Abstract
Dendritic cells (DCs) need to migrate in the interstitial environment of peripheral tissues to reach secondary lymphoid organs and initiate a suitable immune response. Whether and how inflamed tissues instruct DCs to emigrate is not fully understood. In this study, we report the unexpected finding that the epithelial-derived cytokine TSLP triggers chemokinesis of resting primary human DCs in a cell-autonomous manner. TSLP induced the polarization of both microtubule and actin cytoskeletons and promoted DC 3-dimensional migration in transwell as well as in microfabricated channels that mimic the confined environment of peripheral tissues. TSLP-induced migration relied on the actin-based motor myosin II and was inhibited by blebbistatin. Accordingly, TSLP triggered the redistribution of phosphorylated myosin II regulatory light chain to the actin cortex, indicating that TSLP induces DC migration by promoting actomyosin contractility. Thus, TSLP produced by epithelial cells in inflamed tissue has a critical function in licensing DCs for cell-autonomous migration. This indicates that cytokines can directly trigger cell migration, which has important implications in immune physiopathology and vaccine design.
Introduction
Competence of dendritic cells (DCs) to induce the differentiation of naive T cells into effector T cells relies on their ability to migrate from the peripheral sites of inflammation to the secondary lymphoid organs where T-cell priming takes place.1,2 During this process, DCs must emigrate out of peripheral tissue and move through a variety of narrow spaces, such as tight intercellular junctions in epithelia, basal membrane, extracellular matrix, and endothelia. This motility is in part orchestrated by chemokine gradients, such as CCL19 or CCL21, which dictate the directionality of the movement toward lymphoid organs where these chemokines are abundantly expressed.3 Whether endogenous signals produced by injured tissue at the inflammatory site can instruct DCs to migrate is currently unknown.
Cytokines are proteins that act through specific surface receptors to modulate critical cellular functions, such as cell proliferation, differentiation, and survival.4 They are important components of the inflammatory microenvironment. Their precise function in inducing or modulating cell migration has not been elucidated. Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine that strongly activates DCs and initiates a Th2 type of CD4 T-cell response.5 It plays a critical role in allergic diseases and, in particular, atopic dermatitis where it is highly produced by keratinocytes in human lesions6 and mouse models.7,8 Thus, TSLP mediates a cross-talk between inflamed epithelia and the innate immune response.5
Previous studies from our group and others suggested that TSLP may be associated with Langerhans cell migration in situ6 and ex vivo.9 In this study, we demonstrate that TSLP is sufficient to induce the polarization, and 3-dimensional and confined migration of human DC in vitro, through the actin-based motor protein, myosin II. This constitutes a novel property of cytokines in triggering a cell-autonomous DC migration in interstitial spaces.
Methods
Blood DC purification and culture
CD11c+ DCs were purified to 99% by FACS sorting from buffy coats of healthy adult volunteer blood donors (Crozatier Blood Bank) as previously described.6 Freshly sorted CD11c+ DCs were cultured in RPMI containing 10% FCS, 1% pyruvate, 1% HEPES, and 1% penicilin-streptomycin. Cells were seeded at 1 × 106/mL in flat-bottom 96-well plates in the absence (untreated cells) or presence of 50 ng/mL TSLP (R&D Systems), 2.5 ng/mL TNF (R&D Systems), 20 μg/mL influenza virus (H1N1, A/PR/8/34 strain; Charles River Laboratories), 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich), or 100 ng/mL GM-CSF (BruCells).
Immunofluorescence
To determine the cytoskeleton architecture, DCs were cultured on poly-lysine–coated coverslips for 24 hours and examined by epifluorescence microscopy. Cells were fixed in 4% paraformaldehyde in PBS for 20 minutes at room temperature, permeabilized by 1% Triton X-100 in PBS for 5 minutes, and blocked with 1% BSA in PBS for 20 minutes at room temperature. For localization of filamentous actin, cells were incubated with Cy3-phalloidin (Invitrogen) for 30 minutes. Counting of the number of polarized DCs from 5 different donors assessed polarization index. Polarization was expressed as proportion polarized cells respect to total number of cells. Localization of α-tubulin was achieved by incubation for 1 hour with a rat anti–human α-tubulin antibody (Serotec). Myosin II was detected by a rabbit anti–human myosin II heavy chain antibody (BTI) followed by incubation for 30 minutes with Alexa-488 goat anti–rabbit (Invitrogen). Coverslips were mounted in ProLong Gold antifade reagent (Invitrogen). Fluorescence images were obtained by an epifluorescence microscope (Leica) fitted with appropriate filter sets. For phospho-myosin II light chain and actin staining, cells were permeabilized in 0.2% BSA, 0.05% saponin for 10 minutes, and antibodies were diluted in the same solution. Phospho-myosin II light chain was detected with a rabbit anti–phospho-myosin light chain 2 (pMLC2; Rockland) followed by incubation with Alexa-488 goat anti–rabbit antibody. For quantification of pMLC2 association to actin, images were acquired on an epifluorescence Nikon microscope (Eclipse 90i Upright) with a 100× objective. After deconvolution, colocalization was quantified on each plane by the colocalization analysis program of Metamorph and represented as the percentage of pMLC2 area colocalized with actin area. For each cell analyzed, the result is expressed as a mean of all the planes.
Transwell DC migration
Uncoated or collagen type I (5 μg/mL rat tail collagen type I; BD Biosciences) coated transwells (Costar, 3-μm pores) were placed in 96-well plates filled with 200 μL of DC culture medium. Overnight treated DCs (1 × 106/mL) with TSLP, TNF, LPS, influenza virus, or GM-CSF were resuspended in 50 μL of this solution, added to the upper chamber of the transwells, and incubated at 37°C for 6 hours. CCL20 (500 ng/mL; R&D Systems) was added to the lower chamber as a positive control to induce DC migration where mentioned. After 6 hours, cells in the upper and the lower chambers of the transwell were counted. Results were expressed as percentage of total DCs.
DC migration in microchannels
The microfluidic device was fabricated in polydimethylsiloxane (PDMS).10 The PDMS piece, with embedded microchannels and holes for the inlet and outlet ports, and a glass Iwaki chamber (Milian) were activated in a plasma cleaner (PDC-32G Harrick) and bonded to each other. The chambers were left under strong vacuum for 5 minutes in the plasma cleaner, and plasma was turned on to render the top surface of the PDMS and the inlet and outlet holes hydrophilic. Fibronectin solution at 50 μg/mL was placed on top of the inlet and outlet ports. The solution spontaneously invaded the channels, and all air bubbles were resorbed into the PDMS because of the previous vacuum treatment. Fibronectin was incubated for 1 hour at room temperature, then washed with PBS, and then replaced by cell culture medium. The cells were concentrated, and micropipette tips containing the cells were inserted in the inlet port. Cells fell inside the port, bound to the bottom coverslip, and started migrating. They entered the channels spontaneously, without any mechanical or chemical stimulation.
Phase-contrast images at various positions in the chambers were recorded with time lapses of 1-2 minutes during 6-10 hours, using an automated microscope (Nikon Eciplse TE1000-E, and Olympus X71, with a Marzhauser motorized stage and an HQ2 Roper camera) equipped with an environmental chamber for temperature, humidity, and CO2 (Life Imaging Services). Cells remained alive and motile during the entire period of recording.
Kymograph extraction and instantaneous velocity analysis
Without any intervention from the user, a program written in C++, taking as input an image sequence, provides as output a set of kymographs corresponding to each channel by automatically performing motion compensation, background subtraction, channel detection, and kymograph computation. A number of parameters are accessible when the program is started. Each resulting kymograph is an image that contains, in each horizontal line, the gray values found along a given channel at a given time point. The consecutive time points form the consecutive lines of the image, with time zero at the top. This allowed us to reduce large datasets into a smaller set of images. Specifically, 1 image per channel was obtained, which contains all the necessary information for cell movement analysis. The space dimension perpendicular to the channel length that contains no information was suppressed. The program first performed image cleaning: indeed, at 10× magnification, with phase-contrast microscopy, 4-μm-wide channel displays a strong contrast, and cells in the channels are hardly visible on original images. Moreover, because of fast movements of the stage to get enough positions recorded at a high temporal rate, image sequences displayed slight jiggling because of repositioning errors. Subpixel phase correlation11 and robust multiresolution estimation of translation motion model were used for registration. Then background subtraction was done before the computation of the kymograph. The background was estimated by taking the average intensity along the image sequence. To produce kymographs, first, channels were detected using the Hough transform. An average width for the lines was defined as the half the distance between 2 channels (this parameter can be varied). Intensities of the kymographs were then defined as the maximum intensity inside the bound of the line encountered perpendicularly to it.
Kymographs were then analyzed using homemade routines in MATLAB. Cell signature was identified in each line, and the cells' center of mass and boundaries were found. Statistics and graphs were extracted from the data using MATLAB.
Blebbistatin treatment of DCs
To study the role of myosin II in the morphology of TSLP-DCs, cells were incubated for 12 hours in TSLP with or without 50μM blebbistatin on poly-lysine–coated slides to permit the polarization of cells. To analyze the importance of myosin II in DCs migration, untreated or pretreated DCs for 24 hours were incubated during the migration time with 50μM blebbistatin in collagen-coated transwells. For the migration analysis in microchannels, cells were pretreated with 50μM blebbistatin and then concentrated and inserted in the microchannels.
Statistical analysis
Statistical analysis of the differences between experimental groups was performed using a nonparametric ANOVA (Mann-Whitney test). Differences were considered statistically significant when P was < .05. Results were expressed as mean ± SD.
Results
TSLP directly induces chemokinesis of primary human DCs
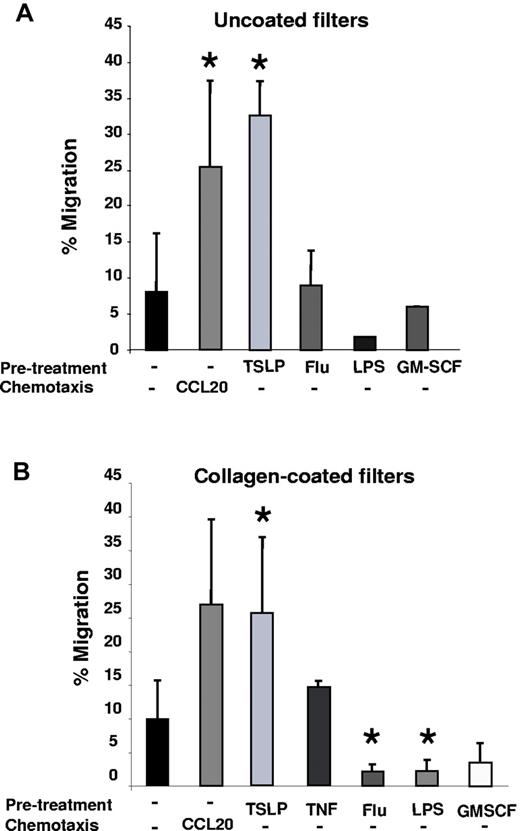
To address the role of TSLP in DC migration, we analyzed its impact on in vitro migration of nonactivated and activated primary human DCs using uncoated semipermeable filters (Transwells). We observed that ∼ 7% of untreated DCs showed a spontaneous baseline migration (Figure 1A). Two TLR ligands, LPS and influenza virus (flu), which strongly activate DCs, did not induce DC migration (Figure 1A). This equally applied to GM-CSF, a cytokine produced by epithelial cells and leukocytes during inflammation. On the contrary, TSLP-treated DCs (TSLP-DCs) became highly efficient for migration in the absence of exogenous chemokines, with up to 30% migrating cells, equivalent to a CCL20-driven chemotaxis (Figure 1A). Thus, TSLP was able to induce 2-dimensional migration of human DCs ex vivo.
TSLP induces chemokinesis of resting DCs in a cell-autonomous manner. (A) Purified blood DCs were precultured in medium (untreated), TSLP, influenza virus (Flu), LPS, or GM-CSF. After 24 hours, cells were washed, counted, and seeded in equal numbers in the upper chamber of an uncoated transwell system in the absence of chemotactic factors. After 6 hours, DCs migrating into the lower chamber were harvested and counted. Data are represented as percentage of input DCs. In the positive control, CCL20 was used in the lower chamber during migration as a chemotactic factor. Data are mean ± SD; n = 7. *P < .05 vs untreated. (B) Primary blood DCs were precultured in medium, TSLP, TNF, influenza virus (Flu), LPS, or GM-CSF. After 24 hours, cells were washed, counted, and seeded in equal numbers in the upper chamber of a collagen-coated transwell system in the absence of chemotactic factors. After 6 hours, DC migration was quantified. Data are represented as percentage of input DCs. In the positive control, CCL20 was added in the lower well. Data are mean ± SD; n = 5. *P < .05 vs untreated.
TSLP induces chemokinesis of resting DCs in a cell-autonomous manner. (A) Purified blood DCs were precultured in medium (untreated), TSLP, influenza virus (Flu), LPS, or GM-CSF. After 24 hours, cells were washed, counted, and seeded in equal numbers in the upper chamber of an uncoated transwell system in the absence of chemotactic factors. After 6 hours, DCs migrating into the lower chamber were harvested and counted. Data are represented as percentage of input DCs. In the positive control, CCL20 was used in the lower chamber during migration as a chemotactic factor. Data are mean ± SD; n = 7. *P < .05 vs untreated. (B) Primary blood DCs were precultured in medium, TSLP, TNF, influenza virus (Flu), LPS, or GM-CSF. After 24 hours, cells were washed, counted, and seeded in equal numbers in the upper chamber of a collagen-coated transwell system in the absence of chemotactic factors. After 6 hours, DC migration was quantified. Data are represented as percentage of input DCs. In the positive control, CCL20 was added in the lower well. Data are mean ± SD; n = 5. *P < .05 vs untreated.
Next, we analyzed the ability of TSLP-DCs to migrate through the 3-dimensional space of collagen type I–coated filter. TSLP-DCs became highly efficient for migration (Figure 1B) contrary to TNF-DCs, which migrated comparably to untreated DCs. LPS, flu, and GM-CSF did not induce human DC migration (Figure 1B), similar to uncoated transwells (Figure 1A). TSLP-induced DC migration was detectable as soon as 3 hours after TSLP exposure (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), in accordance with the up-regulation of TSLP receptor expression by human DCs in culture (data not shown). TSLP-DC migration was dose-dependent and could be activated at TSLP concentrations > 1 ng/mL TSLP (supplemental Figure 1B). To discriminate between chemokinesis and chemotaxis, we added TSLP to the lower chamber of the transwell. This did not affect the migration of untreated DCs, excluding chemotactic properties of TSLP (data not shown).
The fact that TSLP could induce DC migration independently of exogenous factors indicated a cell-autonomous mechanism. However, this does not exclude a role for TSLP-induced molecules acting in an autocrine manner. To address whether TSLP itself was required or whether TSLP-induced secreted factors could recapitulate the increase in DC migration, we performed experiments using TSLP-DC supernatants in the presence and absence of an anti-TSLP blocking monoclonal antibody (mAb; supplemental Figure 2). First, we assessed CD80 expression as a marker of DC activation. TSLP induced a strong up-regulation of CD80, which was inhibited by anti-TSLP mAb (supplemental Figure 2A). TSLP-DC supernatants, which contained both TSLP and TSLP-induced secreted molecules, had a similar effect on CD80 expression compared with TSLP alone. This effect was almost completely blocked by anti-TSLP mAb, indicating that TSLP-induced secreted molecules were not sufficient to recapitulate the maturation effect of TSLP (supplemental Figure 2A). In a parallel set of experiments, we assessed DC migration in transwell under similar conditions as for CD80 expression (supplemental Figure 2B). TSLP and TSLP-DC supernatant both increased DC migration, and their effect was inhibited by anti-TSLP blocking mAb (supplemental Figure 2B). This showed that TSLP was required for the induction of a cell-autonomous DC migration and that TSLP-induced secreted molecules could not recapitulate this effect.
TSLP induces a marked polarization of the cytoskeleton of DCs
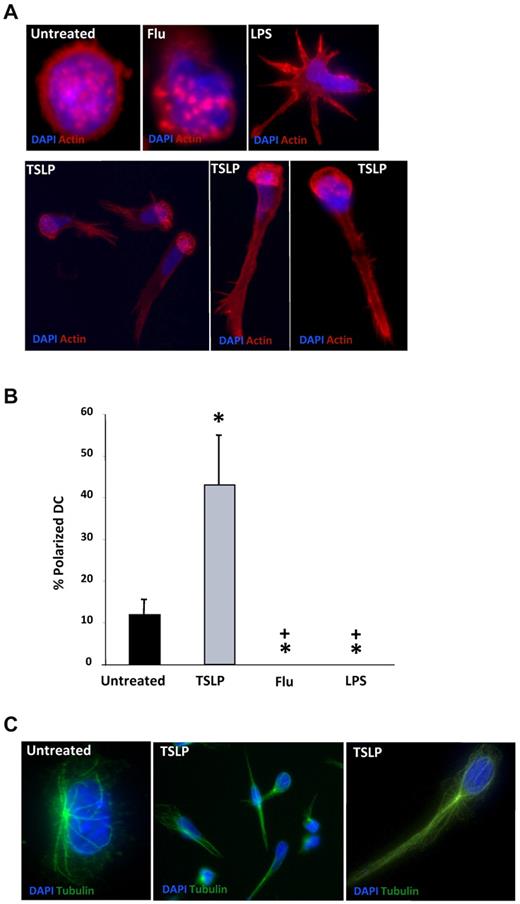
The cell-autonomous TSLP-induced DC motility suggested that cell-intrinsic mechanisms might be involved. Because cell polarization is an essential feature for cell locomotion, we questioned whether TSLP could induce the polarization of human DCs. To address this hypothesis, we performed a detailed analysis of TSLP-DC morphology. Untreated human DCs cultured on poly-lysine-coated coverslips appeared mostly round, with actin filaments enriched at the cell cortex as well as in podosomes, which are actin-rich adhesion structures that form close contacts with the substrate and are most likely involved in cell motility12,13 (Figure 2A). A similar morphology was observed in DCs activated by influenza virus (Figure 2A). LPS induced the formation of multiple dendritic expansions and the dissolution of podosomes, as previously described14 (Figure 2A). Remarkably, TSLP treatment increased the fraction of polarized cells from 12% (spontaneous polarization) to 43% (P < .05; Figure 2A-B). In these cells, the nucleus was located at one cell pole, whereas the other was formed by a long and thin uropod. Podosomes were dissoluted in many cells, but when present they clustered predominantly in the perinuclear area where actin filaments were enriched (Figure 2A). As observed for the actin cytoskeleton, the microtubule network was also reorganized in TSLP-treated DCs, with the microtubule-organizing center localized behind the nucleus at the level of the uropod, as previously described in polarized T cells (Figure 2C). Microtubule-organizing center polarization was not observed in untreated or LPS-treated DCs (Figure 2C; supplemental Figure 1C). Interestingly, spontaneous DC polarization was significantly inhibited by TLR ligands, such as flu and LPS (Figure 2B). In conclusion, TSLP induced an important reorganization of the cell cytoskeleton, leading to the polarization of human DCs, which may license DCs for migration.
TSLP induces polarization of the DC cytoskeleton. (A) DCs on poly-lysine–coated coverslips were cultured in medium (untreated), influenza virus (Flu), LPS, or TSLP. Cells were stained for actin (red) and DAPI (blue) and observed under a fluorescence microscopy. Podosomes appeared as round actin-stained formations. Actin skeleton was reorganized in a polarized manner in TSLP-DCs. Data are from one representative of 5 independent experiments. (B) After 24 hours of culture, DCs with a polarized actin skeleton were quantified. Results are represented as percentage of total DCs. Data are mean ± SD; n = 5. *P < .05 vs untreated. +P < .05 vs TSLP. (C) After 24 hours of culture, DCs were stained with an anti–α-tubulin mAb (green) and DAPI (blue). TSLP-DCs showed a reorganization of the microtubules. Data are from 1 representative of 5 independent experiments.
TSLP induces polarization of the DC cytoskeleton. (A) DCs on poly-lysine–coated coverslips were cultured in medium (untreated), influenza virus (Flu), LPS, or TSLP. Cells were stained for actin (red) and DAPI (blue) and observed under a fluorescence microscopy. Podosomes appeared as round actin-stained formations. Actin skeleton was reorganized in a polarized manner in TSLP-DCs. Data are from one representative of 5 independent experiments. (B) After 24 hours of culture, DCs with a polarized actin skeleton were quantified. Results are represented as percentage of total DCs. Data are mean ± SD; n = 5. *P < .05 vs untreated. +P < .05 vs TSLP. (C) After 24 hours of culture, DCs were stained with an anti–α-tubulin mAb (green) and DAPI (blue). TSLP-DCs showed a reorganization of the microtubules. Data are from 1 representative of 5 independent experiments.
TSLP induces myosin II-dependent DC motility
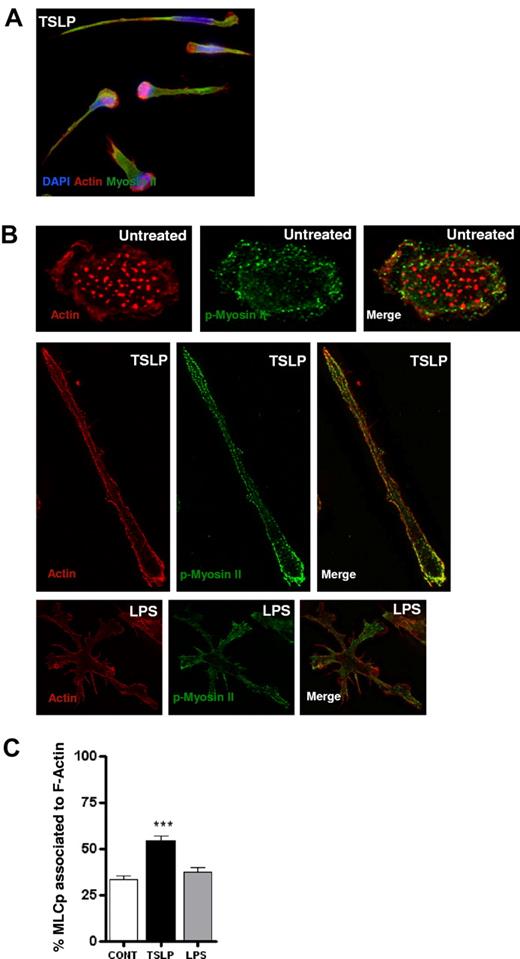
It was recently shown that the actin-based motor protein myosin II is essential for the 3-dimensional movement of mouse leukocytes.15 Thus, we addressed its role in TSLP-induced human DC motility. Myosin II consists of 2 heavy chains whose N-terminus contains a globular head that includes the actin- and adenosine triphosphate (ATP)-binding sites, and of 2 regulatory light chains (MLCs), whose phosphorylation status controls the activity of the protein motor. When phosphorylated, MLC binds to myosin II heavy chain and triggers ATP hydrolysis followed by head displacement toward the plus-end of actin filaments, resulting in actomyosin contraction. In TSLP-DCs, myosin II heavy chain showed a cortical distribution and was found to be preferentially associated with actin filaments in the cell uropod (Figure 3A), as was described for mouse leukocytes.15,16 Moreover, myosin II accumulated also in the nuclear edge of DCs (Figure 3A), suggesting contractibility of this area. Importantly, TSLP-induced myosin II redistribution was also observed for activated phospho-MLCs in TSLP-DCs (Figure 3B). Quantification after 3-dimensional reconstruction showed an increase in the colocalization of phospho-MLC and actin after TSLP treatment (Figure 3C), suggesting an increased actomyosin contractility.
TSLP induces redistribution and colocalization of actin and phospho-myosin II light chain. (A) After 24 hours of culture, TSLP-DCs were stained for myosin II (green), actin (red), and DAPI (blue). TSLP-DCs acquired a polarized morphology, and actin and myosin filaments largely colocalized. Data are from 1 representative of 3 independent experiments. (B) DCs on poly-lysine–coated coverslips were cultured in medium, TSLP, or LPS for 20 hours. Cells were stained for F-actin (red) and pMLC9 (green). Images were acquired as z-series, deconvoluted, and reconstructed as a maximum-intensity projection of all the planes. (C) Quantification of the mean percentage of pMLC area colocalized with F-actin area. For each cell, the percentage is a mean of all the planes. Data are mean ± SEM of 2 independent experiments; n = 20. ***P < .005.
TSLP induces redistribution and colocalization of actin and phospho-myosin II light chain. (A) After 24 hours of culture, TSLP-DCs were stained for myosin II (green), actin (red), and DAPI (blue). TSLP-DCs acquired a polarized morphology, and actin and myosin filaments largely colocalized. Data are from 1 representative of 3 independent experiments. (B) DCs on poly-lysine–coated coverslips were cultured in medium, TSLP, or LPS for 20 hours. Cells were stained for F-actin (red) and pMLC9 (green). Images were acquired as z-series, deconvoluted, and reconstructed as a maximum-intensity projection of all the planes. (C) Quantification of the mean percentage of pMLC area colocalized with F-actin area. For each cell, the percentage is a mean of all the planes. Data are mean ± SEM of 2 independent experiments; n = 20. ***P < .005.
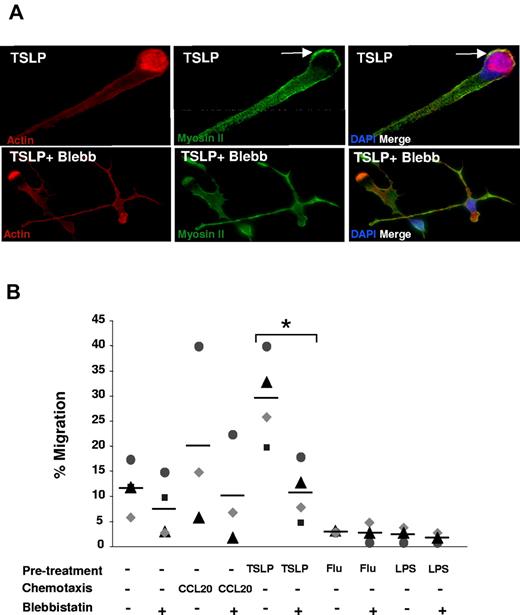
To directly address the role of myosin II in TSLP-induced DC polarization, we inhibited myosin II-ATPase activity using its specific inhibitor blebbistatin.17 Inhibition of myosin II prevented TSLP-induced DC polarization (Figure 4A). Blebbistatin-treated DCs displayed an elongated cellular shape with multidirectional extensions.18 Last, we questioned whether loss of cytoskeleton polarization affected the migration of DCs. Blebbistatin treatment decreased the TSLP-DC migration through collagen-coated semipermeable filter (from 30% to 11%; P < .01; Figure 4B). Migration of TSLP-DCs was inhibited by doses as low as 20μM blebbistatin (supplemental Figure 3). We conclude that myosin II activity is essential for both polarization and migration of human DCs induced by TSLP.
Myosin II is required for TSLP-induced polarization and motility. (A) After 24 hours of culture in the absence or presence of blebbistatin (50μM), TSLP-DCs were stained for myosin II (green), actin (red), and DAPI (blue). Blebbistatin inhibited TSLP-induced cytoskeleton rearrangements with a loss of cell polarization. Data are from 1 representative of 3 independent experiments. (B) DCs were cultured as described in Figure 1A, in the absence or presence of blebbistatin. An increased migration (6 hours) was observed after TSLP pretreatment, which was significantly decreased in the presence of blebbistatin (50μM). Each dot represents cell counts of individual experiments and bars represent the mean. *P < .05.
Myosin II is required for TSLP-induced polarization and motility. (A) After 24 hours of culture in the absence or presence of blebbistatin (50μM), TSLP-DCs were stained for myosin II (green), actin (red), and DAPI (blue). Blebbistatin inhibited TSLP-induced cytoskeleton rearrangements with a loss of cell polarization. Data are from 1 representative of 3 independent experiments. (B) DCs were cultured as described in Figure 1A, in the absence or presence of blebbistatin. An increased migration (6 hours) was observed after TSLP pretreatment, which was significantly decreased in the presence of blebbistatin (50μM). Each dot represents cell counts of individual experiments and bars represent the mean. *P < .05.
TSLP promotes DC migration in the confined environment of microfabricated channels
In vivo, DCs migrate in confined spaces, and recent evidence shows that the geometry of tissues directly impacts the requirements and mechanisms involved in cell movement.15 To assess whether TSLP may impact human DC migration in tissues, we used micro-fabricated channels that mimic the constrained interstitial space of peripheral tissues.19 Live cell imaging of DC migration revealed that TSLP-DCs were more efficient in reaching the border and entering the 4-μm-wide channels than untreated cells (Figure 5A; supplemental Videos 1-2). This effect of TSLP resulted in increased numbers of DCs traveling along microchannels at a given time after TSLP pretreatment (Figure 5A). For each migration experiment, sequential pictures of cells migrating along microchannels were visualized as kymographs, which were used to analyze persistence and calculate instantaneous velocities (Figure 5B). Once inside the channels, TSLP-DCs were more persistent and displayed a more regular and continuous movement compared with untreated DCs (changes of direction in movement inside channels: 10% of TSLP-DCs vs 32% of untreated-DCs; Figure 5B-C). No significant migration was observed when treating DCs with flu (supplemental Video 3). We found no significant difference between the median instantaneous speed of TSLP-DCs and untreated DCs, both reaching ∼ 11 μm/min in both conditions (5D). Similar results were obtained for maximal DC velocities (Figure 5D). We conclude that TSLP promotes the motility of DCs in confined environments but did not modify their velocity.
TSLP promotes DC migration in the confined environment of microchannels. (A) After 20 hours of culture in medium or TSLP, DCs were added in the starting chamber of the microchannel system. They were allowed to spontaneously enter the fibronectin-coated 4-μm-wide channels, and phase-contrast images were recorded. The number of cells entering the channels dramatically increased after TSLP activation of DCs (arrow indicates an individual cell inside a microchannel). (B) Representative kymograph of an untreated DC and a TSLP-DC generated by sequential pictures of a DC within a microchannel. We can see the untreated DC changing direction several times during the recording. Raw phase-contrast images were processed to analyze cell movement as described in “Kymograph extraction and instantaneous velocity.” (C) After 20 hours of culture, DCs were allowed to spontaneously enter the fibronectin-coated 4-μm-wide channels, and phase-contrast images were recorded. Changes of direction through the channels were quantified for individual cells. TSLP-DCs exhibited a more straight movement. Only 10% of TSLP-DCs show changes in direction inside the microchannels. (D) Kymographs obtained in panel C were processed and analyzed to extract instantaneous speed of individual cells. The distributions of median and maximal speed of DCs precultured in control medium (left panels) or with TSLP (right panels) are not significantly different (P > .05). Data are from one representative of 3 independent experiments.
TSLP promotes DC migration in the confined environment of microchannels. (A) After 20 hours of culture in medium or TSLP, DCs were added in the starting chamber of the microchannel system. They were allowed to spontaneously enter the fibronectin-coated 4-μm-wide channels, and phase-contrast images were recorded. The number of cells entering the channels dramatically increased after TSLP activation of DCs (arrow indicates an individual cell inside a microchannel). (B) Representative kymograph of an untreated DC and a TSLP-DC generated by sequential pictures of a DC within a microchannel. We can see the untreated DC changing direction several times during the recording. Raw phase-contrast images were processed to analyze cell movement as described in “Kymograph extraction and instantaneous velocity.” (C) After 20 hours of culture, DCs were allowed to spontaneously enter the fibronectin-coated 4-μm-wide channels, and phase-contrast images were recorded. Changes of direction through the channels were quantified for individual cells. TSLP-DCs exhibited a more straight movement. Only 10% of TSLP-DCs show changes in direction inside the microchannels. (D) Kymographs obtained in panel C were processed and analyzed to extract instantaneous speed of individual cells. The distributions of median and maximal speed of DCs precultured in control medium (left panels) or with TSLP (right panels) are not significantly different (P > .05). Data are from one representative of 3 independent experiments.
To assess whether the increased ability of TSLP-DCs to enter microchannels results, at least in part, from TSLP-induced polarization, we analyzed whether TSLP-induced human DC motility was compromised when inhibiting myosin II activity. Blebbistatin treatment of TSLP-DCs reduced by 50% their ability to enter microchannels (Figure 6A). Similar results were obtained using ML7/Y27632, a combination of 2 MLC phosphorylation inhibitors (Figure 6A), confirming the specificity of the inhibitory process to myosin II. 4,6-Diamidino-2-phenylindole (DAPI)/annexin V staining showed no significant change in DC viability when either myosin II inhibitors were added to TSLP, indicating that the inhibition of DC migration was not the result of an increased cell death (supplemental Figure 4). The velocity of TSLP-treated DCs was also decreased on myosin II inhibition, confirming the involvement of actomyosin contraction in DC migration in confined environments. In addition, blebbistatin modified the velocity distribution of TSLP-treated DCs: whereas 70% of TSLP-DCs exhibited velocities > 10 μm/min, only 32% of blebbistatin-treated TSLP-DCs did so (Figure 6B). We conclude that myosin II activity is required for TSLP-treated DCs to initiate a polarized movement and maintain high speed during motion along microchannels. Altogether, our results revealed that TSLP has the unexpected capacity to induce DC polarization in a myosin II-dependent manner, thereby licensing DCs to migrate in confined spaces.
Myosin II-ATPase activity is essential for DC confined migration. (A) After a pretreatment of 20 hours with TSLP in the absence or presence of blebbistatin (50μM) or a mix of ML7/Y27632 (10μM/10μM), DCs were loaded in the entry chamber of the channels. The number of DCs entering the channels during a 12-hour time-lapse movie was quantified. TSLP induced a 4-fold increase in the capacity of DCs to enter microchannels compared with control. Blebbistatin and ML7Y27632 significantly inhibited TSLP-induced migration. Data are mean ± SD; n = 3. **P < .01. ***P < .005. (B) After 20 hours of culture in the absence or presence of blebbistatin (50μM), DCs were loaded in the entry chamber and allowed to spontaneously enter in the channels. Phase-contrast images were recorded. Kymographs were processed and analyzed to extract instantaneous speed of individual cells. Distribution of the median speed of DCs precultured in TSLP in the absence or presence of blebbistatin is significantly different (P < .05). Data are from 1 representative of 2 independent experiments.
Myosin II-ATPase activity is essential for DC confined migration. (A) After a pretreatment of 20 hours with TSLP in the absence or presence of blebbistatin (50μM) or a mix of ML7/Y27632 (10μM/10μM), DCs were loaded in the entry chamber of the channels. The number of DCs entering the channels during a 12-hour time-lapse movie was quantified. TSLP induced a 4-fold increase in the capacity of DCs to enter microchannels compared with control. Blebbistatin and ML7Y27632 significantly inhibited TSLP-induced migration. Data are mean ± SD; n = 3. **P < .01. ***P < .005. (B) After 20 hours of culture in the absence or presence of blebbistatin (50μM), DCs were loaded in the entry chamber and allowed to spontaneously enter in the channels. Phase-contrast images were recorded. Kymographs were processed and analyzed to extract instantaneous speed of individual cells. Distribution of the median speed of DCs precultured in TSLP in the absence or presence of blebbistatin is significantly different (P < .05). Data are from 1 representative of 2 independent experiments.
Discussion
DC migration is a complex process that involves multiple cell-intrinsic and extrinsic factors.2,20 Chemokines are important players that orchestrate migration and mostly act through chemoattraction, guiding the responding cell toward its target tissue destination.3,21 The contribution of local inflammation in peripheral tissue is generally viewed as permitting and/or facilitating the action of chemokines in several ways: (1) danger signals can induce DC maturation and the up-regulation of chemokine receptors1,2 ; (2) inflammatory mediators, such as leukotrienes, can potentiate the effect of certain chemokines22 ; and (3) edema and vasodilation have a facilitating effect by promoting the nonspecific influx but also emigration of various immune cell types. In our study, we provide new evidence for an active role of the inflamed tissue in instructing DCs to migrate through the production of the cytokine TSLP.
It is commonly accepted that DC activation and migration are coupled, essentially through the induction of CCR7,1 which would guide DCs toward secondary lymphoid organs through a CCL21 gradient.3 Accordingly, DC-activating cytokines, such as GM-CSF and TNF, promoted Langerhans cell emigration in skin explants.23-25 Our data challenge this view in many aspects. First, we did not observe any effect of GM-CSF and TNF in triggering a cell-autonomous DC migration. This suggests that cofactors provided by the tissue environment in the skin explants may be required. Alternatively, these cytokines may act indirectly on other skin cell types, which would support subsequent DC migration. Second, TLR ligands (LPS and flu) did not induce DC migration, although they promote strong DC maturation. This is in keeping with another study showing that early LPS activation inhibited mouse DC migration,26 which may favor an efficient antigen uptake and processing at the site of inflammation.19 Thus, some DC-activating factors induce maturation without migration, whereas TSLP efficiently couples both functional responses. This suggests that the interplay between TLR ligands and proinflammatory cytokines at the inflammatory site may be important to coordinate maturation and migration in DCs.
To demonstrate the ability of TSLP to induce a 3-dimensional migration, we used microfabricated channels that enable cell confinement during motion and thus mimic the microenvironment encountered by DCs in the constrained interstitial spaces of peripheral tissues and lymphoid organs.19 Microchannels impose a directional migration to DCs, which facilitates the automatic extraction from important cell numbers of accurate measurable parameters, which cannot be achieved in systems, such as 3-dimensional artificial collagen matrices.19 We could demonstrate that, although TSLP did not increase the cell velocity compared with untreated DCs, it triggered the entry and subsequent migration of DCs inside the microchannels. This may reflect the first steps in the emigration of DCs out of the epithelium that necessitates the passage through constricted intercellular areas, the basal membrane, and the fibrotic connective tissue of the dermis.
The finding that epithelial-derived TSLP can instruct DCs to migrate reveals inflamed tissue as an important player in the complex regulation of DC migration. It will be important to identify other tissue-derived factors that may share with TSLP the ability to induce cell-autonomous chemokinesis and confined migration of DCs. The chemokinetic properties of TSLP open numerous perspectives for our understanding and pharmacologic manipulation of DC migration in settings, such as vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zofia Maciorowski and Annick Viguier for cell sorting; Dr Amigorena, Dr Ghislain, Dr Hivroz, and Dr Lantz for careful reading of the manuscript and helpful discussions; Dr Matthew Krummel for insightful advices and help on the manuscript; and the staff members of the Nikon Imaging Center and of the PICT-IBiSA imaging facility in Institut Curie.
This work was supported by the European Community Sixth Framework Program (EXT 014162), the city of Paris (Subvention Jeunes Chercheurs, Mairie de Paris), the Fondation pour la Recherche Médicale, and the Association pour la Recherche contre le Cancer. M.P., A.-M.L.-D., and V.S. were supported by InNaBioSanté for MICEMICO project and by Agence Nationale de la Recherche. M.L.H. was supported by a fellowship from the Association pour la Recherche contre le Cancer and from Agence Nationale de la Recherche. C.M.-C. was supported by a fellowship from the French Ministry of Research. B.H. was supported by the German Research Foundation (RU729).
Authorship
Contribution: M.-I.F. designed and performed research and drafted the manuscript; M.L.H. helped with experiment design, performedexperiments, and analyzed migration data; C.M.-C., E.V., and M.-H.D. performed experiments; M.P. designed migration device and analyzed data; A.-M.L.-D. and B.H. gave conceptual advice and helped with experiment design; and V.S. supervised research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.-I.F. is Laboratoire d'immunologie, CHU Sainte-Justine, Montreal, QC.
Correspondence: Maria-Isabel Fernandez, Inserm U932, Paris, F-75248, France; e-mail: isabel.fernandez.hsj@ssss.gouv.qc.ca; and Vassili Soumelis, Inserm U932, Paris, F-75248, France; e-mail: vassili.soumelis@curie.net.
References
Author notes
M.-I.F. and M.L.H. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal