Abstract
X-linked hyper IgM syndrome (XHM) is a combined immune deficiency disorder caused by genetic alterations in CD40 ligand. The purpose of this study was to investigate the safety and efficacy of recombinant CD40 ligand (rCD40L) in the treatment of the disease. Three children were administered rCD40L subcutaneously 3 times per week at 0.03 mg/kg for 22 weeks, and after a 12-week drug-free interval, the dose was increased to 0.05 mg/kg for an additional 22 weeks of treatment. Although specific antibody responses to T cell–dependent antigens was lacking, administration of rCD40 resulted in acquisition of the capacity to mount cutaneous delayed type hypersensitivity reactions that disappeared during the drug-free interval as well as the postbiologic follow-up period. With rCD40L treatment, patient T cells developed a new capacity to respond to T-cell mitogens with synthesis of IFN-γ and TNF-α. Intracellular cytokine staining studies showed that both CD4+ and CD8+ T cells participated in this response. Finally, CD40L therapy was associated with changes in lymph node size and architecture based on comparison of biopsies taken before and after therapy. This clinical study showed that rCD40L is capable of improving T cell–immune function in patients with XHM.
Introduction
X-linked hyper-IgM syndrome (XHM) is a rare inherited immune deficiency disorder caused by mutations in the gene encoding CD40 ligand (CD40L [CD154]).1-3 The cognate interaction between CD40L expressed on activated CD4+ T cells and CD40 expressed constitutively on B cells leads to B-cell proliferation, somatic hypermutation in the immunoglobulin variable region, and immunoglobulin class switch recombination from IgM to IgG, IgA, and IgE.4-6 Humans with mutations in the genes encoding CD40L have skewed IgM antibody responses and a markedly diminished or absent IgG response to protein antigens.7-9 CD40 engagement on the surface of dendritic cells also influences dendritic cell maturation and activation.8,10 It thus promotes the expression of factors that promote T-cell priming such as costimulatory molecules B7.1 (CD80) and B7.2 (CD86), IL-12 secretion, and the release of chemokines.10,11 Indeed, humans and mice with CD40L deficiency exhibit defective T-cell function that manifests by a marked predisposition to develop opportunistic infections with Pneumocystis jerovici, Toxoplasma gondii, and Cryptosporidium species as well as an increased tendency to develop malignancies.12-15
The IgG/IgA deficiency and associated humoral immune abnormalities in patients with XHM is effectively controlled by γ globulin replacement therapy (intravenous immunoglobulin [IVIG] therapy).13,16 However, despite such treatment, the majority of patients die in the second decade of life as a consequence of impaired T-cell function.13,17 Bone marrow transplantation is an effective treatment for patients with XHM, especially if performed early in life before the onset of secondary opportunistic infection,18,19 although the need for matched related donors and the associated morbidity and mortality of the transplant impact on its clinical application.20,21
Identification of CD40L as an important signaling molecule led to the development of a soluble form of recombinant CD40 ligand (rCD40L) that could be used in various clinical situations.22,23 This trimeric form of human rCD40L was generated using an isoleucine zipper that is capable of activating the CD40 receptor (Amgen). We first conducted preclinical studies in a murine model of XHM in which the CD40 ligand was disrupted through genetic engineering revealed that administration of CD40 agonists or recombinant murine CD40L trimer reversed the core immunologic abnormalities. More specifically, treated animals exhibited improved survival and were able to mount antigen-specific antibody responses. Furthermore, we found that in vitro addition of rCD40L induced monocytes in peripheral blood mononuclear cells of XHM patients to produce IL-12 as well as up-regulate the expression of CD80/86, which in turn allowed their T cells to produce TNF-α and IFN-γ.24 Stimulated by these observations, we next performed an open label treatment study of recombinant human CD40L replacement at 2 successive doses in patients with XHM.
Methods
Patients and protocols
Patients were studied at the Clinical Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (protocol 00-I-006). The diagnosis of XHM was established by medical and family history, immunoglobulin profile, and by sequencing the gene encoding CD40 ligand. Three XHM patients ages 8, 13, and 10 years were enrolled in the study. At the time of enrollment, patients underwent a physical examination, complete blood count, serum chemistry screening, and computerized tomograms (CTs) of the chest and abdomen to establish their baseline medical condition, and, when possible, a lymph node biopsy. Blood was collected during the treatment period, and a chest CT and lymph node biopsy were performed at the end of treatment. Patients were maintained on γ globulin substitution therapy (IVIG) and antibiotic prophylaxis with trimethoprim/sufamethazole; no adjustments in dose or frequency of γ globulin infusions were permitted during the study period. The Institutional Review Board of the National Institute of Allergy and Infectious Diseases approved the open protocol, and informed assent and consent was obtained from all patients and their parents, respectively, before enrollment in the study in accordance with the Declaration of Helsinki.
Treatment
The 3 XHIM patients received rCD40L by subcutaneous injection on alternate days (Monday, Wednesday, Friday) on a single-step dose escalation schedule. Each patient received rCD40L at 0.03 mg/kg for 22 weeks, followed by a 12-week drug-free interval, and then a second course of rCD40L at 0.05 mg/kg for an additional 22 weeks. The treatment schedule was based on data from 2 other XHM patients (see below), previous studies in cancer patients, and from preclinical development research in nonhuman primates. Patients were monitored weekly for evidence of drug toxicity using the National Cancer Institute Common Toxicity Criteria Version 2.0 (available at http://ctep.cancer.gov/) during treatment, and every 4 weeks during the postbiologic observation period. Recurrent grade 2 toxicity or any grade 3 or 4 toxicity was defined as a major adverse event and required discontinuation from the study. Isolated grade 2 toxicity was not considered a major adverse event but did necessitate withholding rCD40L treatment until the toxicity resolved.
To establish an appropriate and safe rCD40L dose, 2 XHM patients who were not subsequently included in the study were initially evaluated for toxicity on administration of rCD40L. These patients were administered 6 doses of rCD40L at 0.01 mg/kg/day on a Monday, Wednesday, and Friday schedule for 2 weeks, followed by a drug-free week. They were then administered 0.03 mg/kg/day rCD40L using the same schedule for 3 weeks followed by a drug-free week (for a total of 21 additional doses). rCD40L was well tolerated at both dose levels, thus permitting the study of the 3 patients that are the focus of this report.
Immune assessment
Before beginning the study, the patients were assessed for their ability to mount delayed type hypersensitivity reactions (DTH) by subcutaneous injection of purified candida and mumps “recall” antigens. Intracutaneous DTH skin tests with these proteins were repeated at 10 and 20 weeks into both treatment periods, and at 8 weeks into the drug-free treatment period as well as after cessation of rCD40L administration. In addition, T-cell responses to recall antigens was evaluated by immunizing the patients with 2.5 mg of keyhole limpet hemocyanin (KLH) during weeks 8, 12, and 16 of rCD40L administration followed by intracutaneous DTH skin testing. To further assess for antigen-specific antibody responses to bacteriophage φX-174 was administered during the dose escalation period at weeks 44 and 60. For this purpose, antigen-specific IgG antibody levels in serum samples obtained before study and 14 days after immunization were determined by ELISA.
In vitro cytokine responses were evaluated before and during the course of the study. Peripheral blood mononuclear cells (PBMCs) were obtained using Ficoll-Hypaque density gradient lymphocyte separation medium (Pharmacia) and standard techniques.25 PBMCs were stimulated in vitro with anti-CD3 antibody (OKT3: gift from Ortho Biotech) or with the superantigens staphylococcal enterotoxin A (SEA) or staphylococcal enterotoxin B (SEB)26 using previously described methods. After 36 hours of culture, supernatants were harvested and assayed for levels of IFN-γ and TNF-α by specific ELISA (R&D Systems) according to the manufacturer's instructions. Intracellular cytokine detection was performed as described previously.27 Mutation analysis in B cells was performed by methods described previously.28 Photo micrographic images were acquired with a Nikon Eclipse 50i microscope equiped with an Olympus (Olympus America Inc) DP71 camera and software. Objectives employed were: 2×/0.1 numerical aperture (NA); 4×/0.2 NA; 10×/0.45 NA; 20×/75 NA; 40×/0.95 NA. Final image preparation was performed wiht Adobe Photoshop CS4 extended Version 11.0.2.
Animals
CD40L-deficient mice were generated as described previously.29 Mice were maintained under specific pathogen-free conditions. All experimental procedures were in accordance with the Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. To assess immune responses mice, 2 immunizations with 4 μg of CRM197 mutant diphtheria toxin vaccine in RIBI adjuvant (MBL International) were administered 10 weeks apart. Blood samples were obtained 2 weeks after the second vaccination, and antigen-specific antibodies were determined by specific ELISA.
Results
Murine CD40 ligand treatment improves survival of CD40L-deficient mice
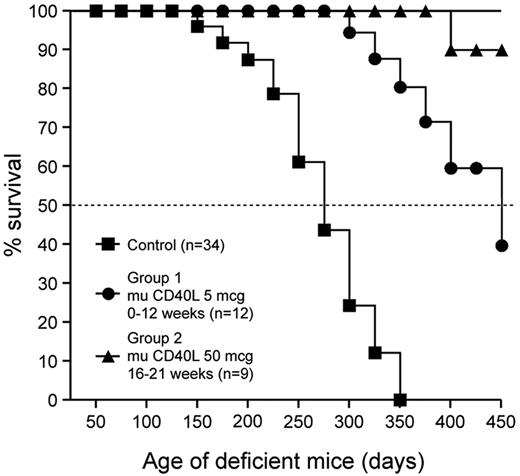
In keeping with the immunologic findings in XHM patients, CD40 ligand knockout (CD40L KO) mice are incapable of neutralizing antibody responses and are susceptible to pulmonary pneumocystis infection as a consequence of defects in T-cell priming. To determine whether treatment with murine CD40L ameliorates the disease phenotype in CD40L KO mice, 2 groups were administered murine CD40L at doses denoted in the Figure 1. A similar-aged control group received treatment with buffer and was housed in the same specific-pathogen-free facility. Mice were monitored for morbidity and mortality until day 450 or death. Murine CD40L treatment improved the survival incidence of group 1 (42%) and group 2 (89%) CD40L KO compared with the control CD40L KO group (0%) at the termination of the experiment on day 450. Mean survival times for the 2 groups treated with CD40L are significantly greater (P < .01) than that of the control group. The majority of control CD40L KO mice and group 1 CD40L KO mice died from pulmonary pneumocystis infection. Thus, the improved survival in CD40L-deficient mice with murine CD40L treatment is dose dependent and is probably reflective of restored cellular immunity. Murine CD40L treatment also restored immunoglobulin class switch and antigen-specific antibody responses in CD40L KO mice (supplemental Tables 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, treatment with murine CD40L restores the core immunologic abnormalities in CD40L-deficient mice, and these findings are consistent with a previous study using a CD40 agonist antibody.30
Murine CD40L treatment increases the survival of CD40L-deficient mice. Mean survival times for the 2 groups treated with CD40L are significantly greater (P < .01) than that of the control group.
Murine CD40L treatment increases the survival of CD40L-deficient mice. Mean survival times for the 2 groups treated with CD40L are significantly greater (P < .01) than that of the control group.
Patient characteristics
The 3 XHM patients enrolled in this study had histories of recurrent otitis media and frequent upper respiratory bacterial infections that occasionally required hospitalization. One patient developed P jiroveci infection at 6 months of age and another developed disseminated coccidioidomycosis at 5 years of age that ultimately required maintenance fluconazole suppressive therapy. Laboratory evaluation revealed that the 3 patients had normal numbers of B cells and T cells, but in each case these cell populations exhibited evidence of failure of immunoglobulin class switch: the B cells expressed surface IgD and IgM but did not express surface IgG or IgA. In addition, as described, the CD4+ T cells were predominantly CD45RA+ with very limited CD45RO+ expression.24 All patients had markedly decreased IgG levels (< 250 mg/dL) before initiation of monthly IVIG therapy, and all were maintained on antibiotic prophylaxis for Pneumocystis pneumonia. This treatment regimen was continued during the study.
All 3 patients completed both phases of rCD40L treatment as specified in the protocol without serious adverse events, and each patient received a total of 132 subcutaneous injections. In previous studies of cancer patients, dose-related, transient serum elevations in liver transaminases were seen during treatment with rCD40L.31 In that study, grade 3 or 4 elevations in alanine transaminase were observed in 14% and 28% of patients receiving 0.05 and 0.10 mg/kg/day, respectively.31 At a dose of 0.10 mg/kg/day, the half-life of rCD40L was 24.8 hours.31 Based on these considerations and our experience with the initial 2 XHM patients, we initiated treatment of the 3 study XHM patients with rCD40L at a dose of 0.03 mg/kg on an alternate-day dosing schedule. None of the patients experienced elevations in serum transaminases above baseline values at this lower dose of CD40L. One patient developed a skin infection at the rCD40L injection site that responded to antibiotic therapy. Another patient experienced mild injection site reactions that were obviated by rotating the site of administration, treating the patient with nonsteroidal anti-inflammatory drugs, or both. Grade 1 elevation of liver enzymes was noted in all 3 patients at the 0.05-mg/kg dose of CD40L. The elevations in liver enzymes and the local skin reactions resolved within 1 week of completing treatment. During the study, the patients did not develop opportunistic infections or require hospitalization for any complications of the underlying disease.
Effect of treatment on DTH reactions
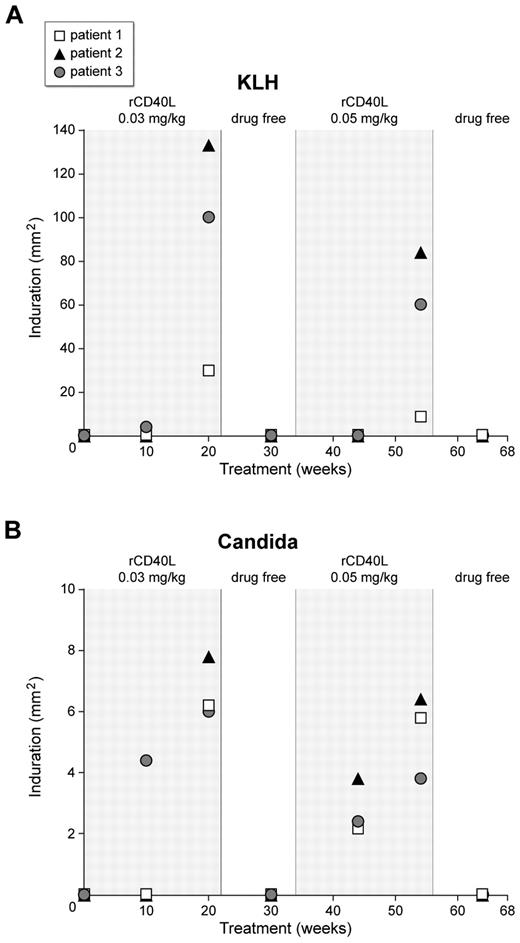
DTH reactions are mediated by CD4+ T cells reacting to a recall antigen presented by Langerhan's cells in the skin or dendritic cells in the draining lymph node.32,33 We have reported previously that XHM patients have deficient or absent DTH reactions because of the absence of CD40L–CD40 interactions on the surface of T cells and antigen-presenting cells, respectively.24 Consistent with this, the 3 XHM patients in this study lacked DTH reactions to injected recall antigens, candida, mumps, or purified protein derivative (PPD) antigen. In addition, they exhibited an expected negative baseline DTH response to KLH because of a lack of prior exposure. To assess whether administration of rCD40L could restore absent DTH responses in XHM patients, we immunized the patients with KLH at weeks 8, 12, and 16 to induce DTH responses to this antigen. We evaluated DTH response to KLH and to the recall antigens, candida, mumps, and PPD, on weeks 10 and 20 during rCD40L treatment and 8 weeks into the drug-free interval (Figure 2). One patient developed DTH responses to KLH, mumps, and candida antigens at week 10, and the level of response (as assessed by the degree of induration) was further increased on retesting at week 20. The other 2 patients developed positive DTH reactions to KLH and candida antigen at week 20 but not to mumps or PPD antigens. It is not known whether the patient who developed DTH responses to mumps antigen had received prior MMR vaccination. Notably, DTH responses were absent in all patients 8 weeks into the drug-free interval period, were restored during the dose escalation period, and lost again during the second follow-up period. These results demonstrate that the positive skin reactions were not a result of a booster response to repeat antigen challenge and that the maintenance of restored antigen-specific T-cell immune responses in XHM will probably require chronic rCD40L administration.
Development of positive DTH reactions to KLH and Candida in the 3 XHM participants who received rCD40L. The patients were administered rCD40L subcutaneously 3 times per week at 0.03 mg/kg for 22 weeks, and after a 12-week drug-free interval, the dose of rCD40L was increased to 0.05 mg/kg for an additional 22 weeks of treatment. Note the absent reactions during the drug-free interval and the posttreatment period, indicating the positive results during rCD40L treatment period were not because of repeated skin testing.
Development of positive DTH reactions to KLH and Candida in the 3 XHM participants who received rCD40L. The patients were administered rCD40L subcutaneously 3 times per week at 0.03 mg/kg for 22 weeks, and after a 12-week drug-free interval, the dose of rCD40L was increased to 0.05 mg/kg for an additional 22 weeks of treatment. Note the absent reactions during the drug-free interval and the posttreatment period, indicating the positive results during rCD40L treatment period were not because of repeated skin testing.
Restored TH1 function after rCD40L administration
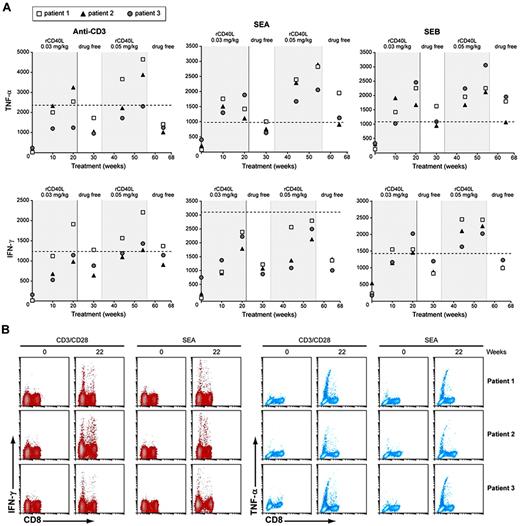
We have shown previously that T cells from XHM patients are deficient in the production of T helper 1 (TH1) effector cytokines after activation and that this impairment in T-cell function can be at least partially restored by the ex vivo addition of rCD40L.24 To determine whether in vivo administration of rCD40L can drive naive XHM T cells to produce TH1 cytokines, we cultured patient and control PBMCs with anti-CD3 (or with the superantigens SEA and SEB) for 36 hours and then measured TNF-α or IFN-γ secreted into the culture fluid by specific ELISA. As shown in Figure 3A, before treatment with rCD40L, the patients' T cells exhibited markedly reduced levels of TNF-α and IFN-γ secretion to all 3 stimuli. However, 10 weeks after initiation of rCD40L treatment the T cells displayed increased capacity to produce both TNF-α and IFN-γ, albeit still at a level below that produced by control cells. Furthermore, the capacity to produce these effector cytokines generally improved with the dose escalation step and persisted 10 weeks after rCD40L treatment had been stopped. We also assessed the ability of rCD40L treatment to prime CD4+ T-cell subsets by measuring intracellular TNF-α and IFN-γ by flow cytometry after stimulation with anti-CD3/CD28 (Figure 3B). Consistent with the ELISA results, before treatment the activated patient T cells were deficient in generating cells with intracellular TNF-α or IFN-γ. However, after 20 weeks of rCD40L treatment, cells positive for intracellular TNF-α and IFN-γ were found in both the CD4+ and CD8+ T-cell subsets after stimulation with anti-CD3/CD28. These results demonstrate that in vivo rCD40L treatment can at least partially restore deficient T-cell function.
T cells priming with rCD40L treatment in XHM patients. (A) PMBCs (2 × 106) from patients with XHM and normal controls were stimulated with anti-CD3 or SEA or SEB. Cytokine measurements were made at 36 hours by ELISA. Healthy adult controls (n = 20) were used to determine the reference range because pediatric controls were not available. The lower limit of the 95% confidence interval range is represented by the dotted line. Reference range values for TNF-α production are as follows: anti-CD3, 2 343 to 23 968; SEA, 1062 to 6598; and SEB, 1084 to 6198. Reference range for IFN-γ production is as follows: anti-CD3, 1 242 to 17 703; SEA, 3 105 to 11 010; and SEB, 1 421 to 16 354. (B) Intracellular TNF-α and IFN-γ production in CD4+ and CD8+ T cells after in vitro stimulation with anti-CD3/28 or SEA for 6 hours. After stimulation, PBMCs were fixed, permeabilized, and stained with antibodies for surface markers and intracellular cytokines. Gating on CD3+CD8+ cells the plots depict surface staining for CD8 and intracellular TNF-α or IFN-γ; CD8− cells are equivalent to CD4+ cells.
T cells priming with rCD40L treatment in XHM patients. (A) PMBCs (2 × 106) from patients with XHM and normal controls were stimulated with anti-CD3 or SEA or SEB. Cytokine measurements were made at 36 hours by ELISA. Healthy adult controls (n = 20) were used to determine the reference range because pediatric controls were not available. The lower limit of the 95% confidence interval range is represented by the dotted line. Reference range values for TNF-α production are as follows: anti-CD3, 2 343 to 23 968; SEA, 1062 to 6598; and SEB, 1084 to 6198. Reference range for IFN-γ production is as follows: anti-CD3, 1 242 to 17 703; SEA, 3 105 to 11 010; and SEB, 1 421 to 16 354. (B) Intracellular TNF-α and IFN-γ production in CD4+ and CD8+ T cells after in vitro stimulation with anti-CD3/28 or SEA for 6 hours. After stimulation, PBMCs were fixed, permeabilized, and stained with antibodies for surface markers and intracellular cytokines. Gating on CD3+CD8+ cells the plots depict surface staining for CD8 and intracellular TNF-α or IFN-γ; CD8− cells are equivalent to CD4+ cells.
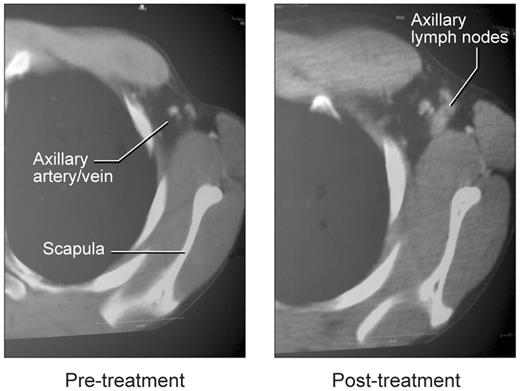
Effect of rCD40L treatment on lymph node architecture
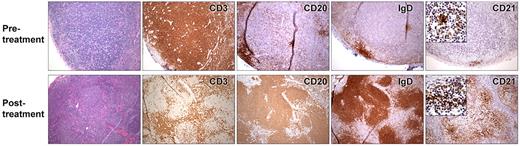
To assess the effect of rCD40L on lymph node architecture, we compared the histology of pretreatment and posttreatment tissue in one patient and examined posttreatment tissue in the other 2 patients (Figure 4). Before treatment, all 3 patients lacked palpable axillary lymph nodes, and little or no lymphoid tissue could be identified based on CT examination (Figure 5). One patient had an inguinal lymph node biopsy before rCD40L therapy that revealed small, scattered aggregates of IgM+/IgD+ B cells. However, no germinal centers were seen, and the B cells were primarily located in abortive follicles that contained only scant follicular dendritic cells. This histologic finding is similar to that described in previous studies of lymph nodes of XHM patients.8,34
Lymph node biopsies from patient 3 taken before (top) and during treatment (bottom) with rCD40L. Pretreatment, CD3 immunostaining reveals paracortical hyperplasia. Immunostaining with anti-CD20, IgD, and CD21 shows a paucity of B cells and diminutive primary follicles. With rCD40L treatment, a reorganization of lymph node architecture is noted. CD3-positive T cells are now primarily located in parafollicular region, whereas B cells are located in organized primary follicles. Development of prominent network of CD21-positive dendritic follicular cells in the primary follicles is also visible with rCD40L treatment. However, the B cells remain invariably naive as evidenced by their expression IgD. The immunostaining pattern of a reference control reactive lymph node is available in supplemental Figure 1.
Lymph node biopsies from patient 3 taken before (top) and during treatment (bottom) with rCD40L. Pretreatment, CD3 immunostaining reveals paracortical hyperplasia. Immunostaining with anti-CD20, IgD, and CD21 shows a paucity of B cells and diminutive primary follicles. With rCD40L treatment, a reorganization of lymph node architecture is noted. CD3-positive T cells are now primarily located in parafollicular region, whereas B cells are located in organized primary follicles. Development of prominent network of CD21-positive dendritic follicular cells in the primary follicles is also visible with rCD40L treatment. However, the B cells remain invariably naive as evidenced by their expression IgD. The immunostaining pattern of a reference control reactive lymph node is available in supplemental Figure 1.
Appearance of lymph nodes in left axilla with rCD40L treatment. Before treatment, the arrow indicates the location of the axillary artery and vein on contrast-enhanced CT studies. The image on the right shows emergence of axillary nodes.
Appearance of lymph nodes in left axilla with rCD40L treatment. Before treatment, the arrow indicates the location of the axillary artery and vein on contrast-enhanced CT studies. The image on the right shows emergence of axillary nodes.
A substantially different picture was obtained at the end of rCD40L treatment when all 3 patients had easily visualized lymph nodes on CT examination (Figure 5). These were particularly evident in the axillary areas, possibly because the majority of rCD40L injections were given in the forearm. Biopsy and histologic examination of these nodes revealed prominent cortical primary follicles associated with a robust development of follicular dendritic cells (Figure 4). However, as in the pretreatment specimen, no germinal centers were observed, and only IgM+/IgD+ B cells were present. There was no change in spleen size or in the number of circulating lymphocytes. Consistent with the lack of induction of germinal center development, the patients did not mount IgG responses to challenge with KLH or bacteriophage φX-174. Serum immunoglobulin levels were unchanged, and IgE as well as IgA levels remained below the level of detection (supplemental Table 3). Immunophenotyping studies before and after rCD40L treatment, revealed that all peripheral blood B cells remained invariably naive with surface coexpression of IgD and IgM. The failure of memory B-cell differentiation in these patients was further confirmed in 2 of the 3 patients with analysis of somatic mutations in the heavy chain V3-23 Ig V gene35 (supplemental Table 4). These results show that although in vivo rCD40L treatment at the dose level used in this study induces development of primary follicles and a follicular dendritic cell network, it does not result in the development of germinal centers or a further differentiation of naive B cells in the periphery.
Discussion
XHM syndrome is a combined immune deficiency disorder caused by mutations in the gene encoding CD40L.1,2 The humoral component of the immune deficiency is because of failure of activated CD4+ T cells to express CD40L, preventing the stimulation of B cells via CD40 and blocking the somatic hypermutation in the immunoglobulin variable region that enables antibody affinity maturation and immunoglobulin class switching from IgM to IgG, IgA, or IgE.4,5,36 CD40L also interacts with CD40 on antigen-presenting cells to induce production of factors such as IL-12 and the expression of costimulatory molecules such as B7 that collectively mediate the differentiation and maturation of T cells.10,11,37 Thus, failure to express CD40L results in impaired T-cell function and increases the risk for opportunistic infections and malignancy.12-14 Therefore, unlike pure humoral immune deficiencies associated with mutations in Bruton's tyrosine kinase38,39 or activation-induced cytidine deaminase,40,41 γ globulin replacement therapy (IVIG) is less satisfactory for the management of XHM because of the significant T-cell abnormalities.13,16 Despite γ globulin substitution therapy the expected survival rate for XHM is < 20% by the age of 25, emphasizing the need for new medical therapies for this disorder.13
A clinical trial of one potential new therapy, subcutaneous administration of rCD40L, is reported here. Importantly, all patients were clinically stable during both 22-week treatment periods and the 12-week posttreatment follow-up. There were no serious adverse events and injection site reactions were effectively managed by site rotation and occasional use of oral nonsteroidal anti-inflammatory agents.
The rCD40L treatment regimen used in this study resulted in improvement in both T-cell and B-cell immunity. All 3 patients demonstrated the capacity to mount DTH reactions to the recall antigen candida antigen and to develop a recall response to KLH. This latter response is significant because it was lost 8 weeks after discontinuation of rCD40L therapy and therefore seems to be directly linked to rCD40L therapy and not simply the result of booster immunization by repeated exposure to a recall antigen. Delayed-type hypersensitivity is a major mechanism of defense against various opportunistic infections with P jerovicii, T gondii, and Cryptosporidium species. Whether the degree of improved T-cell function resulting from rCD40L treatment is sufficient to offer protection against these infections awaits further investigation. The observed effect of rCD40L treatment on in vivo T-cell function was also accompanied by a demonstrable effect on in vitro T-cell function. Patient peripheral T cells (both CD4+ and CD8+ T cells) acquired the ability to produce both IFN-γ and TNF-α in response to antigen receptor stimulation while the patient was receiving rCD40L. It is important to note that the magnitude of this response was considerably less than that of normal controls, however, it was sustained > 8 weeks after discontinuation of rCD40L, suggesting that relatively long-lived memory cells had been induced. The fact that DTH responses were not sustained for an equally long period may be associated with the requirement for restimulation of the cells with the induction of adhesion molecule expression necessary for the movement of cells into sites of inflammation.
Follicular dendritic cells are stromal cells that contribute directly to the survival and activation of B cells; it is in follicular dendritic cell areas that B cells undergo somatic mutation, positive and negative selection, isotype switching, and differentiation into high-affinity plasma cells and memory B cells. Recombinant CD40L therapy contributed to a marked expansion of follicular dendritic cells within the primary follicles of lymph nodes. Although these histologic changes represent evidence of improved B-cell function in patients with XHM, they fell short of changes that might have clinically important effects, such as germinal center formation or serum IgG responses to T cell–dependent antigens such as φX-174. Nonetheless, the observed changes in patient lymph nodes and evidence of immunoglobulin class switch recombination in CD40L-deficient mice treated with murine rCD40L (supplemental Table 1) suggest that a more persistent dosing schedule, higher dosing schedule, or both could produce additional positive impact on patient B-cell function.
The expression of CD40L is tightly regulated on the surface of CD4+ T cells and constitutive expression of CD40L in mice led to the development of lymphomas that involved multiple organs.42,43 While such adverse events were not noted in CD40L-deficient mice that received murine rCD40L in a 450-day treatment study, XHM patients enrolled in future clinical trials that use rCD40L will need careful monitoring. At the 0.05-mg/kg dose, transient elevations in liver transaminases were noted in our study patients. Although reversible, future studies of rCD40L should incorporate frequent evaluations for liver toxicity. This leads to the question as to which group of patients should enroll into future studies involving rCD40L. Studies with the potential to offer clinical benefit should have priority. Cryptosporidium infection in XHM patients often leads to liver failure and hematopoietic stem cell transplantation in this setting is associated with poor clinical outcomes.44 In light of improved T-cell function resulting from rCD40L administration, rCD40L has the potential to be an important treatment option for XHM patients with biliary Cryptosporidium.
In conclusion, this “proof of principle” study of rCD40L therapy for XHM patients provides convincing evidence that such therapy is well tolerated in the doses used. rCD40L treatment resulted in a degree of reconstitution of T-cell priming and T cell–mediated effector functions that could favorably affect patient resistance to opportunistic infection and immune surveillance for cancer. Thus, continued investigation of CD40 agonists in XHM and related disorders of B-cell terminal differentiation is warranted.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and family members who participated in this study, Warren Strober and Cliff Lane for helpful discussions, and Harry Malech for careful review of the manuscript.
The project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400, and supported by the intramural program of NIAID/NIH.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
National Institutes of Health
Authorship
Contribution: A.J., J.A.K., D.L.N., V.D., S.L., and T.A.F. designed research; A.J., J.A.K., D.L.N., V.D., S.L., T.A.F., S.A.M., S.P., W.F., X.F., D.W.W., R.H., M.R.B., J.J.S., S.L., V.D., H.A.H., and H.O. performed research; and A.J. and T.A.F. wrote the paper.
Conflict-of-interest disclosure: W.F. is an employee of Amgen. The company did not fund the study. The remaining authors declare no competing financial interests.
Correspondence: Ashish Jain, Laboratory of Host Defenses, NIAID, NIH, 10 Center Dr, Rm 5W 3950, Bethesda, MD 20892; e-mail: ajain@nih.gov.