Abstract
ZAP-70 in chronic lymphocytic leukemia (CLL) has been associated with enhanced B-cell receptor (BCR) signaling, survival, and migration. We investigated whether ZAP-70 can directly govern migration and the underlying mechanisms. In the ZAP-70 stably transfected Ramos cell line, IgM stimulation, but no IgD, enhanced phosphorylation of ERK1/2, Akt and Syk, and delayed IgM and CD79b internalization. In contrast, in the Raji cell line, where ZAP-70 was constitutively phosphorylated, ERK1/2, but not Akt, was phosphorylated, suggesting that MAPK pathway mediates ZAP-70 effects. BCR stimulation modulated the expression of CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L, which were up-regulated in ZAP-70–positive CLL primary subclones. The most dramatic change after BCR engagement in ZAP-70-transfected cells was CCR7 up-regulation, this being impaired by ERK1/2 inhibition and translating into both increased signaling and migration toward CCL21. Primary CLL subclones with high ZAP-70 expression showed increased migration toward CCL21. In conclusion, ZAP-70 ectopic expression led to enhanced BCR signaling after IgM stimulation and increased the expression of CCR7 predominantly via ERK1/2, increasing the response and migration toward CCL21. In primary CLL samples, cellular subsets with high ZAP-70 expression had increased expression of adhesion molecules and chemokine receptors in addition to an enhanced ability to migrate toward CCL21.
Introduction
ZAP-70 protein is a 70-kDa member of the Syk family of protein tyrosine kinases that was first identified as a crucial element for proximal signaling from the T-cell receptor.1 Similar to Syk protein after the B-cell receptor (BCR) stimulation, ZAP-70 is recruited to the phosphorylated immunoreceptor tyrosine activation motifs of the ζ- and CD3-chains present in the T-cell receptor, where it subsequently becomes phosphorylated and initiates several signaling cascades.2 Expression of ZAP-70 protein was considered to be restricted to T lymphocytes and natural killer cells. However, it has also been found expressed in normal B-cell precursors and in some subsets of activated B cells.3-6 Among B cell–derived malignancies, ZAP-70 is mainly expressed in chronic lymphocytic leukemia (CLL; 37%-57% of cases),7-9 B-acute lymphoblastic leukemia (B-ALL; 56%-59% of cases)4,10 and Burkitt lymphoma (8%-31% of cases).11,12
An increased ZAP-70 expression in CLL has been associated with particular adverse biologic features, such as the presence of unmutated IgHV genes7 or high CD38 expression,13 and correlates with a poor clinical outcome.7-9 In the same vein, ZAP-70 expression in B-ALL correlates with a short survival as well.10 Although ZAP-70 expression in B-cell malignancies has an adverse prognostic influence, its role in the biology of the tumoral B cell is not fully defined. In this regard, the expression of ZAP-70 protein in CLL cells has been related to an enhanced BCR signaling.13-17 In addition, increased ZAP-70 expression has been associated with increased migration capabilities of CLL cells toward different chemokines, such as CXCL12, CCL19, and CCL21,18-20 and with increased signaling and survival on CXCL12 treatment.18,21 However, whether these increased migrative capabilities are a direct effect of ZAP-70 expression or a mere reflection of the distinct biology features of ZAP-70–expressing cells needs to be further investigated.
To ascertain the direct implication of ZAP-70 in B-cell signaling and migration, we analyzed the phenotypic effects of ectopic ZAP-70 expression in a B-cell system and studied the expression of adhesion molecules and chemokine receptors in CLL primary cells with high or low ZAP-70 expression within the same patient. Herein, we report that IgM, but not IgD, stimulation mobilizes and activates ZAP-70, which in turn enhances BCR-induced ERK1/2 and Akt phosphorylation and delays IgM and CD79b internalization. Moreover, we show that ZAP-70 induces the expression of the chemokine receptor CCR7 via ERK1/2 activation, thus directly enhancing the capacity of signaling and migration of the ZAP-70–expressing B cells toward CCL21. Finally, we show that CLL cells with higher ZAP-70 expression within the same patient have a different expression profile of adhesion molecules and chemokine receptors and enhanced migration capacity toward CCL21.
Methods
Cell lines and primary cells
The Burkitt lymphoma B-cell lines Raji and Ramos were obtained from ATCC and were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2mM l-glutamine, and 1mM sodium pyruvate at 37°C in a 5% CO2 atmosphere.
Mononuclear cells from peripheral blood of patients with CLL were obtained by Ficoll-Paque Plus (GE Healthcare) density gradient from 40 patients after informed consent. This procedure was done following the requirement of the local clinical investigation ethical committee and the principles of the Declaration of Helsinki.
Constructs, transfections, and stable cell line generation
The GFP-ZAP-70 expression vector (pEGFP-N2ZAP-70) was generated by subcloning the full-length human ZAP-70 coding sequence from the SRαpuroZAP-70 plasmid (kindly provided by Dr V. Di Bartolo, Institut Pasteur, Paris, France) into the pGlow-TOPO vector (Invitrogen). The ZAP-70 fragment was then subcloned into the EcoRI site at the polylinker of the mammalian expression vector pEGFP-N2 (Clontech), which allowed the expression of a GFP-ZAP-70 fusion protein (97 kDa).
Raji and Ramos cells were stably transfected with plasmids expressing either GFP-ZAP-70 fusion protein or GFP only as a control. For this, cells were resuspended in 200 μL Optimix electroporation buffer (Thermo Hybaid), electroporated (150 μF/300 V), subsequently selected for the presence of the plasmids in standard growth medium containing 1.2 mg/mL of G418 (Invitrogen), and further sorted by GFP expression.
Immunoblotting
BCR was stimulated with 5 μg/mL F(ab′)2 anti-IgM (Invitrogen), or anti-IgD (Southern Biotechnology). CCR7 was stimulated using synthetic human CCL21 (PeproTech). ERK1/2 and Akt were inhibited with PD98059 (Cell Signaling Technology) and LY294002 (Sigma-Aldrich), respectively. Cell lysates from Ramos and Jurkat cells treated with phosphatase-inhibitor pervanadate (3mM H2O2/1mM NaVO4) for 5 minutes at 37°C were used as positive controls for phospho-proteins. Cells were lysed for 30 minutes at 4°C in 100 μL lysis buffer (20mM Tris pH 7.4, 1mM EDTA, 140mM NaCl, 1% NP-40 supplemented with 2mM sodium vanadate and 1 times protease inhibitor cocktail; Sigma-Aldrich). Protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad). Equal amounts of denatured protein were resolved by 10% SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Membranes were blocked for 1 hour at room temperature in 5% milk/TBST. Membranes were incubated overnight with primary antibodies for phospho-ZAP-70Tyr319/SykTyr352, phospho-AktSer473, phospho-ERK1/2Thr202/Tyr204, Akt, and ERK1/2 (Cell Signaling Technology), ZAP-70 (clone 2F3.2; Upstate Biotechnology), Syk (Santa Cruz Biotechnology), and GAPDH (Abcam). Immunodetection was done with antirabbit or antimouse IgG HRP-linked antibodies (Dako North America) and the ECL chemiluminescence detection system (GE Healthcare). Chemiluminescent images were acquired with the LAS-3000 system and analyzed using Image Gauge Version 4.0 software (Fuji Photo Film).
Flow cytometry
Cell surface antigens were detected using the following fluorochrome-labeled antibodies: IgM-phycoerythrin (PE), IgD-PE, CD19-PE–Texas Red, CD5-PE-cyanine 5.5, CXCR3-allophycocyanin (APC), CXCR4-APC, CD44-APC, CD49d-APC, CD62L-APC (BD Biosciences), CCR7-APC, CXCR5-APC (R&D Systems), CD79b-PE, and CD3-PE-cyanine 7 (Beckman Coulter). For intracellular ZAP-70 detection in primary CLL cells, IntraSure kit and primary antibody anti–ZAP-70-PE (BD Biosciences) were used. Cells were acquired in a Navios cytometer (Beckman Coulter), and the results were analyzed using FCS Express Version 4 software (De Novo Software).
Confocal microscopy
Cells were seeded at a density of 1.2 × 106 cells/mL in standard growth media on poly-L-lysine–coated glass coverslips for 30 minutes at 37°C and mounted in an Attoflour chamber (Invitrogen). The chamber was placed under a Leica TCS SP5 confocal microscope (Leica Microsystems), and all images were acquired with a 63× glycerol immersion objective lens. Cells were stimulated with 20 μg/mL F(ab′)2 anti-IgM, or IgD at 37°C and 5% CO2. Anti–human IgM-PE or IgD-PE was used to detect the receptor on surface. Image treatment was performed using the Image Processing Leica Confocal and ImageJ Version 1.37 software (Wayne Rasband, National Institutes of Health).
Calcium flux measurement
For calcium mobilization measurement, cells were washed 3 times with loading buffer (HBSS, 10mM D-glucose, 1.3mM CaCl2, 1.1mM MgCl2) and incubated with 2μM Fluo-4 AM (Invitrogen) in the presence of 0.02% Pluronic F-127 (Invitrogen). Afterward, cells were washed twice with loading buffer, resuspended in RPMI with 25mM HEPES, and kept at room temperature for 15 minutes; 250 ng/mL of CCL21 was added to the samples, and the mean fluorescence intensity (MFI) was recorded every 40 seconds for 11 minutes by flow cytometry in a FACSCalibur cytometer.
Chemotaxis assay
Chemotaxis assays were performed across bare polycarbonate membranes. Briefly, for cell lines, a total of 100 μL containing 5 × 105 cells was added to the top chamber of a 6.5-mm-diameter transwell culture insert with a pore size of 5 μm (Corning). Filters were then transferred to wells containing 600 μL of standard growth medium with or without 1 μg/mL CCL21. Cells were allowed to migrate for 15 hours at 37°C in 5% CO2. Transmigrated cells in the lower chamber were resuspended and collected for counting with a FACSCalibur cytometer under a defined flow rate for 5 minutes. The migration index was calculated as the number of cells transmigrating with chemokine divided by the number of transmigrating cells in the absence of chemokine. Chemotaxis assays of primary CLL cells from 7 patients were performed, adding 400 μL of RPMI-0.5% BSA containing 1.5 × 107 cells to the top chamber of a 24-mm-diameter transwell culture insert. Cells were allowed to migrate toward media containing 1 μg/mL CCL21 for 6 hours, and the percentage of CLL cells expressing ZAP-70 was then determined in both upper and lower chambers by flow cytometry.
Statistical analysis
Results are shown as mean ± SEM of at least 3 replicates. For statistical comparisons between groups, the Mann-Whitney test was used, and P < .05 was considered significant. Paired-sample parametric test (t test) was used to compare the differential expression of molecules between the ZAP-70 high and low CLL subpopulations within the same patient. Analyses were performed using the biostatistics software package SPSS Version 17 (IBM). Results were graphed with GraphPad Prism Version 5.0.
Results
Intensity and duration of Akt and ERK1/2 phosphorylation are enhanced in ZAP-70–expressing B cells after IgM, but not IgD, stimulation
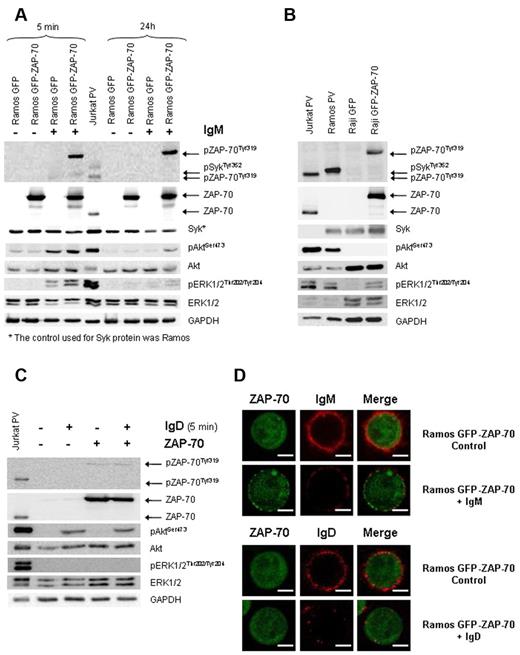
To investigate the phenotypic effects of ZAP-70 expression in B cells, we stably transfected the Burkitt cell lines Ramos and Raji with the pEGFP-N2ZAP-70 vector or the control vector pEGFP-N2. The expression of GFP protein could be detected by flow cytometry in all stable transformants, whereas ZAP-70 expression was only detected in Ramos and Raji stably transfected with the GFP-ZAP-70 expression vector. Proper expression of the 97-kDa fusion protein was verified by Western blot using anti–ZAP-70 antibody in both ZAP-70–transfected B-cell lines (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Although most CLL cells coexpress surface IgM and IgD,22 there is much evidence that BCR stimulation can lead to different signaling depending on whether IgM or IgD is stimulated.23 Remarkably, almost all CLL cases respond to IgD ligation, whereas usually only CLL cells expressing unmutated IgHV genes respond to IgM.22,24-26 In this regard, the expression of ZAP-70 protein in CLL has been related to an enhanced signaling through the BCR on IgM stimulation.13-17 However, the role of ZAP-70 in IgD-BCR signaling has not been investigated. Therefore, we analyzed the effects on BCR signaling of surface IgM and IgD stimulation according to the presence of ZAP-70. First, Ramos cells were stimulated with 5 μg/mL F(ab′)2 anti-IgM for 5 minutes and 24 hours, and afterward the phosphorylation of key mediators of BCR signaling was examined by Western blot. In ZAP-70 stable transfectants, we found phosphorylation of ZAP-70 at activating tyrosine Tyr319 along with an increased intensity and duration of the BCR-induced phosphorylation of Syk, Akt, and ERK1/2 kinases (Figure 1A). The selected Raji cell line lacked surface IgM and IgD and constitutively expressed phosphorylated ZAP-70 at Tyr319 after transfection. Remarkably, in this cell line, we observed that ERK1/2 protein, but no Akt protein, was also constitutively phosphorylated (Figure 1B). Altogether, these results confirm the participation of ZAP-70 in the IgM-BCR signaling; they indicate that ZAP-70 is able to activate the MAPK pathway independently of BCR stimulation, and suggest that Akt activation may require BCR stimulation.
ZAP-70 enhances IgM-BCR signaling but does not participate in IgD-BCR signaling. (A) Ramos stable transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgM for 5 minutes and 24 hours. Enhanced Syk, Akt, and ERK1/2 phosphorylation was observed in IgM-BCR stimulated ZAP-70–expressing cells. (B) Immunoblotting analysis of Raji transfectants expressing constitutive phosphorylated ZAP-70 and ERK1/2. Ramos and Jurkat cells treated with pervanadate (PV) were used as positive controls. (C) Ramos stable transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgD for 5 minutes. On IgD stimulation, activation of Akt, but not of ERK1/2 or ZAP-70, was observed. Jurkat cells treated with PV were used as positive control. (D) Confocal microscopy (original magnification ×63). Ramos GFP-ZAP-70 cells were activated with 20 μg/mL F(ab′)2 anti-IgM or IgD for 40 minutes and then stained with anti–IgM-PE or anti–IgD-PE. ZAP-70 was translocated from the cytoplasm to the membrane and remained on surface after IgM activation while IgM internalization was almost complete (top panel). In contrast, while IgD was also internalized, no mobilization of ZAP-70 was observed after IgD stimulation (bottom panel). The scale bar in the image represents 5 μm.
ZAP-70 enhances IgM-BCR signaling but does not participate in IgD-BCR signaling. (A) Ramos stable transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgM for 5 minutes and 24 hours. Enhanced Syk, Akt, and ERK1/2 phosphorylation was observed in IgM-BCR stimulated ZAP-70–expressing cells. (B) Immunoblotting analysis of Raji transfectants expressing constitutive phosphorylated ZAP-70 and ERK1/2. Ramos and Jurkat cells treated with pervanadate (PV) were used as positive controls. (C) Ramos stable transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgD for 5 minutes. On IgD stimulation, activation of Akt, but not of ERK1/2 or ZAP-70, was observed. Jurkat cells treated with PV were used as positive control. (D) Confocal microscopy (original magnification ×63). Ramos GFP-ZAP-70 cells were activated with 20 μg/mL F(ab′)2 anti-IgM or IgD for 40 minutes and then stained with anti–IgM-PE or anti–IgD-PE. ZAP-70 was translocated from the cytoplasm to the membrane and remained on surface after IgM activation while IgM internalization was almost complete (top panel). In contrast, while IgD was also internalized, no mobilization of ZAP-70 was observed after IgD stimulation (bottom panel). The scale bar in the image represents 5 μm.
To elucidate whether ZAP-70 is also involved in IgD-BCR signaling, Ramos transfectants were stimulated with 5 μg/mL goat F(ab′)2 anti-IgD for 5 minutes. In contrast to what we observed after IgM stimulation, IgD did not induce phosphorylation of ZAP-70 or ERK1/2. However, IgD stimulation induced Akt phosphorylation, which was not enhanced by the presence of ZAP-70 (Figure 1C). Further differences were observed by confocal microscopy after IgM or IgD stimulation of ZAP-70–expressing Ramos cells. After 5 minutes of F(ab′)2 anti-IgM stimulation, we observed mobilization of ZAP-70 from the cytoplasm to the membrane, where it was distributed forming patches (Figure 1D, supplemental Figure 2, supplemental Videos 1-3). Remarkably, 40 minutes later, when the internalization of IgM was almost complete, ZAP-70 still remained associated with the membrane. In contrast, and in accordance with the previous results, BCR stimulation by IgD did not affect the intracellular localization of ZAP-70, thus confirming the lack of ZAP-70 activation on IgD stimulation (Figure 1D). Taken together, these results indicate that the differences between IgD and IgM signaling described in CLL cells could be explained in part by the fact that ZAP-70 has no influence in IgD signaling.
ZAP-70 delays CD79b and IgM internalization after BCR stimulation
The stimulation of the BCR is followed by the activation of several signaling cascades and by a fast internalization of the complex, so that the antigen can be processed and presented to T cells.27 Furthermore, under some conditions of antigen binding, the immunoglobulin is physically uncoupled from the signaling complex formed by CD79a/b molecules,28 being these signaling complexes preferentially retained in the membrane.29 The fact that ZAP-70 binds to CD79a/b phosphorylated immunoreceptor tyrosine activation motifs,13 along with our observation that ZAP-70 remained associated with the membrane long after IgM was internalized, prompted us to study the internalization kinetics of surface immunoglobulin and of CD79b on BCR stimulation. For this, we analyzed by flow cytometry the expression of IgM and CD79b on surface at 10 and 60 minutes after BCR stimulation in Ramos stable transfectants. After 60 minutes of BCR stimulation, Ramos GFP internalized 53% ± 1.9% of surface IgM and 29.4% ± 2.9% of CD79b, whereas in Ramos GFP-ZAP-70 cells the internalization was significantly delayed, with a 25.7% ± 0.7% of IgM and 4.7% ± 1.5% of CD79b being removed from surface (Figure 2A). These phenomena were further confirmed by confocal microscopy where, after F(ab′)2 anti-IgM stimulation at 10, 30, and 60 minutes, we observed a stronger surface IgM staining in the Ramos GFP-ZAP-70 cells, indicating that the internalization of the IgM was slower when ZAP-70 was present (Figure 2B). Overall, these results show that after IgM engagement the BCR complex is uncoupled and that the different components are internalized at different rates. In the presence of ZAP-70, the internalization of both IgM and CD79b is significantly delayed.
ZAP-70 activation delays IgM and CD79b internalization. (A) Stimulation of Ramos transfectants was performed with 5 μg/mL F(ab′)2 anti-IgM for 10 and 60 minutes at 37°C and stopped at 4°C. Density of IgM and CD79b on surface was analyzed by flow cytometry at the indicated time points with IgM-PE and CD79b-PE antibodies (top panel). The internalization kinetics of IgM differed from that of CD79b. The expression of ZAP-70 delayed the internalization of both IgM and CD79b (bottom panel). (B) Confocal microscopy (original magnification ×63). Ramos transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgM, and sequential images at 0, 10, 30, and 60 minutes after IgM-PE labeling were obtained. Density of surface IgM in Ramos GFP-ZAP-70 cells was higher than in Ramos GFP cells. Scale bar represents 5 μm.
ZAP-70 activation delays IgM and CD79b internalization. (A) Stimulation of Ramos transfectants was performed with 5 μg/mL F(ab′)2 anti-IgM for 10 and 60 minutes at 37°C and stopped at 4°C. Density of IgM and CD79b on surface was analyzed by flow cytometry at the indicated time points with IgM-PE and CD79b-PE antibodies (top panel). The internalization kinetics of IgM differed from that of CD79b. The expression of ZAP-70 delayed the internalization of both IgM and CD79b (bottom panel). (B) Confocal microscopy (original magnification ×63). Ramos transfectants were stimulated with 5 μg/mL F(ab′)2 anti-IgM, and sequential images at 0, 10, 30, and 60 minutes after IgM-PE labeling were obtained. Density of surface IgM in Ramos GFP-ZAP-70 cells was higher than in Ramos GFP cells. Scale bar represents 5 μm.
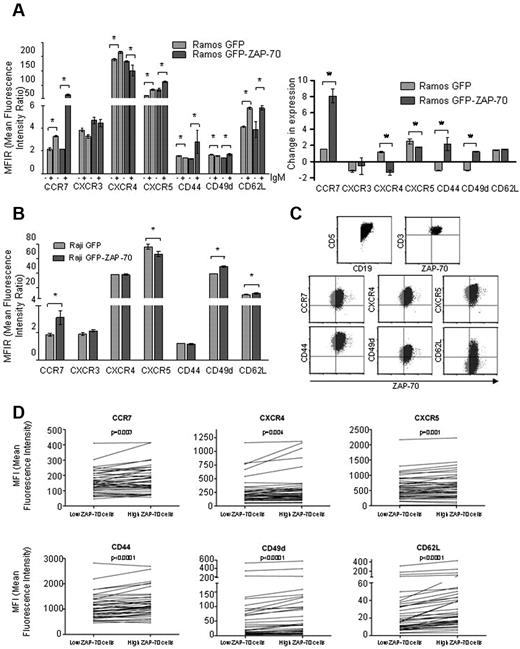
ZAP-70 signaling induces the expression of CCR7 in B cells through ERK1/2 phosphorylation
Along with enhanced BCR signaling, the expression of ZAP-70 in primary CLL cells has been correlated with an increased response to survival and migratory signals in vitro.18-21 Moreover, it has been previously described that patients with CLL with high ZAP-70 expression (> 20%) have an overall increase in expression of CCR7.18 Despite that, the mechanisms by which ZAP-70 is controlling the migration processes are not well defined. To analyze the influence of ZAP-70 in the expression of chemokine receptors and adhesion molecules, we studied CCR7, CXCR3, CXCR4, CXCR5, CD44, CD49d, and CD62L surface levels by flow cytometry after ZAP-70 activation by BCR stimulation. The stimulation of the BCR in Ramos GFP cells significantly increased the levels of CCR7, CXCR4, CXCR5, and CD62L, whereas we observed a modest down-regulation of CD44 and CD49d and no change of CXCR3 after 16 hours (Figure 3A; supplemental Figure 3). Moreover, BCR stimulation of Ramos GFP-ZAP-70 cells increased the expression of CCR7, CXCR5, CD44, CD49d, and CD62L and decreased the expression of CXCR4, whereas CXCR3 expression remained unmodified (Figure 3A; supplemental Figure 3). Interestingly, BCR stimulation of Ramos GFP cells increased CCR7 expression 1.5-fold (MFIR from 2.13 ± 0.12 to 3.3 ± 0.04; P = .02), whereas in Ramos GFP-ZAP-70 cells BCR stimulation enhanced the CCR7 expression 8-fold (MFIR from 2.17 ± 0.005 to 17.5 ± 0.9; P = .028), this molecule being the one with the greatest difference caused by ZAP-70 (Figure 3A right panel). In the Raji cell line, the ectopic expression and activation of ZAP-70 also translated into an increase in CCR7 (MFIR from 1.8 ± 0.09 to 3.1 ± 0.19; P = .0022), CD49d, and CD62L expression (Figure 3B) and a decrease in CXCR5 expression (MFIR from 76.3 ± 3.36 to 66.3 ± 1.79; P = .028), whereas its effect on CXCR3, CXCR4, and CD44 expression was not significant. Of note, the stimulation of the BCR through IgD engagement in Ramos cells did not modulate the levels of CCR7 regardless of ZAP-70 expression, adding more evidence to the differences observed between IgM and IgD signaling (data not shown).
ZAP-70 enhances CCR7 up-regulation after BCR activation. (A) Expression of CCR7, CXCR3, CXCR4, CXCR5, CD44, CD49d, and CD62L was assessed by flow cytometry in Ramos transfectants before and after stimulation for 16 hours with 5 μg/mL F(ab′)2 anti-IgM. Left panel: MFIR (± SEM) of expression before and after IgM stimulation. Right panel: Change of expression relative to unstimulated cells in Ramos GFP and Ramos GFP-ZAP-70. *P < .05. (B) Expression of CCR7, CXCR3, CXCR4, CXCR5, CD44, CD49d, and CD62L was assessed by flow cytometry in Raji cells with or without ZAP-70 expression. (C) ZAP-70, CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L expression was assessed by flow cytometry in peripheral blood mononuclear cells obtained from 40 patients with CLL. The expression of ZAP-70 in CD3+ gated T cells was used as internal control and allowed for the measurement of expression of chemokine receptors and adhesion molecules in CD19+/CD5+ gated CLL cells with high (black) or low (gray) ZAP-70 expression. (D) Expression of CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L was significantly higher in CLL cells with high ZAP-70 expression within the same patient.
ZAP-70 enhances CCR7 up-regulation after BCR activation. (A) Expression of CCR7, CXCR3, CXCR4, CXCR5, CD44, CD49d, and CD62L was assessed by flow cytometry in Ramos transfectants before and after stimulation for 16 hours with 5 μg/mL F(ab′)2 anti-IgM. Left panel: MFIR (± SEM) of expression before and after IgM stimulation. Right panel: Change of expression relative to unstimulated cells in Ramos GFP and Ramos GFP-ZAP-70. *P < .05. (B) Expression of CCR7, CXCR3, CXCR4, CXCR5, CD44, CD49d, and CD62L was assessed by flow cytometry in Raji cells with or without ZAP-70 expression. (C) ZAP-70, CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L expression was assessed by flow cytometry in peripheral blood mononuclear cells obtained from 40 patients with CLL. The expression of ZAP-70 in CD3+ gated T cells was used as internal control and allowed for the measurement of expression of chemokine receptors and adhesion molecules in CD19+/CD5+ gated CLL cells with high (black) or low (gray) ZAP-70 expression. (D) Expression of CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L was significantly higher in CLL cells with high ZAP-70 expression within the same patient.
To determine whether Akt or ERK1/2 pathways were involved in the induction of CCR7 expression on BCR stimulation and ZAP-70 activation, Ramos stable cell lines were stimulated with 5 μg/mL F(ab′)2 anti-IgM after 1 hour of preincubation with either the ERK1/2 inhibitor PD98059 or the Akt inhibitor LY294002 (Figure 4A). As previously observed, BCR stimulation of Ramos GFP-ZAP-70 cells increased the levels of CCR7 expression to MFIR 6.37 ± 0.28 (Figure 4B). Interestingly, after ERK1/2 inhibition by PD98059, the CCR7 up-regulation was significantly impaired, the levels raising only up to MFIR 2.5 ± 0.01 at 100μM for Ramos GFP-ZAP-70 (P = .002). The inhibition of Akt, although significant, had a biologically negligible effect on the expression of CCR7 (MFIR 5.5 ± 0.17 after LY294002 at 10μM; P = .026; Figure 4B right panel). The same pattern of inhibition was observed in Ramos GFP cells, even though the increase of CCR7 after IgM stimulation was only to MFIR 1.47 ± 0.08 (Figure 4B left panel). Taken together, these results show that the MAPK pathway has a prominent role in regulating the overexpression of CCR7 induced by IgM-activated ZAP-70.
CCR7 up-regulation is dependent on ERK1/2 activation. (A) Ramos GFP (left panel) and Ramos GFP-ZAP-70 (right panel) cells were incubated for 1 hour in the presence of 50μM or 100μM PD98059 for ERK1/2 inhibition, and in the presence of 5μM or 10μM LY294002 for Akt inhibition before 5 minutes of stimulation with 5 μg/mL F(ab′)2 anti-IgM. Inhibition of phosphorylation of Akt and ERK1/2 was confirmed by immunoblotting. Jurkat cells treated with PV were used as positive control. (B) IgM stimulation was performed for 4 hours after 1 hour of preincubation with Akt and ERK1/2 inhibitors, and the levels of CCR7 were measured by flow cytometry. Inhibition of ERK1/2 phosphorylation with PD98059 significantly reduced the IgM-mediated induction of CCR7 expression, whereas inhibition of Akt had only a minor effect on CCR7 expression. *P < .05, versus the IgM activated cells. MFIR was calculated relative to unstimulated samples.
CCR7 up-regulation is dependent on ERK1/2 activation. (A) Ramos GFP (left panel) and Ramos GFP-ZAP-70 (right panel) cells were incubated for 1 hour in the presence of 50μM or 100μM PD98059 for ERK1/2 inhibition, and in the presence of 5μM or 10μM LY294002 for Akt inhibition before 5 minutes of stimulation with 5 μg/mL F(ab′)2 anti-IgM. Inhibition of phosphorylation of Akt and ERK1/2 was confirmed by immunoblotting. Jurkat cells treated with PV were used as positive control. (B) IgM stimulation was performed for 4 hours after 1 hour of preincubation with Akt and ERK1/2 inhibitors, and the levels of CCR7 were measured by flow cytometry. Inhibition of ERK1/2 phosphorylation with PD98059 significantly reduced the IgM-mediated induction of CCR7 expression, whereas inhibition of Akt had only a minor effect on CCR7 expression. *P < .05, versus the IgM activated cells. MFIR was calculated relative to unstimulated samples.
Differential expression of adhesion molecules and chemokine receptors by ZAP-70-high and ZAP-70-low cells within CLL primary clones
As we observed that ZAP-70 can directly govern the expression of some chemokine receptors and adhesion molecules, we decided to depict the differential expression of these molecules in CLL cells expressing high and low ZAP-70 within the same patient. For this, we compared the expression by flow cytometry of CCR7, CXCR4, CXCR5, CD44, CD49d, and CD62L in ZAP-70-high versus ZAP-70-low CD19+/CD5+ gated cells from 40 CLL primary cases. The expression of ZAP-70 observed in the CD3+ T cells from each sample was used to define the threshold of high versus low ZAP-70, and then the MFI of each corresponding cellular subset was obtained for each molecule (Figure 3C). Interestingly, we found that the expression of all the adhesion molecules and chemokine receptors analyzed was significantly higher in the subset of CLL cells with higher expression of ZAP-70 (Figure 3D).
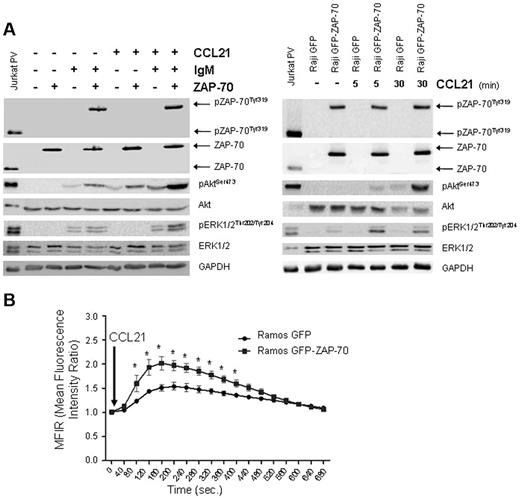
CCR7 signaling is increased in ZAP-70–expressing B-cell lines
CCR7 ligands (chemokines CCL21 and CCL19) are mainly expressed in high endothelial venules and in the T zones from secondary lymphoid organs.30,31 The interaction of CCR7 with its ligands leads to the mobilization of intracellular calcium and activation of the Akt and MAPK pathways.32 To elucidate whether the overexpressed CCR7 in ZAP-70 B cells is functional, we stimulated cells with 1 μg/mL CCL21 for 30 minutes after 4 hours of stimulation with F(ab′)2 anti-IgM (Figure 5A). In Ramos cells with BCR-activated ZAP-70, and therefore increased CCR7 expression, we observed an enhanced phosphorylation of Akt and ERK1/2 on CCL21 stimulation compared with cells with BCR activation only. Moreover, the stimulation of CCR7 after BCR stimulation in Ramos GFP-ZAP-70 cells also induced higher phosphorylation of Akt and ERK1/2 compared with Ramos GFP cells. Besides, a slight increase in Akt phosphorylation was also observed after CCL21 stimulation in Ramos expressing ZAP-70 before BCR stimulation, this suggesting that ZAP-70 could be directly participating in the CCR7 signaling pathway. Moreover, the increase in intracellular calcium concentration was also higher in B cells with activated ZAP-70 (Figure 5B). This enhanced signaling was also observed in the Raji GFP-ZAP-70 cell line, where phosphorylation of both Akt and ERK1/2 was greatly enhanced after CCR7 stimulation by CCL21 compared with Raji GFP cells (Figure 5A).
CCR7 signaling is enhanced in B cells expressing activated ZAP-70. (A) Ramos transfectants prestimulated for 4 hours with 5 μg/mL F(ab′)2 anti-IgM were stimulated with 1 μg/mL CCL21 for 30 minutes (left panel). Only in IgM stimulated Ramos ZAP-70 transfectants, CCL21 stimulation increased the phosphorylation of ERK1/2. CCL21 increased Akt phosphorylation with or without the presence of IgM stimulation. Akt phosphorylation was the highest when ZAP-70 was activated. A slight increase in Akt phosphorylation was observed in ZAP-70–expressing cells before IgM stimulation. In Raji transfectants treated with 1 μg/mL CCL21 for 5 and 30 minutes (right panel), the same enhanced signaling was observed. (B) Ramos transfectants prestimulated with 5 μg/mL F(ab′)2 anti-IgM for 4 hours were stimulated with 250 ng/mL CCL21. MFIR was calculated relative to unstimulated samples. Significant increase in calcium mobilization was observed in BCR-stimulated ZAP-70–positive cells. *P < .05.
CCR7 signaling is enhanced in B cells expressing activated ZAP-70. (A) Ramos transfectants prestimulated for 4 hours with 5 μg/mL F(ab′)2 anti-IgM were stimulated with 1 μg/mL CCL21 for 30 minutes (left panel). Only in IgM stimulated Ramos ZAP-70 transfectants, CCL21 stimulation increased the phosphorylation of ERK1/2. CCL21 increased Akt phosphorylation with or without the presence of IgM stimulation. Akt phosphorylation was the highest when ZAP-70 was activated. A slight increase in Akt phosphorylation was observed in ZAP-70–expressing cells before IgM stimulation. In Raji transfectants treated with 1 μg/mL CCL21 for 5 and 30 minutes (right panel), the same enhanced signaling was observed. (B) Ramos transfectants prestimulated with 5 μg/mL F(ab′)2 anti-IgM for 4 hours were stimulated with 250 ng/mL CCL21. MFIR was calculated relative to unstimulated samples. Significant increase in calcium mobilization was observed in BCR-stimulated ZAP-70–positive cells. *P < .05.
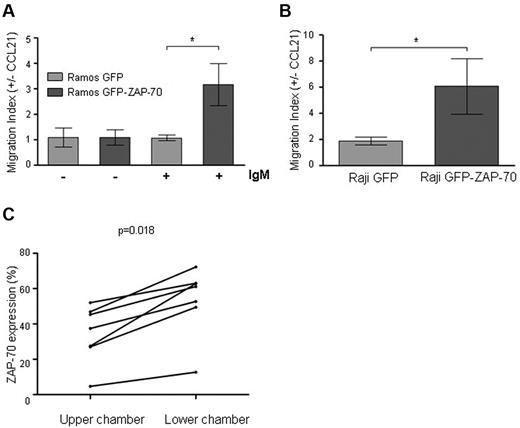
Migration toward CCL21 chemokine is increased in B cells expressing activated ZAP-70
CCR7 ligation can mediate migration and survival of tumoral cells from CLL and B-ALL.18,33-36 Importantly, the up-regulation of CCR7 alone may not be enough for CCR7-mediated migration; in this regard, it has been shown that, in monocyte-derived dendritic cells and B-ALL, additional stimuli are needed after CCR7 up-regulation for responsiveness to CLL19 or CCL21.37,38 Moreover, the levels of chemokine receptors in CLL do not always correlate with the migration toward their ligands.20,36,39 We therefore aimed to analyze whether the increased CCR7 expression and signaling observed after ZAP-70 activation did translate into an increased migrative capacity of the B lymphocytes. For this, we performed transmigration assays across bare polycarbonate transwell culture inserts placed on medium with or without CCL21. Before BCR stimulus, we did not find significant differences caused by ZAP-70 expression in the average proportion of transmigrated Ramos cells toward CCL21 chemokine (migration index: 1.09 ± 0.15 vs 1.09 ± 0.29; P = .93). Moreover, BCR stimulation of Ramos GFP cells, although inducing a significant increase in CCR7 expression (Figure 3A), did not increase the migration toward CCL21 (1.09 ± 0.15 vs 1.07 ± 0.12). In contrast, after BCR stimulation, the presence of ZAP-70 increased 2.9 times the migration index (1.07 ± 0.12 vs 3.15 ± 0.82; P = .016; Figure 6A). In accordance, the migration index of Raji GFP-ZAP-70 cells was 3.2 times higher than the one observed for Raji GFP (1.9 ± 0.3 vs 6.08 ± 2.13; P = .046; Figure 6B). These results show that, in this cell system, induction of CCR7 expression by ZAP-70 activation is a sufficient condition to increase the B-cell migrative capacity.
Migration toward CCL21 is enhanced after ZAP-70 activation. (A) Ramos transfectants either stimulated (6 hours, 5 μg/mL F(ab′)2 anti-IgM) or unstimulated, and (B) Raji transfectants were subjected to migration assay toward CCL21 (1 μg/mL) for 15 hours at 37°C in 5% CO2 atmosphere. The absolute number of transmigrated cells was determined by flow cytometry, acquiring cells under a defined flow rate. Results are expressed as migration index, calculated as the number of cells transmigrating with chemokine divided by the number of transmigrating cells toward media only. ZAP-70–activated cells showed a significantly higher migrative capacity. *P < .05. (C) Peripheral blood mononuclear cells from 7 patients with CLL were subjected to migration assay toward CCL21 (1 μg/mL) for 6 hours at 37°C in 5% CO2 atmosphere. The percentage of CD19+/CD5+ CLL cells expressing ZAP-70 was determined in the cellular fraction remaining in the upper chamber and in the cellular fraction of transmigrated cells for each patient sample by flow cytometry. CLL cells transmigrating toward CCL21 after 6 hours had a significantly higher percentage of ZAP-70–positive cells (P = .018).
Migration toward CCL21 is enhanced after ZAP-70 activation. (A) Ramos transfectants either stimulated (6 hours, 5 μg/mL F(ab′)2 anti-IgM) or unstimulated, and (B) Raji transfectants were subjected to migration assay toward CCL21 (1 μg/mL) for 15 hours at 37°C in 5% CO2 atmosphere. The absolute number of transmigrated cells was determined by flow cytometry, acquiring cells under a defined flow rate. Results are expressed as migration index, calculated as the number of cells transmigrating with chemokine divided by the number of transmigrating cells toward media only. ZAP-70–activated cells showed a significantly higher migrative capacity. *P < .05. (C) Peripheral blood mononuclear cells from 7 patients with CLL were subjected to migration assay toward CCL21 (1 μg/mL) for 6 hours at 37°C in 5% CO2 atmosphere. The percentage of CD19+/CD5+ CLL cells expressing ZAP-70 was determined in the cellular fraction remaining in the upper chamber and in the cellular fraction of transmigrated cells for each patient sample by flow cytometry. CLL cells transmigrating toward CCL21 after 6 hours had a significantly higher percentage of ZAP-70–positive cells (P = .018).
Primary CLL cells with high ZAP-70 expression have enhanced migrative capacity toward CCL21 compared with CLL cells with low ZAP-70 expression from the same patient.
Peripheral blood mononuclear cells from patients with CLL and high ZAP-70 expression (≥ 20%) have shown increased migration capacity toward CCL19 and CCL21.18 In this sense, we found that primary CLL cells with higher expression of ZAP-70 within the same patient had an increased expression of adhesion molecules and chemokine receptors. Therefore, we investigated whether CLL subclones with higher ZAP-70 expression had also increased migratory potential. For this, we performed chemotaxis assays toward CCL21 using peripheral blood mononuclear cells from 7 patients and measured the expression of ZAP-70 of the transmigrated cells. Of note, after 6 hours of migration, we observed that, for all the cases analyzed, the percentage of ZAP-70–positive cells was significantly higher in the cells that had migrated compared with the cells present in the upper chamber, indicating that ZAP-70–positive CLL cells have an enhanced ability to respond to and to migrate toward CCL21 (Figure 6C).
Discussion
The expression of ZAP-70 protein and its correlation with adverse prognosis in some subsets of B lymphocyte–derived malignancies prompted the analysis of its function in a B lymphocyte setting. ZAP-70 and Syk proteins belong to the same protein family and have similar function in T and B lymphocytes, respectively. Therefore, several parallelisms in their role in BCR signaling have been found, with ZAP-70 involved in enhancing the IgM-BCR signaling, thus mimicking the role of Syk.13-17 Moreover, in primary CLL cells, other biologic features have been correlated with ZAP-70 expression, such as increased response to migrative and survival stimuli.18-21 However, it has not been elucidated whether these features are a reflection of the distinct biologic characteristics of cells expressing ZAP-70 or directly governed by ZAP-70. Therefore, we ascertained the effects on BCR signaling and migration induced by the ectopic expression of ZAP-70.
In our report, we showed that ZAP-70 enhances the signaling through IgM-BCR, increasing and prolonging Akt and ERK1/2 phosphorylation. In contrast, in a B-cell line lacking surface immunoglobulin where ectopic ZAP-70 became constitutively phosphorylated, we found that ERK1/2, but not Akt, was also activated, suggesting that ZAP-70 would downstream signal through the MAPK pathway independently of BCR stimulation. Even though the majority of CLL cases coexpress surface IgM and IgD,22 there is no information on the role of ZAP-70 in IgD BCR signaling. In CLL, BCR signaling mediated by engagement of IgM has been found to be different from that observed after IgD stimulation: whereas only CLL cases with unmutated IgHV genes usually respond to IgM stimulation,22,24,25 virtually all cases respond to IgD22,24,26 ; moreover, the response triggered by IgD or IgM ligation in the same sample can be qualitatively different.23 ZAP-70 enhances IgM signaling in B cells, as previously described13-17 and further confirmed in this report. Intriguingly, however, in our cell system, ZAP-70 did not participate in IgD signaling. On IgD stimulation, ZAP-70 did not become phosphorylated, did not translocate from the cytoplasm to the cellular membrane, and did not enhance Akt phosphorylation. In addition, as opposed to signaling after IgM stimulation, IgD stimulation did not induce the phosphorylation of ERK1/2 protein. Altogether, these results show that ZAP-70 is not participating in IgD-mediated signaling, this probably being responsible for some of the differences described between IgM and IgD signaling in CLL cells.
In B lymphocytes, ZAP-70 protein binds to the signaling molecules of the BCR, namely, CD79a and CD79b,13 and delays BCR internalization after stimulation.15 Herein, we observed that IgM and CD79b had different internalization kinetics, indicating that in our system BCR components are uncoupled and independently removed from the surface after BCR stimulation. Interestingly, both components of the BCR, particularly CD79b, were retained longer in the membrane of ZAP-70–expressing cells compared with BCR stimulated cells without ZAP-70 expression. These results show that CD79b-bound ZAP-70 is upholding longer the presence of both components of the BCR (CD79b and IgM) in the membrane, thus allowing for a sustained activation of the cells that express ZAP-70.
Several signals from the microenvironment of lymph node and bone marrow, mainly regulated by chemokines, can influence the accumulation and survival of B lymphocytes, including B-ALL and CLL cells.33,34,40-43 Of note, in B lymphocytes, the activation of the BCR can lead to a modulation in the expression of different chemokine receptors.20,25,44,45 In our B cells without ZAP-70, we found that the stimulation of the BCR caused variable modifications in the surface expression levels of several chemokine receptors and adhesion molecules, including the increase in CCR7 expression. The increase in the expression of CCR7 was regulated by the activation of the MAPK pathway, as it was shown by the impairment of CCR7 up-regulation after ERK1/2 pharmacologic inhibition. This inhibition did not completely abrogate CCR7 up-regulation, indicating that other molecules are also involved in CCR7 regulation. In addition, Raji GFP-ZAP-70 cells, with constitutive ZAP-70 activation, also had a significantly higher level of CCR7 expression. Interestingly, these cells also had phosphorylated ERK1/2 but not phosphorylated Akt, thus supporting again that the MAPK pathway is playing a role in the ZAP-70–dependent up-regulation of the CCR7 receptor. It has been described that CCR7 is variably expressed in B-ALL34,38 and overexpressed in CLL, where it has been associated with adverse prognostic characteristics, such as clinical lymphadenopathy and increased ZAP-70 expression.18,39,46 However, it has not been depicted whether CLL cells expressing ZAP-70 are those with a high expression of CCR7. In our paper, we found higher CCR7 expression in CLL cells with high ZAP-70 expression within the same patient sample, this indicating that ZAP-70 could be responsible for the increased expression of CCR7 observed in CLL patients with increased ZAP-70 expression as we observed in our cellular system. Moreover, CLL cells with high ZAP-70 had also significantly increased expression of additional adhesion molecules and chemokine receptors that had been found implicated in the interaction of CLL cells with their microenvironment, regarded as having a prominent role in CLL pathogenesis and progression.33,34,40-43
CCR7 ligands, chemokines CCL19 and CCL21, are expressed in secondary lymphoid organs and can increase the migrative capacity and survival of CCR7-positive cells,47 including primary cells from patients with B-ALL and CLL.33-36 In this regard, we found that CCR7 signaling after CCL21 ligation was greatly increased in transfected cells with activated ZAP-70, probably because these cells harbor higher levels of CCR7 on surface. However, we cannot exclude a direct participation of ZAP-70 protein in CCR7 signal transduction because we observed enhanced Akt phosphorylation on CCL21 binding without BCR-mediated ZAP-70 activation and, thus, with no up-regulation of the CCR7 levels. Indeed, ZAP-70 and Syk proteins have been implicated in BCR-independent signaling through CXCR4 and CXCR3,48-50 chemokine receptors with a molecular structure similar to that of CCR7.
Although ligation of chemokines to their receptors can induce survival and migration, additional stimuli may be needed to induce cells to migrate, as it has been reported for monocyte-derived dendritic cells and B-ALL cells.37,38 In addition, levels of chemokine receptor do not always correlate with migrative capacity toward ligands, as it has been shown for CXCR4 and CCR7 in CLL.20,36,39 Remarkably, in our experiments, we did find a significantly higher migration toward CCL21 of B cells expressing activated ZAP-70. Therefore, up-regulated CCR7 expression in ZAP-70–positive cells would induce them to migrate toward a more favorable microenvironment, such as lymph nodes or secondary lymphoid organs, where they would receive additional survival and proliferative signals. Indeed, we did observe a significant enrichment of ZAP-70–positive cells in primary CLL cells migrating toward CCL21 in an in vitro chemotaxis system.
In conclusion, this study described the participation of ZAP-70 in IgM-BCR signaling and the lack of involvement of ZAP-70 in IgD-mediated signaling. Moreover, we showed that ZAP-70 was responsible for an ERK1/2-dependent up-regulation of the chemokine receptor CCR7, this leading to an enhanced signaling and migration on CCR7 ligation with CCL21. ZAP-70 was also involved in the regulation of CXCR4, CXCR5, CD44, CD49d, and CD62L, molecules that had significantly higher levels in CLL primary subclones with high ZAP-70 expression; moreover, the primary CLL subclones with high ZAP-70 expression had enhanced migration capacity toward CCL21. These results are extending the knowledge of ZAP-70 function in B cells and are contributing to explain the adverse clinical outcome of B lymphoproliferative disorders with increased ZAP-70 expression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Vicenzo Di Bartolo (Department of Immunology, Institut Pasteur, Paris, France) who kindly provided SRαpuroZAP-70 plasmid; Dr Pablo Engel for discussion of data (Department of Cell Biology, Immunology and Neurosciences, University of Barcelona, Barcelona, Spain); Neus Abella, Anna Bosch, and Maria Calvo from confocal microscopy facilities (Serveis Científicotècnics, Universitat de Barcelona-IDIBAPS) for technical assistance in the time lapse microscopy experiments; and Isabel Crespo and Cristina López (Unitat de Citòmica-IDIBAPS) for their expert technical assistance in flow cytometry and cell sorting.
This work was supported by Instituto de Salud Carlos III, Fondo de investigaciones Sanitarias (FIS 05/213 and FIS 08/0211), and Fundació Marató de TV3 (05/1810). M.J.B. is a recipient of a PhD fellowship (SFRH/BD/28698/2006) from Ministério da Ciência, Tecnologia e Ensino Superior, Portugal.
Authorship
Contribution: E.C. designed the research, performed experiments, analyzed data, designed figures, and wrote the paper; C. Codony designed the research and supervised the study; C. Carpio performed experiments and analyzed data; M.J.B., P.A., and N.P. analyzed data; and F.B. and M.C. designed the research, supervised the study, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesc Bosch, Department of Hematology, University Hospital Vall d'Hebron, Pg de la Vall d'Hebron 119-129 (08035), Barcelona, Spain; e-mail: fbosch@vhebron.net.
References
Author notes
F.B. and M.C. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal