.
IC50 values for celecoxib and E7123 in DLBCL cell lines
| DLBCL cell line . | IC50 values, μM . | |
|---|---|---|
| Celecoxib . | E7123 . | |
| DB | 46.83 ± 3.4 | 17.11 ± 0.9 |
| WSU-DLCL-2 | 49.34 ± 1.6 | 16.03 ± 1.2 |
| KARPAS-422 | 63.55 ± 6.9 | 17.66 ± 1.6 |
| HT | 51.92 ± 3.4 | 13.48 ± 0.4 |
| TOLEDO | 30.97 ± 2.7 | 13.08 ± 1.6 |
| NU-DHL-1 | 54.75 ± 4.4 | 17.82 ± 1.3 |
| 0CI-LY-19 | 58.79 ± 3.9 | 16.12 ± 0.7 |
| DLBCL cell line . | IC50 values, μM . | |
|---|---|---|
| Celecoxib . | E7123 . | |
| DB | 46.83 ± 3.4 | 17.11 ± 0.9 |
| WSU-DLCL-2 | 49.34 ± 1.6 | 16.03 ± 1.2 |
| KARPAS-422 | 63.55 ± 6.9 | 17.66 ± 1.6 |
| HT | 51.92 ± 3.4 | 13.48 ± 0.4 |
| TOLEDO | 30.97 ± 2.7 | 13.08 ± 1.6 |
| NU-DHL-1 | 54.75 ± 4.4 | 17.82 ± 1.3 |
| 0CI-LY-19 | 58.79 ± 3.9 | 16.12 ± 0.7 |
Values are mean ± SE of at least 6 independent determinations.
IC50 indicates the concentration of compound that causes the half-maximal inhibition of viability with respect to untreated cells.
We further analyzed the growth inhibitory effect of celecoxib and E7123 in 3 DLBCL cell lines (HT, DB, and KARPAS-422). We first explored the capacity of each compound to induce apoptosis by using Hoechst nuclear staining (Figure 1B). As expected, the percentage of apoptosis in vehicle-treated cells was negligible (< 1%). After 24-hour exposure to celecoxib, cells started to show typical signs of apoptosis (nuclear condensation and formation of apoptotic bodies). In contrast, after only 4 hours, E7123 induced nuclear condensation in most of the cells, whereas a smaller proportion of cells showed peripheral chromatin condensation or apoptotic bodies.
E7123 inhibits signaling through FA proteins and induces nuclear translocation of p130Cas earlier than celecoxib
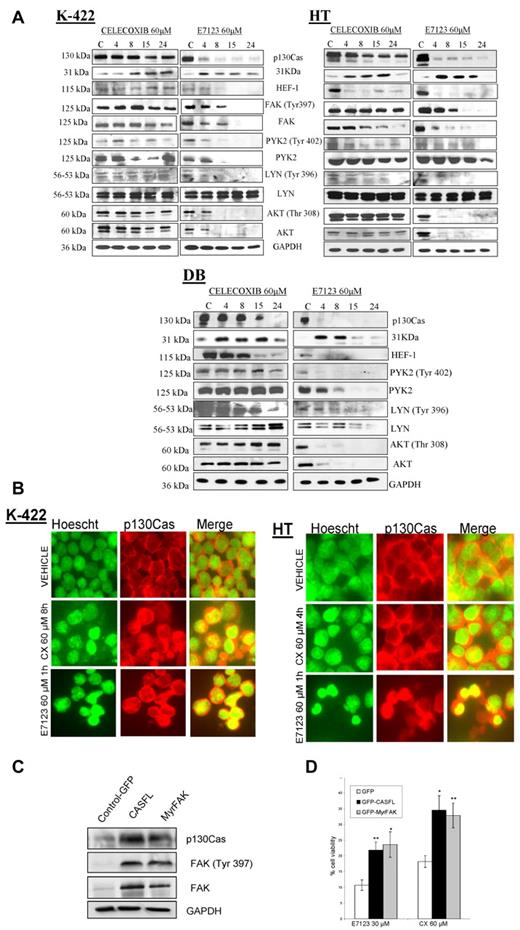
We found that all studied DLBCL cell lines showed expression and/or enhanced phosphorylation of at least one of the evaluated FA proteins (FAK, PYK2, p130Cas, HEF1, and LYN; supplemental Figure 2). We evaluated whether the mechanism of action of celecoxib or E7123 in DLBCL cells was associated with the deregulation of these proteins in HT, KARPAS-422, and DB cell lines (Figure 2A). Both compounds induced a strong reduction of full-length p130Cas after 4-hour exposure, generating a 31-kDa fragment that is a product of its proteolysis. Celecoxib reduced HEF1 expression levels in the HT cell line at 4 hours and induced LYN dephosphorylation in HT and DB cells after 8 or 15 hours of exposure. However, it did not induce any change in FAK, PYK2, or AKT expression or phosphorylation levels in any cell line. In contrast, E7123 reduced HEF-1 expression as well as FAK, PYK2, LYN, and AKT phosphorylation, after 4-hour exposure in all cell lines.
FA involvement in the mechanism of action of celecoxib and E7123 in DLBCL cell lines. (A) Immunoblot analysis of FA proteins was performed in KARPAS-422, HT, and DB cells treated with vehicle (V) or 60μM of celecoxib or E7123 for 4, 8, 15, or 24 hours. Expression levels of FAK are not shown in DB cells because they were undetectable. Equal loading was ensured by immunoblotting against GAPDH. (B) Immunolocalization of p130Cas in HT and KARPAS-422 (K-422) cell lines after treatment with 60μM celecoxib (CX) or E7123 for the period times at which we observed up-regulation of the 31-kDa fragment by Western blot (4-8 hours for celecoxib and 1 hour for E7123). p130Cas was stained with anti-p130Cas-tetramethylrhodamine isothiocyanate antibody (red, second column), the nucleus was stained with Hoechst (green, first column), and colocalization was detected by merging (yellow, third column; original magnification ×630). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Molecular analysis of DB transfectants. Equal loading was checked by immunobloting against GAPDH. (D) Alteration of cell viability in infected cells (GFP, CASFL, and MyrFAK) after 4-hour exposure to the drug. The overexpression of wild-type p130Cas and active MyrFAK partially reverted the cytotoxic effect of celecoxib and E7123 in DB cells (Mann-Whitney U test): *P < .05; **P < .01. Error bars represent SE.
FA involvement in the mechanism of action of celecoxib and E7123 in DLBCL cell lines. (A) Immunoblot analysis of FA proteins was performed in KARPAS-422, HT, and DB cells treated with vehicle (V) or 60μM of celecoxib or E7123 for 4, 8, 15, or 24 hours. Expression levels of FAK are not shown in DB cells because they were undetectable. Equal loading was ensured by immunoblotting against GAPDH. (B) Immunolocalization of p130Cas in HT and KARPAS-422 (K-422) cell lines after treatment with 60μM celecoxib (CX) or E7123 for the period times at which we observed up-regulation of the 31-kDa fragment by Western blot (4-8 hours for celecoxib and 1 hour for E7123). p130Cas was stained with anti-p130Cas-tetramethylrhodamine isothiocyanate antibody (red, second column), the nucleus was stained with Hoechst (green, first column), and colocalization was detected by merging (yellow, third column; original magnification ×630). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Molecular analysis of DB transfectants. Equal loading was checked by immunobloting against GAPDH. (D) Alteration of cell viability in infected cells (GFP, CASFL, and MyrFAK) after 4-hour exposure to the drug. The overexpression of wild-type p130Cas and active MyrFAK partially reverted the cytotoxic effect of celecoxib and E7123 in DB cells (Mann-Whitney U test): *P < .05; **P < .01. Error bars represent SE.
Based on previous reports describing that the 31-kDa fragment, a product of p130Cas cleavage, could be translocated to the nucleus to induce cell death,26 we determined whether celecoxib and E7123 could also alter the subcellular distribution of p130Cas or its 31-kDa proteolytic fragment in DLBCL cell lines. The duration of cell exposure to the compound was chosen as the time required for the detection of the 31-kDa fragment in these cells by Western blot (Figure 2A; supplemental Figure 3), being 1 hour for E7123 and 4 or 8 hours for celecoxib. Cells were exposed to each drug or vehicle, and p130Cas was immunolocalized with an antibody that recognizes both full-length p130Cas and the 31-kDa proteolyzed fragment. We stained the nuclei with Hoechst dye to facilitate the observation of the subcellular localization of p130Cas (Figure 2B). In cells exposed to vehicle, p130Cas was mainly located in the cytosol and the plasma membrane, whereas in cells treated with celecoxib or E7123, we observed nuclear staining of p130Cas, even though some cytosolic p130Cas was still present.
Exogenous overexpression of p130Cas or active FAK in DB cells reverts the antitumor effect of celecoxib and E7123
To evaluate whether p130Cas or FAK mediate the cell death induced by celecoxib or E7123, we induced wild-type p130Cas (CASFL) or constitutively active FAK (MyrFAK) overexpression by lentiviral infection in the DB cell line. First, we analyzed the expression of p130Cas and FAK as well as the tyrosine phosphorylation of FAK by Western blot in these cells (Figure 2C). As expected, the CASFL-infected DB cells showed higher levels of p130Cas expression than control cells. Similarly, the MyrFAK-infected DB cells had higher FAK expression and activation levels than control DB cells. Interestingly, we also observed up-regulation of FAK expression and phosphorylation in CASFL-infected cells and p130Cas overexpression in MyrFAK-infected cells. We treated transduced DB cells, overexpressing p130Cas, or active FAK, with celecoxib or E7123 and compared the inhibition of cell viability with control (GFP)-infected DB cells. The viability of CASFL- or MyrFAK-transduced DB cells exposed to celecoxib or E7123 was significantly higher than that of GFP-infected DB cells (Figure 2D). This result indicated that p130Cas or active FAK overexpression partially blocked celecoxib and E7123 cytotoxic effect in DB cells.
E7123 induces caspase-independent cell death mediated by induction of MPT, whereas celecoxib induces caspase-dependent apoptosis
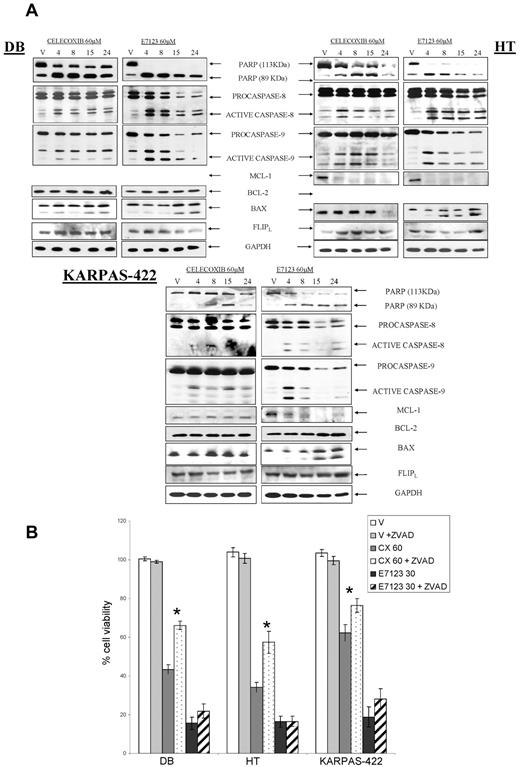
To explore the mechanism of action of celecoxib or E7123-induced cell death, we examined the activation of caspases and the proteolysis of PARP. We also assessed the regulation of the antiapoptotic Bcl-2 and Mcl-1 and the proapoptotic Bax proteins, after drug exposure (Figure 3A). Celecoxib induced proteolysis of PARP after 4, 8 or 15 hours of exposure, depending on the cell line, and activated the extrinsic and intrinsic apoptotic pathways by inducing procaspase-8 and procaspase-9 proteolysis. Celecoxib down-regulated Mcl-1 expression only in HT cells and did not induce any change in Bax expression levels. In contrast, E7123 induced PARP proteolysis, activation of both procaspases, down-regulation of Mcl-1 expression, and an increase in Bax expression at 4 hours in all cell lines. Bcl-2 and the caspase-8 inhibitor FLIPL remained unaltered after each drug exposure. We also analyzed whether the inhibition of caspases could revert the antitumor effect induced by celecoxib and E7123. When cells were pretreated with the pancaspase inhibitor Z-VAD-FMK (ZVAD), there was a significant reduction in celecoxib antitumor effect (P < .05) in all 3 DLBCL cell lines (Figure 3B). However, no significant differences in cytotoxicity were observed after exposure to E7123. Therefore, although celecoxib and E7123 induced the activation of both procaspases, E7123 antitumor activity was independent of their activation.
Signal transduction associated with apoptotic induction and effect of caspase inhibition in the antitumor effect of celecoxib or E7123 on DLBCL cell lines. (A) Immunoblot analyses of DB, HT, and KARPAS-422 cells treated with vehicle (V), 60μM celecoxib, or E7123 for different time periods (4, 8, 15, and 24 hours). Equal loading was checked by immunobotting with GAPDH. Expression levels of Mcl-1 in DB cells and Bcl- 2 in HT cells are not shown because they were undetectable. (B) Antitumor effect of 60μM celecoxib (CX) or 30μM E7123 after 4-hour exposure in DB, HT, and KARPAS-422 cells pretreated for 1 hour with the pancaspase inhibitor ZVAD at 50μM. Error bars represent SE. Statistical analysis was performed using the Mann-Whitney test: *P < .05.
Signal transduction associated with apoptotic induction and effect of caspase inhibition in the antitumor effect of celecoxib or E7123 on DLBCL cell lines. (A) Immunoblot analyses of DB, HT, and KARPAS-422 cells treated with vehicle (V), 60μM celecoxib, or E7123 for different time periods (4, 8, 15, and 24 hours). Equal loading was checked by immunobotting with GAPDH. Expression levels of Mcl-1 in DB cells and Bcl- 2 in HT cells are not shown because they were undetectable. (B) Antitumor effect of 60μM celecoxib (CX) or 30μM E7123 after 4-hour exposure in DB, HT, and KARPAS-422 cells pretreated for 1 hour with the pancaspase inhibitor ZVAD at 50μM. Error bars represent SE. Statistical analysis was performed using the Mann-Whitney test: *P < .05.
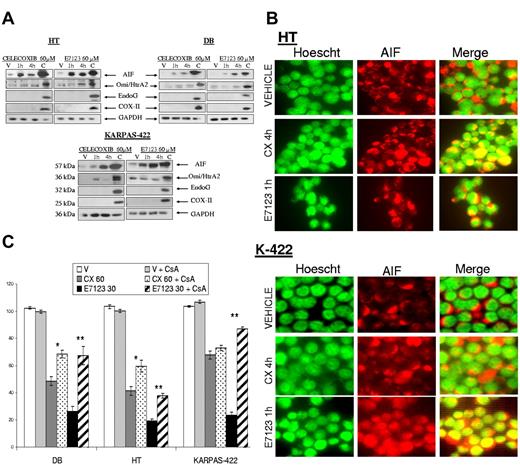
Because E7123-induced apoptosis showed no dependency on caspases, we explored whether AIF, EndoG, or Omi/HtrA2, mitochondrial effectors of caspase-independent cell death,27 contributed to the antitumor effect of E7123. These proteins must be released from the mitochondria to perform their function. Thus, we evaluated their expression levels in cytosolic extracts after vehicle or drug exposure (Figure 4A). We observed that both celecoxib and E7123 induced AIF release from the mitochondria after 1-hour exposure in all cell lines. Omi/HtrA2 expression was slightly up-regulated in KARPAS-422 cells after celecoxib treatment and in HT after E7123 exposure. EndoG remained unaltered in all cell lines after each drug exposure. Because AIF must be translocated to the nucleus to induce cell death,27 we explored AIF localization by immunofluorescence in DLBCL cells exposed to vehicle or drug (Figure 4B). AIF showed a cytosolic distribution in control vehicle-treated cells; however, when cells were exposed to celecoxib or E7123, AIF was localized mainly in the nucleus. The nuclear translocation appeared earlier in E7123 (1 hour) than in celecoxib (4 hours)–treated cells. To analyze the consequences of the inhibition of AIF release from the mitochondria in celecoxib and E7123 antitumor effect, we preincubated cells with CsA, an MPT inhibitor, before drug exposure (Figure 4C). MPT inhibition significantly (P < .01) rescued cell death induced by E7123 in all cell lines. The celecoxib effect was also blocked in HT and KARPAS-422 cell lines after CsA pretreatment (P < .05), although cell viability recovery after celecoxib exposure was lower than that observed after E7123 exposure in most cell lines.
Involvement of AIF release from the mitochondria in the antitumor effect of celecoxib or E7123 in DLBCL cell lines. (A) Immunoblot analyses of AIF, Omi/HtrA2, and EndoG in the cytosolic fraction extracts after incubating HT, DB, and KARPAS-422 cells with 60μM of compound or vehicle for 1 or 4 hours. The remaining extract containing mitochondrial and nuclear fractions of vehicle-treated cells was used as a control for the presence of the proteins in the mitochondria. The absence of COX-II expression indicated no mitochondrial contamination of the cytoplasmic fraction. GAPDH was used as a cytoplasmic fraction marker as well as a control for protein loading. (B) Immunolocalization of AIF after cell exposure to celecoxib (CX) or E7123. HT and KARPAS-422 cells were treated with vehicle, 60μM celecoxib for 4 hours, or 60μM E7123 for 1 hour. AIF was stained with anti–AIF-tetramethylrhodamine isothiocyanate antibody (red, second column), the nucleus was stained with Hoechst (green, first column), and colocalization was detected by merging (yellow, third column; original magnification ×630). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Cytotoxic effect of 60μM celecoxib or 30μM E7123 after 4-hour exposure in DB, HT, KARPAS-422 cells pretreated with 10μM of CsA, an MPT inhibitor, for 30 minutes. Error bars represent SE. Statistical analysis was performed using the Mann-Whitney test: *P < .05; **P < .01.
Involvement of AIF release from the mitochondria in the antitumor effect of celecoxib or E7123 in DLBCL cell lines. (A) Immunoblot analyses of AIF, Omi/HtrA2, and EndoG in the cytosolic fraction extracts after incubating HT, DB, and KARPAS-422 cells with 60μM of compound or vehicle for 1 or 4 hours. The remaining extract containing mitochondrial and nuclear fractions of vehicle-treated cells was used as a control for the presence of the proteins in the mitochondria. The absence of COX-II expression indicated no mitochondrial contamination of the cytoplasmic fraction. GAPDH was used as a cytoplasmic fraction marker as well as a control for protein loading. (B) Immunolocalization of AIF after cell exposure to celecoxib (CX) or E7123. HT and KARPAS-422 cells were treated with vehicle, 60μM celecoxib for 4 hours, or 60μM E7123 for 1 hour. AIF was stained with anti–AIF-tetramethylrhodamine isothiocyanate antibody (red, second column), the nucleus was stained with Hoechst (green, first column), and colocalization was detected by merging (yellow, third column; original magnification ×630). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Cytotoxic effect of 60μM celecoxib or 30μM E7123 after 4-hour exposure in DB, HT, KARPAS-422 cells pretreated with 10μM of CsA, an MPT inhibitor, for 30 minutes. Error bars represent SE. Statistical analysis was performed using the Mann-Whitney test: *P < .05; **P < .01.
To prove that ZVAD inhibited E7123-induced caspase activition, we evaluated by Western blot, PARP cleavage, and caspase activation in HT, DB, and KARPAS-422 cells exposed to vehicle or the compound with or without ZVAD. ZVAD blocked E7123-induced caspase-8 activation and PARP cleavage and partially reverted caspase-9 proteolysis (supplemental Figure 4). We also evaluated whether CsA had an inhibitory effect on caspase activity. CsA did not inhibit caspase-8 activation or PARP cleavage. However, it slightly inhibited E7123-induced procaspase-9 proteolysis, probably because cytochrome c, which is required for caspase-9 activation in the cytosol,28 cannot be released from the mitochondria as a consequence of MPT inhibition (supplemental Figure 4).
E7123-mediated caspase-independent AIF release induces peripheral chromatin condensation and large-scale DNA fragmentation
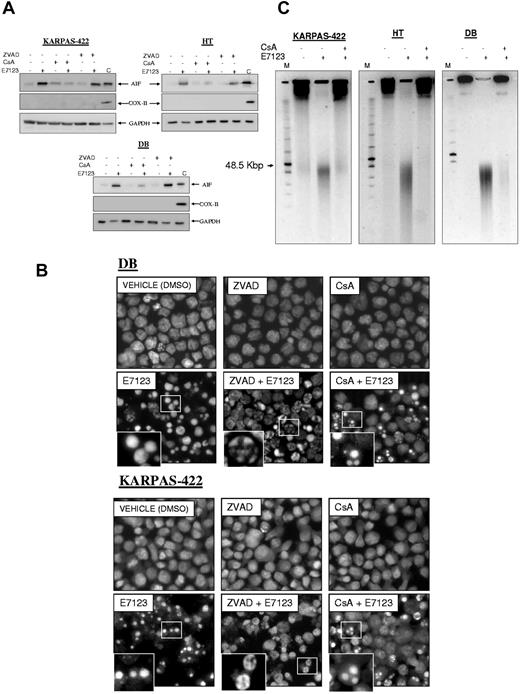
To test whether AIF release from the mitochondria was caspase-independent, we performed Western blot analysis on cytosolic extracts of DB, HT, and KARPAS-422 cells exposed to vehicle or E7123 with or without ZVAD (Figure 5A). We observed that ZVAD treatment did not block E7123-induced AIF release. Thus, AIF release from the mitochondria to the cytosol was a caspase-independent process. As expected, CsA clearly blocked AIF release to the cytosol, as a consequence of MPT inhibition.
AIF-induced nuclear condensation and large-scale DNA fragmentation of DLBCL cells exposed to E7123. (A) Immunoblot analyses of AIF in cytosolic fraction extracts of HT, DB, and KARPAS-422 cells exposed to 60μM of E7123 during 4 hours with or without pretreatment with 1 hour 50μM ZVAD or 30 minutes 10μM CsA. The remaining extract containing mitochondrial and nuclear fractions of vehicle-treated cells was used to control (C) for the presence of the evaluated proteins in the mitochondria. The absence of COX-II expression indicated no mitochondrial contamination of the cytoplasmic fraction. GAPDH was used as a cytoplasmic fraction marker, as well as a control for protein loading. (B) Hoechst staining of DB and KARPAS-422 cells after 4-hour exposure to 60μM E7123 with or without pretreatment with ZVAD 50μM for 1 hour or CsA 10μM during 30 minutes (original magnification ×400). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Pulse-field gel electrophoresis of DB, HT, and KARPAS-422 cells treated with vehicle or 60μM E7123 during 4 hours. Pretreatment with 10μM of CsA during 30 minutes blocked the 50-kb pair DNA fragments induced by E7123.
AIF-induced nuclear condensation and large-scale DNA fragmentation of DLBCL cells exposed to E7123. (A) Immunoblot analyses of AIF in cytosolic fraction extracts of HT, DB, and KARPAS-422 cells exposed to 60μM of E7123 during 4 hours with or without pretreatment with 1 hour 50μM ZVAD or 30 minutes 10μM CsA. The remaining extract containing mitochondrial and nuclear fractions of vehicle-treated cells was used to control (C) for the presence of the evaluated proteins in the mitochondria. The absence of COX-II expression indicated no mitochondrial contamination of the cytoplasmic fraction. GAPDH was used as a cytoplasmic fraction marker, as well as a control for protein loading. (B) Hoechst staining of DB and KARPAS-422 cells after 4-hour exposure to 60μM E7123 with or without pretreatment with ZVAD 50μM for 1 hour or CsA 10μM during 30 minutes (original magnification ×400). Slides were viewed with an inverted microscope (Axiovert 200M). Images were acquired using a digital camera (Coolsnap; Photometrics) and processed with MetaMorph Version 5.01 software. (C) Pulse-field gel electrophoresis of DB, HT, and KARPAS-422 cells treated with vehicle or 60μM E7123 during 4 hours. Pretreatment with 10μM of CsA during 30 minutes blocked the 50-kb pair DNA fragments induced by E7123.
Once in the cytosol, AIF enters the nucleus inducing peripheral chromatin condensation and “large-scale” DNA fragmentation (to ∼ 50 kbp) typical signs of AIF-mediated cell death.29,30 To assess E7123-induced changes in nuclear morphology, we performed Hoechst staining of DB and KARPAS-422 cells exposed to vehicle or to the drug with or without ZVAD or CsA (Figure 5B). Cells treated with E7123, without inhibitors, showed mainly nuclear condensation. Some cells showing peripheral chromatin condensation or apoptotic bodies were also detected, in accordance with our findings in Figure 1B. E7123 induced peripheral chromatin condensation in most cells, when they were pretreated with ZVAD. However, when exposed to CsA, peripheral chromatin condensation was completely absent, and these dead cells showed clear apoptotic bodies (Figure 5B)
Using pulse-field gel electrophoresis, we detected that all cell lines (KARPAS-422, HT, and DB) exposed to E7123 developed large-scale DNA fragmentation to ∼ 50 kbp (Figure 5C). These E7123-induced DNA fragments were inhibited by blocking AIF release from the mitochondria with CsA.
Thus, E7123 induced peripheral chromatin condensation, especially when caspase activity was inhibited, and “large-scale” DNA fragmentation, suggesting that the caspase-independent cell death induced by this compound in DLBCL is mediated by AIF.
E7123 induces tumor growth suppression in a xenograft model of DLBCL
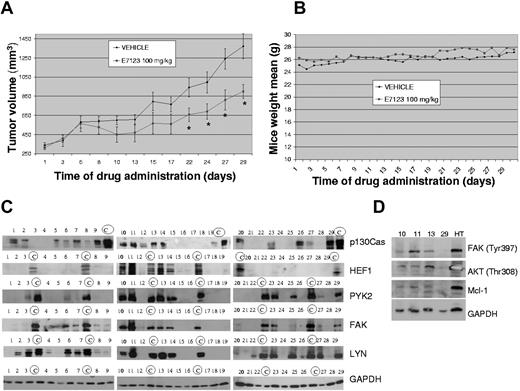
To determine the E7123 in vivo antitumoral effect, we generated a xenograft model of DLBCL by subcutaneous injection of DB cells. Swiss-nude mice were inoculated with 10 × 106 DB cells per flank and monitored weekly until the formation of tumors (20-24 days). They were then treated orally with 100 mg/kg of E7123 or vehicle. We used PEG-400:FBS 3:1 as a vehicle because it showed no toxicity in healthy mice and permitted the total solubilization of E7123 without changing its antitumor activity in vitro (data not shown). After 22 days of E7123 treatment, a significant tumor growth suppression was observed compared with vehicle treatment (P < .05). The effect was maintained until the 30th day of administration, when mice were killed (Figure 5A). To assess E7123 toxicity in vivo, body weight of mice administered with vehicle or E7123 was monitored daily. No weight loss was observed in mice exposed to E7123 compared with those exposed to vehicle (Figure 5B).
Most DLBCL patient samples show expression of FA proteins and activation of downstream signaling
To evaluate whether inhibition of FA signaling by E7123 could be a therapeutic approach in DLBCL, we studied the expression levels of p130Cas, HEF1, FAK, PYK2, and LYN in 29 DLBCL patient samples by Western blot (Figure 6A). We observed that 76% of the patient samples showed expression of at least one of the studied FA proteins (Figure 6A). p130Cas, LYN, and FAK were the most expressed proteins, representing 58%, 55%, and 51%, respectively, of the evaluated samples. However, a lower percentage of patient samples, 34% and 13%, showed expression of PYK2 and HEF1, respectively. Because of the lack of biopsy material, only 4 of these samples were further evaluated to assess activation of the pathway inhibited by E7123 (Figure 6B). We observed expression of p-FAK and the downstream proteins p-AKT and Mcl-1 in 3 of the 4 samples.
Antitumor activity of E7123 in an in vivo xenograft model and FA protein signaling in DLBCL patient samples. (A) Swiss-nude mice were injected in the flank with 10 × 106 HT cells and treated with vehicle or 100 mg/kg of E7123 as described in “Animal experiments.” Tumor volumes were plotted against days of treatment. A significant reduction of tumor volume can be observed after 22 doses. Error bars represent SD. Statistical analysis was performed using the Mann-Whitney test. *P < .05. (B) Weights of each mouse were monitored daily, and the mean weight of each group was plotted against days of treatment to assess E7123 toxicity. (C) Immunoblot analyses of p130Cas, HEF1, FAK, PYK2, and LYN in whole protein extracts of 29 DLBCL patient samples. Positive control samples for each particular protein are marked with a circled “C.” GAPDH was used as a control for protein loading. (D) Immunoblot of phosphorylated FAK, AKT, and Mcl-1 on 4 DLBCL patient samples. GAPDH was used as a control for protein loading.
Antitumor activity of E7123 in an in vivo xenograft model and FA protein signaling in DLBCL patient samples. (A) Swiss-nude mice were injected in the flank with 10 × 106 HT cells and treated with vehicle or 100 mg/kg of E7123 as described in “Animal experiments.” Tumor volumes were plotted against days of treatment. A significant reduction of tumor volume can be observed after 22 doses. Error bars represent SD. Statistical analysis was performed using the Mann-Whitney test. *P < .05. (B) Weights of each mouse were monitored daily, and the mean weight of each group was plotted against days of treatment to assess E7123 toxicity. (C) Immunoblot analyses of p130Cas, HEF1, FAK, PYK2, and LYN in whole protein extracts of 29 DLBCL patient samples. Positive control samples for each particular protein are marked with a circled “C.” GAPDH was used as a control for protein loading. (D) Immunoblot of phosphorylated FAK, AKT, and Mcl-1 on 4 DLBCL patient samples. GAPDH was used as a control for protein loading.
Discussion
In this paper, we found that the celecoxib derivative E7123 showed in vitro and in vivo antitumor activity against human DLBCL cell lines. Its novel mechanism of action includes deregulation of FA and induction of caspase-independent cell death.
E7123 inhibited FA signaling in DLBCL cell lines. It induced dephosphorylarion of FAK, PYK2, and LYN, down-regulation of HEF1, and proteolysis of p130Cas in a 31-kDa fragment. The nuclear translocation of this fragment may contribute to E7123-induced cell death because, in the nucleus, the 31-kDa fragment induces apoptosis by repressing E2A transcription factors.26 E2A proteins are highly expressed in B cells and play a critical role in B-cell development,31 giving additional support to our finding. We also demonstrated that p130Cas and active FAK mediate E7123 activity because their overexpression in a DLBCL cell line significantly blocked E7123-induced cytotoxicity. This finding is consistent with our previous observation that p130Cas overexpression blocks the cytotoxic activity of celecoxib in colon carcinoma cells.21 Similar observations have been reported in other malignant cells overexpressing p130Cas9,32 or FAK33 after different agent exposure. Our finding that most DLBCL patient samples showed expression of at least one of the studied FA proteins indicates that E7123 may be effective in patients. Interestingly, normal B cells express p130Cas,18 HEF1,18 FAK,19 PYK2,34 and LYN,35 which become phosphorylated after B-cell receptor activation and contribute to the receptor signaling. Because chronic active B-cell receptor signaling is a pathogenic mechanism in a large number of DLBCLs,36 FA proteins may also be required for DLBCL survival. Indeed, other authors have observed FAK expression14 or LYN phosphorylation15 in most DLBCL patients.
E7123 induced caspase-independent cell death. This mechanism of cell death induction is essentially different from that of most current therapies for DLBCL. Almost all anticancer drugs currently used for DLBCL treatment induce cell death by activating the intrinsic caspase-9–dependent apoptosis pathway.5 However, processes that occur during DLBCL transformation inhibit these apoptotic cascades, provoking intrinsic resistance to chemotherapy-induced cell death.37 Caspase-9 apoptosis, for example, can be inhibited by defective p53 activation or Bcl-2 protein overexpression (Bcl-2 or Mcl-1), whereas caspase-8 is blocked by death receptor mutations or c-FLIP overexpression in DLBCL.5,37 For this reason, experimental therapies presently undergoing clinical trials for chemotherapy-refractory DLBCLs are aimed at restoring caspase-9– or caspase-8–dependent apoptosis.5 E7123, in contrast, is capable of inducing cell death by a mechanism that avoids these 2 major apoptotic pathways, representing a possible alternative to treat those DLBCL patients resistant to current therapies.
The caspase-independent cell death induced by E7123 could be the consequence of the FA signaling inhibition. We showed that E7123 induced inhibition of AKT activation and down-regulation of Mcl-1, simultaneously with up-regulation of Bax, in DLBCL cells. This finding is consistent with the report by Woods et al38 that Mcl-1 inhibition induces Bax activation and anoikis in metastatic lung cancer cells. Moreover, activation of AKT stabilizes Mcl-1 and prevents permeabilization of the outer mitochondrial membrane in murine pro-B cells,39 whereas its inhibition results in a conformational change in Bax and the induction of caspase-independent cell death.40 On the other hand, FA signaling is closely linked to AKT phosphorylation in several malignancies.41,42 These observations support the argument that, in our in vitro DLBCL model, inhibition of FA proteins may induce Mcl-1 down-regulation and Bax activation through AKT dephosphorylation, leading to MPT and the subsequent AIF release, which, in turn, triggers caspase-independent cell death. In 3 of the 4 DLBCL patient samples we could evaluate, we found FAK and AKT phosphorylation as well as Mcl-1 expression, suggesting that the pathway inhibited by E7123 could be activated in these patients. However, we will need to further explore this pathway in a larger number of patient samples to estimate which percentage of DLBCL patients could be candidates for E7123 treatment. E7123 inhibition of AKT and Mcl-1 could have clinical relevance because both proteins are associated with poor survival in DLBCL patients.43,44 Moreover, the mechanism of cell resistance to rituximab is associated with up-regulation of Mcl-1 and AKT45 ; and, in some lymphoid malignancies, down-regulation of Mcl-1 enhances the therapeutic effect of this monoclonal antibody.46 Interestingly, E7123 showed a potent antitumor activity against HT, a cell line that has been described as rituximab-resistant.45 Altogether, these findings could provide a rational basis for further studying E7123, either alone or in combination with the established chemotherapeutic agents in DLBCL.
The fact that this drug can be formulated for oral administration is an important step toward its possible future use in DLBCL patients. E7123 induced tumor growth retardation in a mouse model of DLBCL after its oral administration, and it was tolerated without toxicity. Interestingly, the E7123 parent compound, celecoxib, in combination with cyclophosphamide, has shown activity in phase 2 studies with primary relapsed DLBCL,47,48 although close surveillance for arterial and venous thrombotic events has been recommended. These cardiovascular side effects are associated with COX-2 inhibition.49 Interestingly, E7123 showed a significantly higher antitumor activity against DLBCL cells than celecoxib, but it does not inhibit COX-2. Thus, it is probably effective without showing cardiovascular toxicity in its clinical use in DLBCL patients.
In conclusion, E7123 is a new, well-tolerated, and orally bioavailable therapeutic agent that has in vitro and in vivo activity against DLBCL cells. Its mechanism of action differs from that of most therapies currently in clinical use for DLBCL. We consider E7123 merits further investigation as findings to date suggest it may be useful for DLBCL treatment, especially for chemotherapy-resistant or relapsing DLBCL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mónica Gómez and Luis Carlos Navas for their technical support and Carolyn Newey for editing the English (they were supported by funding from Institut d'Investigacions Biomèdiques Sant Pau) as well as the Microbiology Department of Hospital de Sant Pau for technical support with pulse-field gel electrophoresis.
This work was supported by the Instituto de Salud Carlos III (FIS PS09/00965, R.M.; FIS PI080672, J.S.; FI08/00007, R.B.; and Sara Borrell CD09/00014, R.D.-G.), Fundación de Investigación Médica Mútua Madrileña (2006-168, R.M.; MiCINN PIB2010BZ-00563, R.M.; Networking Research Center on Bioengineering, Biomaterials and Nanomedicine NanoCoMets, R.M.; and Marató TV3 100830), Fundación José Carreras, and Fundació d'Investigació Sant Pau.
Authorship
Contribution: R.B. designed and performed research and wrote the manuscript; R.D.-G. and M.V.C. helped to perform in vivo experiments; M.P. helped to perform lentiviral experiments; M.A.P. performed statistical analysis; J.S. and A.G. analyzed and interpreted data; and R.M. and I.C. designed the research, supervised the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ramon Mangues, Grup d'Oncogènesi i Antitumorals, Institut d'Investigacions Biomèdiques Sant Pau, Pavelló 19 1r pis, Ave Sant Antoni Maria Claret 167, 08025 Barcelona, Spain; e-mail: rmangues@santpau.cat.
References
Author notes
R.M. and I.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal