Abstract
Monocytes and T helper (TH) cells rapidly infiltrate inflamed tissues where monocytes differentiate into inflammatory dendritic cells (DCs) through undefined mechanisms. Our studies indicate that TH cells frequently interact with monocytes in inflamed skin and elicit the differentiation of specialized DC subsets characteristic of these lesions. In psoriasis lesions, TH1 and TH17 cells interact with monocytes and instruct these cells to differentiate into TH1- and TH17-promoting DCs, respectively. Correspondingly, in acute atopic dermatitis, TH2 cells interact with monocytes and elicit the formation of TH2-promoting DCs. DC formation requires GM-CSF and cell contact, whereas TH subset specific cytokines dictate DC function and the expression of DC subset specific surface molecules. Moreover, the phenotypes of T cell–induced DC subsets are maintained after subsequent stimulation with a panel of TLR agonists, suggesting that TH-derived signals outweigh downstream TLR signals in their influence on DC function. These findings indicate that TH cells govern the formation and function of specialized DC subsets.
Introduction
DCs are phenotypically and functionally divergent antigen presenting cells that originate from bone marrow precursors such as monocytes.1-6 Encompassing a broad anatomical distribution, DCs continuously sample the microenvironment in search of danger signals recognized by a wide spectrum of pattern recognition receptors (PRRs).1,2,6 Depending on the cytokine milieu and the pathogen encountered, DCs differentially up-regulate costimulatory molecules and secrete a variety of cytokines that dictate the nature of the T cell response.7-10 DC exposure to particular intracellular pathogens or their products, including lipopolysaccharide (LPS) and double-stranded RNA, leads to DC-driven TH1 differentiation primarily through IL-12 secretion.11 Conversely, DCs mediate TH2 differentiation in response to extracellular parasites, such as S mansoni, which likely results from diminished IL-12 secretion and increased OX40L expression.7,8 Several reports have implicated DC-derived IL-1β, IL-6, IL-23, and TGF-β in the polarization of TH17 cells,9,12-17 which mediate defense against extracellular bacteria and various fungal infections.15
Psoriasis and atopic dermatitis are common inflammatory skin diseases characterized by the rapid accumulation of TH cells and DCs.18-22 While both diseases share several pathologic features,18,22 the TH cells implicated in the pathogenesis of each disease are distinct.19,20,23 Infiltration of TH1 and TH17 cells is commonly observed in psoriatic lesions,19 which is accompanied by elevated levels of the TH1 cytokine, IFN-γ, and the TH17 cytokines, IL-17A and IL-22.19 Conversely, acute atopic dermatitis lesions contain elevated levels of TH2 cells and their associated effector cytokines, IL-4, IL-5, and IL-13.20,23 Not surprisingly, DCs that accumulate in each of these diseases exhibit markedly different phenotypes.21,24,25 DCs present in psoriatic lesions express IL-12p40, IL-23, and TNF-α25-27 while DCs present in atopic dermatitis lesions express low or undetectable levels of these cytokines.25 DCs from each of these diseases also differentially express cell surface molecules such as DC-SIGN.21 However, the cell types and/or factors that influence the generation of these DC subsets remain elusive.
Given that memory TH cells and monocytes rapidly infiltrate inflamed tissues2 and that activated TH cells secrete high levels of GM-CSF,28 a cytokine that mediates DC differentiation from monocytes,29,30 we hypothesized that TH cells instruct monocytes to differentiate into DCs. Our data demonstrate that not only do TH cells direct monocytes to differentiate into DCs, but that each TH cell subset drives the formation of phenotypically and functionally distinct DC subsets that resemble DCs previously identified in atopic dermatitis and psoriatic lesions.21,24,25 These data support the hypothesis that memory TH cells influence the nature of the secondary immune response by regulating DC formation and function.
Methods
Cell isolation
Enriched CD4+ T cells and CD14+ monocytes were isolated from PBMCs obtained from healthy blood donors (Stanford Blood Center) by density gradient centrifugation using a custom RosetteSep cocktail (Stem Cell Technologies) containing mAbs against CD235a, CD8, CD19, CD20, CD56, CD66b and TCRγδ. CD14+ monocytes were isolated to > 97% purity using anti-CD14 conjugated microbeads (Miltenyi Biotec), and memory TH cells were purified from the CD14− fraction using a Memory CD4+ T cell Isolation Kit (Miltenyi Biotec). To sort unfractionated TH cells, enriched memory TH cells were stained with fluorescently labeled mAbs against CD14, CD16, CD19, CD25, CD36, CD56, CD123, CD235a, CD45RA, CD45RO (eBioscience), CD4 and CD8 (BD Bioscience) along with propidium iodide (PI; Invitrogen). TH cells were defined as PI−CD4+CD45RA−CD45RO+CD25−/lo. To sort for each TH subset, enriched memory T cells were stained with fluorescently labeled mAbs against CD45RA, CD4 (Invitrogen), CD25, CD127 (Biolegend), CD161, CCR4, CCR6 and CRTH2 (BD Biosciences) along with PI. TH1 cells were defined as PI−CD4+CD45RA−CD25−/loCRTH2−CD161−CCR4−CCR6−; TH2 cells were defined as PI−CD4+CD45RA−CD25−/loCRTH2+; TH17 cells were defined as PI−CD4+CD45RA−CD25−/loCRTH2−CD161+CCR4+CCR6+.
Monocyte/T-cell cocultures
Monocytes (1 × 106) were cultured with or without 1 × 105 autologous or allogeneic TH, TH1, TH2 or TH17 cells in 12-well plates (Corning) containing IMDM medium (Gibco) supplemented with 10% FCS, 2% human AB serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2mM l-glutamine, sodium pyruvate, nonessential amino acids, 50μM 2-ME and, where indicated, 20 ng/mL IL-2 (Peprotech). GM-CSF and IL-4 derived DC (DCGM) were generated as described previously.31 For imaging studies, cells were evaluated at day 6 by Leica DMIRB microscopy with a 40×/0.55 Hoffman Modulation Contrast objective and images were acquired using a digital ORCA-ER camera and Openlab acquisition software. Otherwise, cells were processed on day 6 with 5mM EDTA and subsequently stained with DAPI (Invitrogen), fluorescently labeled isotype control mAbs, or mAbs against CD11c, CD40, CD123 (Biolegend), CD14 (eBioscience), CD3, CD80, CD83, CD86, CD205, CD209 (BD Bioscience) or CD303 (Miltenyi Biotec). Cells were also stimulated at day 6 with 2 μg/mL LPS, 5 μg/mL Pam3CSK4, 10 μg/mL CpG, 10 μg/mL zymosan, 25 μg/mL poly(I:C), 1 μg/mL flagellin, 5 μg/mL imiquimod, 1 × 108 cells/mL heat killed Listeria monocytogenes (HKLM), 1 μg/mL 3M-002 or 2 μg/mL N-glycol MDP for 36 hours and stained with the aforementioned mAbs against DC surface molecules or incubated with 100 μg/mL DQ-OVA (Invitrogen) on ice or at 37°C for an additional 30 minutes. Cells were analyzed on a BD LSRII flow cytometer, and FACS plots and MFIs were generated by FlowJo (Treestar). T cells were excluded according to their FSC:SSC profiles and CD3 expression. Cell-free supernatants were collected from LPS stimulated cultures for measurement of IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, and TNF-α by ELISA. For neutralization studies, isotype control mAbs or mAbs against GM-CSF (Biolegend), CD119 (BD Bioscience), IL-17R, IL-21R (R&D Systems), IL-4R, IL-13, IFN-γ, TNF-α, or CD40L (eBioscience) were added at the initiation of culture at 10 μg/mL. Transwell experiments were performed using 24-well 0.4μM transwell inserts (Corning) with TH cell and monocytes placed in the upper insert and monocytes below the insert or with TH cells placed in the upper insert and monocytes in the lower insert.
Mixed leukocyte reaction (MLR)
Autologous TH cells were magnetically depleted from LPS-stimulated monocytes, DCTh and DCGM using biotinylated anti-CD3 and anti-TCRα/β mAbs followed by anti-biotin microbeads (Miltenyi Biotec). Cells were irradiated (3000 rad) and cultured for 6 days at a 1:5 ratio with allogeneic naive CD4+CD45RA+CD45RO−CD25− T cells isolated from PBMCs with a Naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec). Cultures were pulsed with 1 μCi/well of 3H-thymidine for the last 20 hours and harvested by Harvester 400 (Tomtec), and radioactivity was measured by a 1450 MicroBeta counter (LKB Wallac). For DCTh subsets, monocytes and TH cells were cultured as described in “Monocyte/T-cell cocultures,” and after 6 days, T cell–depleted DCTh (> 95% by magnetic depletion; > 99% by FACS purification) were stimulated with 2 μg/mL LPS for 36 hours and subsequently cultured at a 1:1 ratio with allogeneic naive CD4+CD45RA+CD45RO−CD25− T cells. After 6 days, supernatants were assayed for IFN-γ, IL-5, IL-13, and IL-17A by ELISA.
Immunohistochemistry
Sections were deparaffinized in 3 changes of xylene followed by graded alcohols. Antigen retrieval was performed using pH 6.0 Diva universal retrieval solution (Biocare Medical) inside a pressure cooker (Biocare Medical). Endogenous peroxidase activity was quenched using 3% H202 (Lab Vision). Sections were blocked with serum-free protein blocker (Dako). For immunohistochemical stains in which primary antibodies were raised in different species, sections were incubated with a cocktail of mouse and rabbit primary antibodies inside a moist chamber followed by detection using a MACH-2 detection kit (Biocare Medical). Color development was performed with freshly prepared 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution (Vector Labs) and Ferangi Blue solution (Biocare Medical) before mounting with Faramount Mounting Medium (Dako).
For stains in which the primary antibodies were raised in the same species, the protocol was carried out sequentially. The first antibody was detected with either an alkaline phosphatase or HRP-conjugated polymer kit (Biocare Medical) and developed in fresh DAB solution. Before the addition of the next primary antibody, the first reaction complex was treated with denaturing solution (Biocare Medical).
The primary antibodies used were: mouse monoclonal anti-CD4 (1:10, Vector), rabbit polyclonal anti-CD14 (1:200; Atlas), mouse monoclonal anti-GATA-3 (1:100; Santa Cruz Biotechnology), rabbit monoclonal anti–T-bet (1:100; Santa Cruz Biotechnology) and rabbit polyclonal anti-RORγ (1:40; Novus Biologicals).
Sections were evaluated by an Olympus BX51 microscope with a 40×/0.95 objective and images were acquired using a CRI Nuance VIS Flex camera and CRI Nuance acquisition software. Images were subsequently imported into ImageJ (National Institutes of Health).
ELISA and statistical analysis
Antibody pairs for IL-1β, IL-4, IL-5, IL-10, IL-17A, IL-23p19 (eBioscience), IL-13, and GM-CSF (BD Bioscience) were used for ELISA analysis. BD OptEIA kits were used for IL-6, IL-12p70, and TNF-α measurements. Samples were run in duplicate, and data and statistics were analyzed with GraphPad Prism. A 2-tailed Wilcoxon rank-sum test was used to analyze all pairwise comparisons between conditions. Two-tailed Wilcoxon rank-sum tests were also used to analyze the flow cytometry data.
Results
TH cells are juxtaposed with monocytes in inflamed skin and convert monocytes into DCs
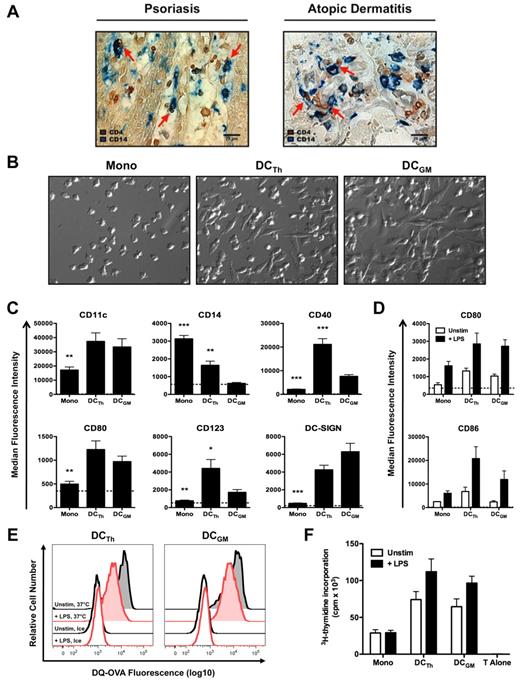
Infiltration of tissues with TH cells is a distinguishing feature of a variety of inflammatory diseases, including atopic dermatitis and psoriasis.19,20,22 To investigate the possible interplay between monocytes and TH cells in situ, skin biopsies from patients with atopic dermatitis and psoriasis were stained by immunohistochemistry with anti-CD4 and anti-CD14 antibodies. In both diseases, CD4+ and CD14+ cells were found in close apposition, suggesting that TH cells interact with monocytes in inflamed skin (Figure 1A).
TH cells are juxtaposed with monocytes in inflamed skin and drive monocyte differentiation into DCs. (A) Representative skin lesions from psoriasis and atopic dermatitis patients were stained with antibodies against CD4 (brown) and CD14 (blue). Images are shown at 400× magnification and were acquired using the equipment described in “Immunohistochemistry.” Red arrows indicate potential monocyte/T cell interactions. Scale bar equals 20 μm. (B-F) CD14+ monocytes were cultured with IL-2 (Mono), autologous TH cells and IL-2 (DCTh), or GM-CSF and IL-4 (DCGM) for 6 days. (B) Hoffman modulation contrast microscopy shown at 400× magnification. Images were acquired using the equipment described in “Monocyte/T-cell cocultures.” Data are representative of > 10 donors. (C) Median fluorescence intensities (MFIs) of DC associated molecules are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM); *P < .05, **P < .01, ***P < .001 compared with DCGM. (D-F) On day 6, Mono, DCTh and DCGM were cultured for 36 hours in the presence (+ LPS) or absence (Unstim) of LPS. (D) MFIs of CD80 and CD86 are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 2 independent experiments and 6 donors (mean and SEM). (E) Unstimulated (black histograms) and LPS-stimulated (red histograms) DCTh and DCGM were incubated with DQ-OVA for 30 minutes on ice (open histograms) or at 37°C (shaded histograms). DQ-OVA expression is shown after excluding TH cells. Data are representative of 2 independent experiments and 2 donors. (F) Allogeneic naive CD4+ T cells were cultured alone (T Alone) or with irradiated Mono, DCTh or DCGM with and without LPS stimulation at a 1:5 APC to T cell ratio in an MLR. After 6 days, 3H-thymidine was added for an additional 20 hours and T cell proliferation was assessed. Data are from 2 independent experiments and 5 donors (mean and SEM).
TH cells are juxtaposed with monocytes in inflamed skin and drive monocyte differentiation into DCs. (A) Representative skin lesions from psoriasis and atopic dermatitis patients were stained with antibodies against CD4 (brown) and CD14 (blue). Images are shown at 400× magnification and were acquired using the equipment described in “Immunohistochemistry.” Red arrows indicate potential monocyte/T cell interactions. Scale bar equals 20 μm. (B-F) CD14+ monocytes were cultured with IL-2 (Mono), autologous TH cells and IL-2 (DCTh), or GM-CSF and IL-4 (DCGM) for 6 days. (B) Hoffman modulation contrast microscopy shown at 400× magnification. Images were acquired using the equipment described in “Monocyte/T-cell cocultures.” Data are representative of > 10 donors. (C) Median fluorescence intensities (MFIs) of DC associated molecules are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM); *P < .05, **P < .01, ***P < .001 compared with DCGM. (D-F) On day 6, Mono, DCTh and DCGM were cultured for 36 hours in the presence (+ LPS) or absence (Unstim) of LPS. (D) MFIs of CD80 and CD86 are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 2 independent experiments and 6 donors (mean and SEM). (E) Unstimulated (black histograms) and LPS-stimulated (red histograms) DCTh and DCGM were incubated with DQ-OVA for 30 minutes on ice (open histograms) or at 37°C (shaded histograms). DQ-OVA expression is shown after excluding TH cells. Data are representative of 2 independent experiments and 2 donors. (F) Allogeneic naive CD4+ T cells were cultured alone (T Alone) or with irradiated Mono, DCTh or DCGM with and without LPS stimulation at a 1:5 APC to T cell ratio in an MLR. After 6 days, 3H-thymidine was added for an additional 20 hours and T cell proliferation was assessed. Data are from 2 independent experiments and 5 donors (mean and SEM).
After infiltrating inflamed tissues, monocytes can differentiate into DCs.3-5 To determine whether TH cells trigger monocyte differentiation, FACS purified memory CD4+CD45RO+CD45RA−CD25−/lo TH cells from healthy donors and autologous CD14+ monocytes were cocultured with IL-2 for 6 days. Monocytes cultured under these conditions (DCTh) underwent morphologic changes similar to those of monocyte-derived DCs generated with GM-CSF and IL-4 (DCGM; Figure 1B). Multicolor flow cytometry analysis indicated that DCTh and DCGM expressed similar levels of DC-associated molecules such as CD11c, CD80 and CD209 (DC-SIGN; Figure 1C). DCGM expressed lower levels of CD14, CD40 and CD123 in comparison to DCTh (Figure 1C). Both cell types expressed high levels of CD11c (Figure 1C) and low or undetectable levels of CD303 (BDCA-2; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which is consistent with the phenotype of myeloid DCs.32 Conversely, monocytes cultured in medium alone, IL-2 alone (Mono) or with TH cells alone did not exhibit an altered morphology and uniformly expressed low levels of DC-associated molecules (Figure 1B-C and supplemental Figure 1A-B). Moreover, monocytes cocultured with TH cells and nominal antigens, such as tetanus toxoid and influenza peptides, or monocytes cocultured with TH cells and IL-15, differentiated into DCs with a phenotype similar to that described in Figure 1B and C, (supplemental Figure 1C,E and data not shown). Addition of IL-2, IL-15, or nominal antigens in this system was necessary for GM-CSF secretion by TH cells (supplemental Figure 1D and data not shown). These data indicate that TH cells instruct monocytes to form DCs under a variety of conditions.
DCTh are functionally similar to DCGM
On activation, DCs increase the expression of costimulatory molecules, exhibit reduced endocytic activity and transform into potent antigen presenting cells.6 To assess the functional capacity of DCTh, cells were cultured in the presence or absence of LPS for 36 hours. Under these conditions, DCTh up-regulated the expression of costimulatory molecules CD80 and CD86 to comparable or higher levels than DCGM (Figure 1D). After LPS stimulation, DCTh and DCGM exhibited reduced endocytic activity as measured by DQ-OVA fluorescence (Figure 1E). As expected, cells that were cultured on ice with DQ-OVA failed to internalize antigen (Figure 1E). To assess the capacity of DCTh to stimulate naive T cell proliferation, T cell–depleted DCTh were irradiated and subsequently cultured with allogeneic naive CD4+CD45RA+CD45RO−CD25− T cells in a mixed leukocyte reaction (MLR). Both LPS-stimulated and unstimulated DCTh induced naive T cell proliferation to a similar extent as DCGM (Figure 1F). Taken together, these data indicate that DCTh and DCGM are functionally similar.
Cell contact, GM-CSF, and TNF-α mediate DCTh formation
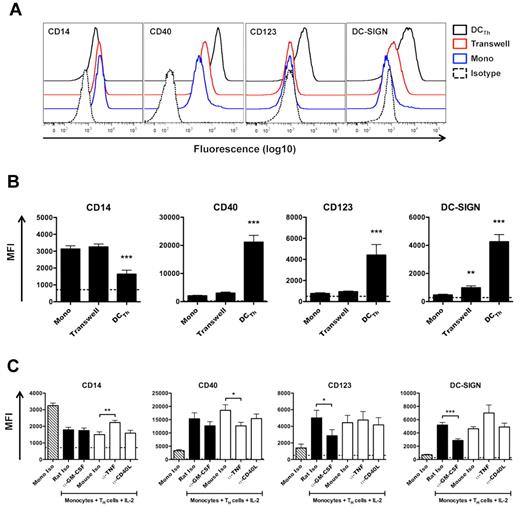
To investigate whether cell contact is required for DCTh differentiation, monocytes and autologous TH cells were cocultured with IL-2 on opposite sides of a 0.4 μm transwell membrane that allowed for the diffusion of soluble molecules, but not cells. Monocytes cultured under these conditions remained CD14hi and did not up-regulate CD40 or CD123 (Figure 2A-B). DC-SIGN also remained low when monocytes were separated from TH cells by a transwell membrane (Figure 2A-B). These data indicate that direct cell-cell contact is required for DCTh differentiation.
Cell contact, GM-CSF, and TNF-α mediate DCTh differentiation. (A, B) CD14+ monocytes were cultured for 6 days with IL-2 (Mono), IL-2 and autologous TH cells (DCTh), or IL-2 and autologous TH cells on opposite sides of a 0.4 μm transwell membrane (Transwell) for 6 days. Cell surface expression of DC associated molecules is shown. (A) Representative FACS plots are shown from 4 independent experiments and > 10 donors. Isotype controls for straining (Isotype) are shown as dashed lines. (B) MFIs are shown. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM), **P < .01, ***P < .001 compared with Mono. (C) CD14+ monocytes were cultured for 6 days with IL-2 and isotype control Ab (Mono Iso) or autologous TH cells, IL-2 and the indicated isotype control or neutralizing Ab. Filled columns indicate rat IgG1 Ab, while open columns indicate mouse IgG1 Ab. Mono Iso (hatched columns) represent combined values of rat IgG1 and mouse IgG1 isotype control Ab. MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM), *P < .05, **P < .01, ***P < .001.
Cell contact, GM-CSF, and TNF-α mediate DCTh differentiation. (A, B) CD14+ monocytes were cultured for 6 days with IL-2 (Mono), IL-2 and autologous TH cells (DCTh), or IL-2 and autologous TH cells on opposite sides of a 0.4 μm transwell membrane (Transwell) for 6 days. Cell surface expression of DC associated molecules is shown. (A) Representative FACS plots are shown from 4 independent experiments and > 10 donors. Isotype controls for straining (Isotype) are shown as dashed lines. (B) MFIs are shown. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM), **P < .01, ***P < .001 compared with Mono. (C) CD14+ monocytes were cultured for 6 days with IL-2 and isotype control Ab (Mono Iso) or autologous TH cells, IL-2 and the indicated isotype control or neutralizing Ab. Filled columns indicate rat IgG1 Ab, while open columns indicate mouse IgG1 Ab. Mono Iso (hatched columns) represent combined values of rat IgG1 and mouse IgG1 isotype control Ab. MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 4 independent experiments and > 10 donors (mean and SEM), *P < .05, **P < .01, ***P < .001.
To identify soluble or cell surface molecules capable of influencing DCTh differentiation, monocytes and autologous TH cells were cocultured with IL-2 and isotype control or neutralizing mAb against GM-CSF, TNF-α or CD154 (CD40L). Analysis of cocultures on day 6 demonstrated that GM-CSF neutralization inhibited DCTh differentiation based on reduced acquisition of DC-SIGN and CD123 (Figure 2C). As expected, GM-CSF neutralization did not affect CD14 levels.30 Conversely, TNF-α neutralization inhibited CD14 down-regulation and CD40 up-regulation (Figure 2C). Neutralization of GM-CSF or, to a lesser extent, TNF-α also prevented the acquisition of DC-like morphology (data not shown). These data suggest that GM-CSF and TNF-α play synergistic roles in DCTh differentiation. Blocking CD40-CD40L interactions did not appear to alter DC differentiation (Figure 2C), which was surprising given the high levels of CD40 expression on DCTh (Figure 1C).
Monocytes predominantly interact with TH1 and TH17 cells in psoriatic skin lesions and with TH2 cells in atopic dermatitis
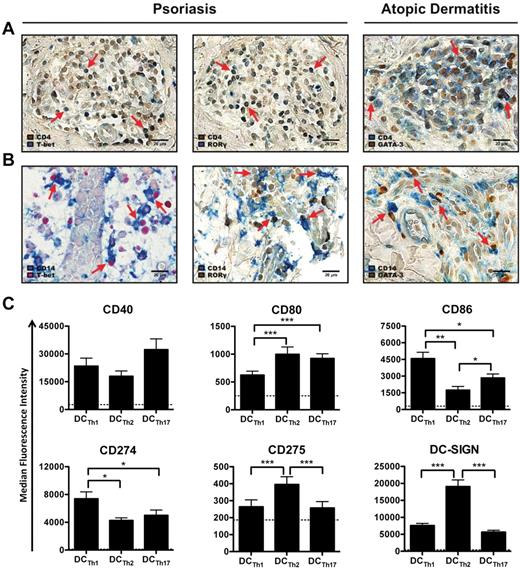
To investigate the potential interactions between TH subsets and monocytes in atopic dermatitis and psoriatic lesions, TH1, TH2, and TH17 cells were identified according to the expression of their master regulatory transcription factors T-bet, GATA-3 and RORγ, respectively.33-35 In both diseases, the majority of T-bet+, GATA-3+ or RORγ+ cells were CD4+, which is consistent with their identity as TH cells (Figure 3A). As expected, infiltrating GATA-3+ cells in atopic dermatitis lesions were present at higher levels than T-bet+ and RORγ+ cells (supplemental Figure 2A and data not shown). Numerous CD14+ monocytes were also present in the infiltrate and were frequently juxtaposed to and/or in multipoint contact with GATA-3+ cells (Figure 3B). In contrast, most infiltrating CD4+ cells in psoriatic skin lesions were T-bet+ or RORγ+ (supplemental Figure 2A), and many of these cells were in close proximity with CD14+ cells (Figure 3B). Activated DCs, identified by CD208 (DC-LAMP) expression, were also found in close vicinity and/or multipoint contact with TH cells in atopic dermatitis and psoriasis (supplemental Figure 2B). Given that DCs that arise in the presence of TH1/TH17 cells in psoriasis are phenotypically and functional divergent from DCs formed in the presence of TH2 cells in atopic dermatitis,21,24,25 we investigated the impact of different TH cell subsets on DCTh differentiation.
TH subsets instruct monocytes to form specialized DC subsets. (A-B) Representative skin lesions from psoriasis and atopic dermatitis patients were stained with Abs against T-bet (left column), RORγ (middle column) or GATA-3 (right column) and (A) CD4 or (B) CD14. Images are shown at 400× magnification. Red arrows indicate examples of (A) double positive cells or (B) monocyte/TH cell interactions. Scale bar equals 20 μm. (C) CD14+ monocytes were cultured with IL-2 and allogeneic TH1 cells (DCTh1), TH2 cells (DCTh2) or TH17 cells (DCTh17). After 6 days, DCTh subsets were analyzed for surface expression of DC-associated molecules. MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001.
TH subsets instruct monocytes to form specialized DC subsets. (A-B) Representative skin lesions from psoriasis and atopic dermatitis patients were stained with Abs against T-bet (left column), RORγ (middle column) or GATA-3 (right column) and (A) CD4 or (B) CD14. Images are shown at 400× magnification. Red arrows indicate examples of (A) double positive cells or (B) monocyte/TH cell interactions. Scale bar equals 20 μm. (C) CD14+ monocytes were cultured with IL-2 and allogeneic TH1 cells (DCTh1), TH2 cells (DCTh2) or TH17 cells (DCTh17). After 6 days, DCTh subsets were analyzed for surface expression of DC-associated molecules. MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001.
Freshly isolated TH1, TH2, and TH17 cells convert monocytes into specialized DCTh subsets
To determine whether TH cell subsets are sufficient to generate DC diversity, we FACS purified TH1, TH2, and TH17 cells from healthy donors36-38 (supplemental Figure 2C-D) and subsequently cocultured each subset with allogeneic monocytes to simulate an inflammatory setting. Similar to monocytes cocultured with unfractionated TH cells, monocytes cocultured with each TH subset differentiated into DCs that resembled the DCs described in Figure 1C (supplemental Figure 2E). More importantly, each TH subset elicited the formation of phenotypically distinct DCTh subsets. DCTh2 routinely expressed higher levels of DC-SIGN and CD275 (ICOSL; Figure 3C), which is consistent with reports indicating that TH2-associated cytokines increase DC-SIGN and CD275 expression.39-41 Conversely, DCTh1 expressed elevated levels of CD86 and the inhibitory receptor, CD274 (PDL-1; B7-H1), but reduced levels of CD80 (Figure 3C).
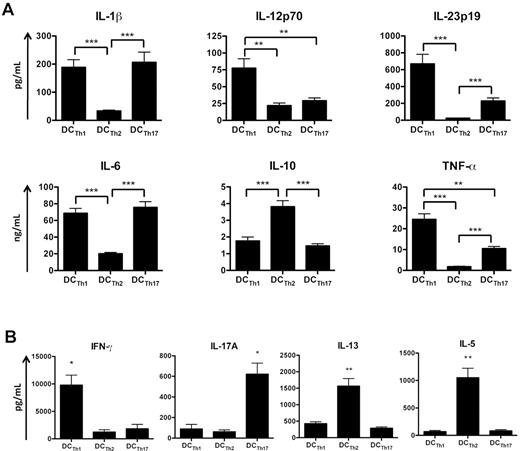
To examine the cytokine secretion profile of each DCTh subset, cell-free supernatants from LPS-stimulated cultures were analyzed by ELISA. Similar to previously purified DCs from psoriatic lesions,25,27 DCTh1 and DCTh17 secreted high levels of IL-1β, IL-6, IL-23, and TNF-α (Figure 4A). DCTh1 also secreted elevated levels of IL-12p70 (Figure 4A), a potent TH1 polarizing cytokine that inhibits TH2 and TH17 differentiation.42,43 Intracellular analysis of IL-1β, IL-12p40, and TNF-α confirmed these results (supplemental Figure 3A-B). Conversely, DCTh2 more closely resembled DCs previously purified from atopic dermatitis lesions21,25 and produced little or no TNF-α, IL-12p70, and IL-23 (Figure 4A). DCTh2 also produced high levels of IL-10 (Figure 4A). Similar cytokine secretion profiles were observed with DCTh generated with autologous TH cells or by DCTh stimulated with LPS in the absence of TH cells (supplemental Figure 3C-D). Overall, these data indicate that cytokines secreted by DCTh1 would be expected to promote a TH1 response,11,42 whereas the secretion of IL-1β, IL-6 and IL-23, but not IL-12p70, by DCTh17 would be expected to promote a TH17 response.12-16 Similarly, the DCTh2 cell surface and cytokine profile favors a TH2 response.7,8,42,43
DCTh subsets promote distinct TH cell responses. CD14+ monocytes were cultured with IL-2 and allogeneic TH1 cells (DCTh1), TH2 cells (DCTh2), or TH17 cells (DCTh17). (A) On day 6 of culture, each DCTh coculture was stimulated with LPS for 36 hours. Supernatants were subsequently collected for measurement of IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, and TNF-α by ELISA. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (B) T cell–depleted DCTh subsets from autologous cocultures were stimulated with LPS at day 6 for 36 hours and subsequently cultured with allogeneic naive CD4+ T cells in an MLR. After 6 days, supernatants were collected for measurement of IL-5, IL-13, IL-17A, and IFN-γ by ELISA. Data are from 4 independent experiments and 4 donors (mean and SEM), *P < .05, **P < .01 compared with all other subsets. See also supplemental Figure 4.
DCTh subsets promote distinct TH cell responses. CD14+ monocytes were cultured with IL-2 and allogeneic TH1 cells (DCTh1), TH2 cells (DCTh2), or TH17 cells (DCTh17). (A) On day 6 of culture, each DCTh coculture was stimulated with LPS for 36 hours. Supernatants were subsequently collected for measurement of IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, and TNF-α by ELISA. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (B) T cell–depleted DCTh subsets from autologous cocultures were stimulated with LPS at day 6 for 36 hours and subsequently cultured with allogeneic naive CD4+ T cells in an MLR. After 6 days, supernatants were collected for measurement of IL-5, IL-13, IL-17A, and IFN-γ by ELISA. Data are from 4 independent experiments and 4 donors (mean and SEM), *P < .05, **P < .01 compared with all other subsets. See also supplemental Figure 4.
DCTh subsets trigger distinct T-cell responses in an MLR
Given the marked differences in the cell surface and cytokine secretion profiles among the DCTh subsets, we investigated whether each subset could differentially impact naive CD4+ T-cell polarization. Magnetically purified DCTh subsets from autologous cocultures were stimulated with LPS for 36 hours and cultured in the presence or absence of allogeneic naive CD4+CD45RA+CD45RO−CD25− T cells in an MLR. After 6 days, cell-free supernatants were assayed for the levels of IL-5, IL-13, IL-17A, and IFN-γ by ELISA. Notably, naive T cells cultured in the presence of DCTh1 secreted elevated levels of the prototypic TH1 cytokine, IFN-γ (Figure 4B). In contrast, DCTh2 induced naive T cells to produce elevated levels of IL-5 and IL-13, indicative of a TH2 response, while naive T cells cocultured with DCTh17 secreted higher levels of IL-17A (Figure 4B). These data were confirmed by intracellular cytokine staining (supplemental Figure 4A). Because magnetic beads were used for DCTh isolation, it was possible that residual TH cells from the original cocultures could confound the cytokine data. However, similar results were obtained with FACS purified DCTh subsets (> 99% purity; data not shown). Furthermore, the stated cytokines were largely undetectable when DCTh subsets were cultured in the absence of naive T cells (supplemental Figure 4B) indicating that the contribution of residual TH cells was negligible. These data indicate that each DCTh subset promotes an immune response that mirrors the effector function of the TH cell that initiated DCTh differentiation.
TH cell specific cytokines and monocyte-TH cell contact regulate the formation of specialized DCTh subsets
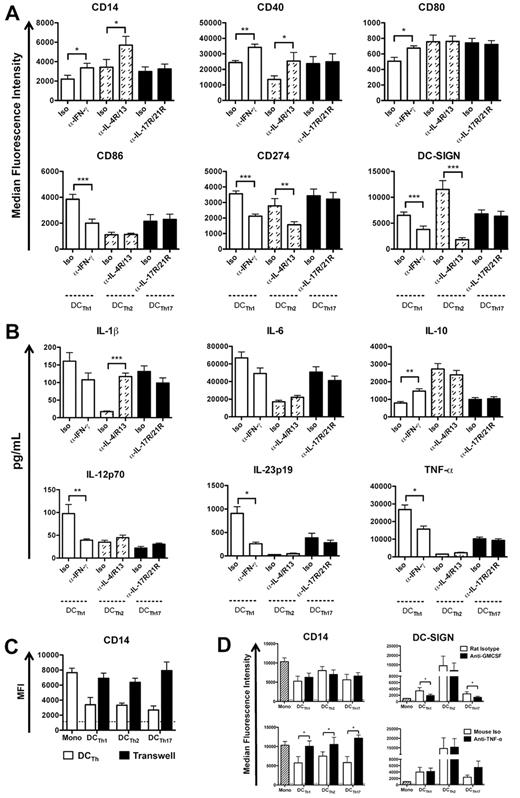
To dissect the mechanisms governing the formation of distinct DCTh subsets, monocytes and allogeneic TH cells were cocultured in the presence of isotype control or TH-specific neutralizing mAb. Analysis of cocultures on day 6 indicated that IFN-γ neutralization in DCTh1 cocultures reduced the acquisition of a DCTh1 phenotype as assessed by decreased CD86 and CD274 expression, but increased CD80 expression (Figure 5A). IFN-γ neutralization in DCTh1 cocultures and IL-4R/IL-13 neutralization in DCTh2 cocultures reduced the acquisition of a DC-like phenotype based on increased CD14 expression and decreased DC-SIGN expression (Figure 5A). Down-regulation of DC-SIGN after IL-4R/IL-13 neutralization is consistent with the high levels of DC-SIGN on DCTh2 in comparison to the other DCTh subsets (Figure 3C). Neutralization of IL-17R/IL-21R or IL-22 did not alter the surface phenotype of DCTh17 according to any of the markers tested (Figure 5A and data not shown).
TH cell specific cytokines and monocyte-TH cell contact regulate the formation of specialized DCTh subsets. (A,B) DCTh1 (open columns), DCTh2 (hatched columns) or DCTh17 (filled columns) were formed in the presence of isotype control (Iso) or the indicated neutralizing antibodies for 6 days. (A) MFIs of DC-associated molecules are shown after excluding TH cells. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (B) On day 6 of culture, each DCTh coculture was stimulated with LPS in the presence of the indicated isotype control (Iso) or neutralizing Ab for 36 hours. Supernatants were subsequently collected for measurement of IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, and TNF-α by ELISA. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (C) CD14+ monocytes were cultured for 6 days with IL-2 (Mono), IL-2 and allogeneic TH cells (DCTh), or IL-2 and allogeneic TH cells on top of a 0.4 μm transwell membrane and CD14+ monocytes below the transwell insert (Transwell). MFIs of cells below the transwell insert are shown. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 1 experiment and 3 donors (mean and SEM). (D) CD14+ monocytes were cultured for 6 days with IL-2 and isotype control Ab (Mono, hatched column) or TH cells, IL-2 and the indicated isotype control (open columns) or neutralizing Ab (filled columns). MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 3 independent experiments and 6 donors (mean and SEM), *P < .05.
TH cell specific cytokines and monocyte-TH cell contact regulate the formation of specialized DCTh subsets. (A,B) DCTh1 (open columns), DCTh2 (hatched columns) or DCTh17 (filled columns) were formed in the presence of isotype control (Iso) or the indicated neutralizing antibodies for 6 days. (A) MFIs of DC-associated molecules are shown after excluding TH cells. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (B) On day 6 of culture, each DCTh coculture was stimulated with LPS in the presence of the indicated isotype control (Iso) or neutralizing Ab for 36 hours. Supernatants were subsequently collected for measurement of IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, and TNF-α by ELISA. Data are from 5 independent experiments and > 10 donors (mean and SEM), *P < .05; **P < .01; ***P < .001. (C) CD14+ monocytes were cultured for 6 days with IL-2 (Mono), IL-2 and allogeneic TH cells (DCTh), or IL-2 and allogeneic TH cells on top of a 0.4 μm transwell membrane and CD14+ monocytes below the transwell insert (Transwell). MFIs of cells below the transwell insert are shown. Dashed lines indicate the average MFIs of isotype control Abs used for staining from all culture conditions. Data are from 1 experiment and 3 donors (mean and SEM). (D) CD14+ monocytes were cultured for 6 days with IL-2 and isotype control Ab (Mono, hatched column) or TH cells, IL-2 and the indicated isotype control (open columns) or neutralizing Ab (filled columns). MFIs are shown after excluding TH cells. Dashed lines indicate the average MFIs of isotype control Ab used for staining from all culture conditions. Data are from 3 independent experiments and 6 donors (mean and SEM), *P < .05.
To determine which TH-specific cytokines influence DCTh cytokine secretion, DCTh subsets were generated as in Figure 4 and stimulated with LPS in the presence of the appropriate isotype control or neutralizing mAb. After 36 hours, cell-free supernatants were analyzed by ELISA. The data indicate that IFN-γ neutralization reduced the acquisition of a DCTh1 phenotype in favor of a DCTh2 phenotype as assessed by increased IL-10 secretion and reduced secretion of IL-12p70, IL-23, and TNF-α (Figure 5B). Similarly, neutralization of IL-4R and IL-13 in DCTh2 cocultures shifted the acquisition of a DCTh2 phenotype toward a DCTh1/DCTh17 phenotype as assessed by increased IL-1β secretion (Figure 5B). Interestingly, neutralization of IL-17R/IL-21R or IL-22 did not alter the cytokine secretion profile of DCTh17 (Figure 5B and data not shown) indicating that as of yet unidentified cytokines or cell surface molecules influence DCTh17 formation and function.
To assess whether direct contact between TH cells and monocytes was required for the formation of DCTh subsets, we separated monocytes from monocyte-TH cell cocultures with a 0.4-μm transwell membrane. Analysis of the cells on day 6 indicated that cell contact was necessary for the formation of each DCTh subset based on the failure of monocytes separated from monocyte-TH cell cocultures to down-regulate CD14 (Figure 5C). We also assessed the role of GM-CSF and TNF-α in the formation of DCTh subsets by adding neutralizing mAb to monocyte-TH cell cocultures. The data indicate that DCTh1 and DCTh17 formation required both GM-CSF and TNF-α, whereas DCTh2 formation was independent of GM-CSF and partly dependent on TNF-α (Figure 5D). Thus, TH specific cytokines and direct TH cell-monocyte contact are required for the full acquisition of a DC phenotype.
TH cells, not PRR agonists, regulate DCTh cytokine secretion profiles
As previously mentioned, DC responses are modulated according to the type of pathogen encountered.7,9,10 To assess whether memory TH cells or PRR engagement dominate DCTh responsiveness, DCTh cocultures were stimulated with a range of PRR agonists (TLR1-9 and NOD2) for 36 hours. Surprisingly, analysis of cell-free supernatants indicated that differential PRR engagement modulated the total amount of cytokine secreted, but did not alter the overall cytokine secretion profile. For example, DCTh2 consistently secreted high amounts of IL-10 and relatively low amounts of IL-1β, TNF-α, and IL-23 regardless of PRR engagement (Figure 6). Instances where IL-10 secretion was higher in DCTh2 such as HKLM or zymosan stimulation were also associated with concomitant increases in IL-1β, IL-6, IL-23, and TNF-α (Figure 6). Similarly, DCTh1 and DCTh17 typically secreted higher levels of IL-1β, IL-6, IL-23, and TNF-α in comparison to DCTh2 (Figure 6). While some minor exceptions exist (ie DCTh1, poly:IC), increases or decreases in the levels of IL-1β, IL-6, IL-23, and TNF-α were generally associated with concomitant increases or decreases in the other cytokines of this group (Figure 6). Taken together, these data indicate that not only do memory TH cells elicit DC formation from monocytes, but that once formed, the DCs retain their functional phenotype regardless of subsequent TLR stimulation.
TH cells, not PRR agonists, regulate DCTh cytokine secretion profiles. DCTh1, DCTh2, and DCTh17 were generated as in Figure 3C. On day 6 of coculture, each DCTh subset was stimulated with the indicated pattern recognition receptor (PRR) or left unstimulated (Unstim). After 36 hours, cell-free supernatants were collected for measurement of IL-1β, IL-6, IL-10, IL-23p19, and TNF-α by ELISA. IL-1β is displayed as log2 values; IL-10 and IL-23 as log5 values; and IL-6 and TNF-α as log10 values. Data are from 2 independent experiments and 4 donors.
TH cells, not PRR agonists, regulate DCTh cytokine secretion profiles. DCTh1, DCTh2, and DCTh17 were generated as in Figure 3C. On day 6 of coculture, each DCTh subset was stimulated with the indicated pattern recognition receptor (PRR) or left unstimulated (Unstim). After 36 hours, cell-free supernatants were collected for measurement of IL-1β, IL-6, IL-10, IL-23p19, and TNF-α by ELISA. IL-1β is displayed as log2 values; IL-10 and IL-23 as log5 values; and IL-6 and TNF-α as log10 values. Data are from 2 independent experiments and 4 donors.
Discussion
Several studies have demonstrated that monocytes infiltrate inflamed tissues and differentiate into CD11c+ DCs in vivo.3-5 However, the mechanisms responsible for DC formation have remained elusive. The present study extends these findings by elucidating the mechanism whereby human TH cells drive DC differentiation from monocytes. Our results demonstrate that TH cells are juxtaposed with monocytes in situ and convert monocytes into DCs in a cell contact, GM-CSF and TNF-α dependent manner. This TH-mediated DC differentiation pathway proceeds upon IL-2 stimulation, which is consistent with our data demonstrating that IL-2 induces GM-CSF secretion from TH cells. This study also indicates that TH cells convert antigen-loaded (tetanus toxoid or influenza antigens) monocytes into DCs in the absence of exogenous stimulation. These data indicate that TH cells may participate in the innate response by initiating a positive feedback loop with monocytes and DCs that govern an intensified and polarized immune response.
One of the striking findings of this study is the capacity of each TH subset to elicit the formation of specialized DC subsets. The data indicate that TH1 cells instruct monocytes to form DCs that secrete IL-12 and express increased CD86 and CD274, whereas TH17 cells elicit the formation of DCs that secrete IL-1β, IL-6 and IL-23, but not IL-12. The cytokines secreted by DCTh1 and DCTh17 are among the key cytokines used in vitro to generate TH1 and TH17 cells, respectively.14,15 Interestingly, TH2 cells drive the formation of TH2-biased DCs that express elevated levels of IL-10, CD275 and DC-SIGN. While CD275 is known to promote IL-4 responses, IL-10 and DC-SIGN have been implicated in the inhibition of TH1 and TH17 responses, respectively.17,44 Subsequent use of DCTh1 and DCTh17 as stimulators in an MLR induced responding CD4+ T cells to produce IFN-γ and IL-17A, respectively. While the identification of DC-derived soluble factors that influence TH2 differentiation remains elusive,10 DCTh2 nevertheless promoted IL-5 and IL-13 secretion from naive T cells. This implies that unidentified soluble factors or cell surface molecules such as OX40/OX40L, CD275 or DC-SIGN may be involved.7,8,41 Gene expression profiling of each DCTh subset may prove useful for identifying additional molecules involved in the formation and functions of DC subsets.
Our data indicate that distinct sets of TH-derived molecules were responsible for DCTh formation and polarization. Whereas all memory TH subsets produced GM-CSF and TNF-α, the 2 cytokines required for generating DC morphology in our system, IFN-γ produced by TH1 cells was required for the induction of DCTh1 while IL-4 and IL-13 produced by TH2 cells played requisite roles in the induction of DCTh2. Interestingly, TH17 cell-derived IL-17, IL-21, or IL-22 were not required for DCTh17 formation. Moreover, monocytes or DCGM cultured with LPS in the presence of TH17-associated cytokines, IL-17A and/or IL-21, did not secrete different cytokines compared with cells cultured with LPS alone (M.N.A., M.T.W., and E.G.E., unpublished data, August 2010). These data suggest that yet to be identified soluble and/or cell surface molecules may be involved in DCTh17 differentiation.
Taken together, our results indicate that TH cells positively regulate the secondary immune response by instructing monocytes to differentiate into DCs, which in turn trigger naive CD4+ T cells to acquire similar effector functions as the TH cell subset that initiated DC differentiation. Such an amplification loop would be beneficial during memory responses, but deleterious if uncontrolled during inflammatory diseases. Psoriasis and atopic dermatitis are 2 diseases that likely involve divergent feedback loops, as evidenced by the accumulation of markedly different TH cells and DC subsets in inflamed skin.18-22 Psoriatic lesions contain primarily TH1 and TH17 cells,19 and possess CD11c+ myeloid DCs that strongly resemble the functional characteristics attributed to DCTh1 and DCTh17.24-27 Most notably, CD11c+ DCs in psoriatic lesions express elevated levels of TNF-α, IL-12p40, and IL-2325,27 and drive the formation of TH1 and TH17 cells ex vivo.24 Conversely, atopic dermatitis lesions contain large numbers of TH2 cells20,22,23 and CD11c+ DCs that share several characteristics with DCTh2.21,25 Importantly, DCs from atopic dermatitis lesions express low or undetectable levels of TNF-α, IL-12p40, and IL-2321,25 and are believed to mediate TH2 differentiation during the acute phase of atopic dermatitis.22 DCs in atopic dermatitis also express higher levels of DC-SIGN compared with DCs from psoriatic lesions,21 which is in accordance with increased DC-SIGN expression observed on DCTh2. These data indicate that CD11c+ DCs present in inflamed skin from classic TH-associated diseases are phenotypically similar to those described in this report.
The numerical predominance of TH cells in lesions from psoriasis and atopic dermatitis patients indicates that these cells may be principally responsible for shaping the local DC repertoire. However, this does not exclude the possibility that monocytes also receive signals from other cell types. Previous studies have shown that NK, CD8+ and NK T cells can also initiate DC differentiation.31,45,46 Similar to TH cells, NK cells colocalize with monocytes in inflamed tissue and direct DC differentiation in a GM-CSF and cell contact dependent manner.46 Comparison of the relative contributions of each of these cell types to inflammatory responses merits further investigation.
The memory immune response is characterized by rapid responses to foreign agents encountered 2 or more times throughout the lifetime of the host. While this response is partially mediated by affinity maturation47 and differential activation requirements,48 our data suggest that memory TH cells may also contribute to the speed and potency of the secondary response by eliciting the formation of specialized DC subsets. Interestingly, stimulation of these DC subsets with a panel of PRR agonists which were previously demonstrated to modulate DC cytokine secretion,7,9,10 affected total cytokine production, but failed to alter the relative ratios of individual cytokines produced by each DCTh subset. These data indicate that, once formed, the phenotype of each DCTh subset is stable and that signals from memory TH cells outweigh subsequent signals from PRR engagement.
While our study elucidates a novel mechanism by which TH cells can amplify the immune response, resolution of this response is essential to prevent ongoing inflammation and autoimmunity. Elimination of the initial immune stimulus, such as a pathogen or allergen, would presumably facilitate resolution. In addition, preliminary data from our laboratory indicate that regulatory T cells can instruct monocytes to differentiate into tolerogenic DCs, thereby providing another mechanistic brake on inflammation. It is also conceivable that TH cells initiate a negative feedback loop after multiple rounds of activation.49 This is supported by a recent report indicating that irradiated TH1 and TH2 clones prompt the formation of DCs that subsequently induce naive T cells to secrete IL-10 and IFN-γ, respectively.50 Thus, whereas TH cells may mediate an amplification pathway during the initiation of a secondary immune response, a variety of mechanisms likely exist to limit the intensity and duration of such responses.
This study identifies an important mechanism responsible for generating DC diversity that also provides a critical link between innate and adaptive immunity. The system we used for generating DC subsets with differential TH polarizing capacity has facilitated the elucidation of cell surface and soluble factors governing the biology of TH-promoting DC subsets. These and additional molecules expressed by DC subsets should prove useful for detecting and quantifying each subset in situ and enable the design of novel therapeutic strategies for TH-associated inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to extend our special thanks to Toru Iwahori and Kartoosh Heydari for their expert assistance in flow cytometry. They thank Donna Jones for secretarial assistance and Claudia Benike, Oliver Crespo, Luis Zuniga, Stephanie Fung and Christina Swanson for careful review of the manuscript.
This work was supported by the following funding sources: National Heart, Lung, and Blood Institute (HL075462), 5 U19 AI050864, 1 U19 AI082719, 1 U19 AI090019, and gifts from the Floren Family Foundation and the Ben May Trust. M.N.A. was funded by National Institutes of Health Fellowship (NRSA) F31 CA142272. M.T.W. was funded by a Mason Case Fellowship.
National Institutes of Health
Authorship
Contribution: M.N.A., M.T.W., and A.L.Z. conceived and executed the studies; M.M.S. and M.G.D. assisted with experimental design and provided technical guidance; D.W. and J.G. performed the IHC studies; T.H.K., D.M.G., and L.T. assisted in the execution of select studies; K.C.D. and J.K. provided psoriasis and atopic dermatitis tissues; P.J.U. supervised select studies. K.S. and E.G.E. conceived and supervised the studies; M.N.A., M.T.W., A.L.Z., M.M.S., D.W., M.G.D., P.J.U., K.S., and E.G.E. analyzed and/or interpreted the data; and M.N.A., M.T.W., and E.G.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.S. is Novo Nordisk Inc.
Correspondence: Dr Edgar Engleman, Stanford University School of Medicine, 3373 Hillview Ave, Palo Alto, CA 94304-1204; e-mail: edengleman@stanford.edu.
References
Author notes
K.S. and E.G.E. contributed equally to this article.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal