Abstract
Thirteen patients with acute myeloid leukemia, 5 with active disease, 2 in molecular relapse, and 6 in morphologic complete remission (CR; median age, 62 years; range, 53-73 years) received highly purified CD56+CD3− natural killer (NK) cells from haploidentical killer immunoglobulin-like receptor–ligand mismatched donors after fludarabine/cyclophosphamide immunosuppressive chemotherapy, followed by IL-2. The median number of infused NK cells was 2.74 × 106/Kg. T cells were < 105/Kg. No NK cell–related toxicity, including GVHD, was observed. One of the 5 patients with active disease achieved transient CR, whereas 4 of 5 patients had no clinical benefit. Both patients in molecular relapse achieved CR that lasted for 9 and 4 months, respectively. Three of 6 patients in CR are disease free after 34, 32, and 18 months. After infusion, donor NK cells were found in the peripheral blood of all evaluable patients (peak value on day 10). They were also detected in BM in some cases. Donor-versus-recipient alloreactive NK cells were shown in vivo by the detection of donor-derived NK clones that killed recipient's targets. Adoptively transferred NK cells were alloreactive against recipient's cells, including leukemia. In conclusion, infusion of purified NK cells is feasible in elderly patients with high-risk acute myeloid leukemia. This trial was registered at www.clinicaltrial.gov as NCT00799799.
Introduction
Treatment of acute myeloid leukemia (AML) in adults is intensive. It is based on multiple cycles of cytosine arabinoside and anthracycline-containing chemotherapy regimens, followed by the option of allogeneic hematopoietic stem cell transplantation for eligible patients. Although complete remission (CR) rates in response to chemotherapy range from 60%-85% in patients < 60 years of age, ∼ 60% of patients subsequently relapse, and the 5-year overall survival (OS) is 40%. In elderly patients, OS falls to 10%, because of the higher prevalence of unfavorable biologic factors, such as poor risk cytogenetics.1
The human lymphocyte subset of natural killer (NK) cells, which are defined by CD56 or CD16 expression and absence of CD3,2 plays a critical role in the innate immune response, particularly in immune control of tumor development and growth.3 NK cells express activating and inhibitory receptors that recognize MHC class I alleles.4 NK cells possess clonally distributed inhibitory receptors termed killer cell immunoglobulin-like receptors (KIRs) that recognize allotypic determinants (KIR ligands)5,6 shared by certain groups of HLA class I alleles. KIR2DL1 recognizes HLA-C alleles with a Lys80 residue (HLA-Cw4 and related; group 2 alleles), KIR2DL2 and KIR2DL3 recognize HLA-C with an Asn80 residue (HLA-Cw3 and related; group 1 alleles), KIR3DL1 is the receptor for HLA-B alleles sharing the Bw4 specificity. NK cells that express, as their only inhibitory receptor for self, a KIR whose ligand is a HLA class I group which is absent on allogeneic targets, sense the missing expression of the self class I KIR ligand and mediate alloreactions.
Preclinical and clinical data from the haploidentical T cell–depleted transplantation setting have shown that haploidentical KIR ligand–mismatched NK cells play a main role as antileukemia effector cells.7 Patients with AML are significantly protected against leukemia relapse when they receive a transplant from NK alloreactive donors.7-11 Furthermore, enriched NK cells can be infused safely as adoptive immunotherapy in patients with leukemia and with cancer after nonmyeloablative and myeloablative immunosuppressive chemotherapy with some cases achieving significant clinical responses.12,13 Notably, these trials have shown that KIR ligand mismatching between donor NK cells and recipient correlated with better response to NK therapy. A recent pilot study of haploidentical KIR-HLA mismatched NK-cell transplantation in childhood AML reported that NK-cell therapy prolonged disease-free survival and OS.14
The present study investigated the feasibility and safety of infusing highly purified NK cells from haploidentical KIR ligand–mismatched donors into a cohort of elderly patients with high-risk AML and has shown, for the first time, donor NK-cell repertoires, trafficking, and function in the peripheral blood (PB), BM, or both of recipients at different time points. Given the critical role of NK alloreactivity in mediating the antileukemia effect after haploidentical stem cell transplantation, adoptive immunotherapy with infusion of functionally active alloreactive NK cells may be of clinical benefit for patients with high-risk AML.
Methods
Patients
Forty-two patients with high-risk AML, as indicated by poor prognostic features at diagnosis, advanced age, or resistant/relapsed disease, were recruited to the study. Inclusion criteria were AML, diagnosed according to World Health Organization classification15 ; age ≥ 18 years old; adequate renal, cardiac, and pulmonary functions; a Karnofsky score ≥ 70% or a World Health Organization score ≤ 1, not eligibile for allogeneic stem cell transplantation. Relatives were screened in the search for one haploidentical KIR ligand–mismatched donor. A suitable donor was available for 18 patients (42.8%), 13 of whom received an infusion of donor NK cells. Table 1 summarizes the characteristics of patients who received infusion, all of whom had received chemotherapy according to institutional guidelines. All subjects gave written informed consent before entering the study in accordance with the Declaration of Helsinki, and all research was approved by the ethical committee of the University of Bologna. The trial was registered at www.clinicaltrial.gov as NCT00799799.
Donor selection
Any consenting healthy family member who fulfilled donor criteria was eligible for leukapheresis. Donors who were classified as NK alloreactive against recipients (NK alloreactive donors) possessed (1) HLA class I KIR ligand(s) that were missing in the recipient, (2) KIR gene(s) for missing self-recognition on recipient targets, and (3) alloreactive NK clones against recipient targets.
HLA typing of recipient and donor(s) tested class I alleles belonging to the 3 class I groups recognized by KIRs (HLA-C group 1, HLA-C group 2, and HLA-Bw4 alleles). In NK alloreactive donors HLA-C and HLA-B typing showed KIR ligand mismatches in the graft-versus-host (GVH) direction, that is, the recipient did not possess either one HLA-C allele group (C1 or C2) or the HLA-Bw4 group which were present in the donor, or both. Donor KIR genotyping was performed to ensure the donor possessed the relevant KIR gene. Donor NK-cell KIR immunophenotyping and functional analyses were also performed (see “KIR genotyping”).
KIR genotyping
KIR typing was performed with a low-resolution PCR with sequence-specific priming assay (KIR Genotyping Kit; Invitrogen) following the manufacturer's instructions. The kit was designed to identify 14 KIR genes (2DL1, 2DL2, 3DL1, 2DL4, 2DL5, 2DS1, 2DS2, 2DS3, 2DS4, 2DS5, 3DL1, 3DL2, 3DL3, 3DS1), 2 pseudogenes (2DP1 and 3DP1), and the common variants of KIR2DL5 (KIR2DL5A, KIR2DL5B), the KIR2DS4 allele (*001/002 and *003) and KIR3DP1 allele (*001/002 and *003).
PCR with sequence-specific priming was performed following the manufacturer's instructions.16 DNA was extracted from blood, NK-cell clone, or BM samples and processed for multiplex PCRs with the use of the ABI AmplfSTR kit that which has 10 polymorphic short tandem repeat PCR marker loci. Short tandem repeat PCR was performed following the manufacturer's instructions. Products were electrophoresed, and fluorescence was measured by ABI 310 Genetic Analyzer. Fluorescence intensity is an estimate of the quantity of amplificant in each electrophoretic peak (band), and single donor chimerism was estimated from the peak area measurements.17
Immunophenotyping
NK-cell purification.
FITC-, phycoerythrin (PE)–, and allophycocyanin-coupled control immunoglobulins or specific antibodies directed at CD56, CD3, CD14, or CD19 were used to evaluate NK purity and yield after immunomagnetic separation as well as the phenotype of circulating and BM cells in patients after the NK-cell infusion.
KIR phenotype.
Four-color immunofluorescence analyses identified KIR−/NKG2A+ versus KIR+/NKG2A−, CD56+/CD3− NK cells. The following mouse monoclonal antibodies: allophycocyanin-conjugated anti-CD56 (IgG1; Miltenyi Biotec), PE-cyanine 7–conjugated anti-CD3 (IgG1; BD Bioscience), unconjugated anti-NKG2A (clone Z199, IgG2b, kindly donated by Alessandro Moretta, University of Genova) developed with FITC-conjugated goat anti–mouse IgG2b antibodies (Southern Biotech), were used in combination with the following PE-conjugated anti-KIR antibodies (Bechman Coulter): either anti-KIR2DL2/3/S2 (clone GL183, IgG1) or anti-KIR2DL1/S1 (clone EB6B, IgG1), or anti-KIR3DL1/S1 (clone Z27, IgG1). Two-color immunofluorescence analyses on NK-cell clones were used to visualize NK cells expressing, as their only inhibitory receptor for self, a KIR for which there was no class I ligand in the recipient. KIR2DL2/3/S2 single-positive NK cells were identified with FITC-conjugated anti-KIR2DL2/3/S2 antibody (clone CH-L, IgG2b; BD Bioscience) in combination with a cocktail of PE-conjugated anti-KIR2DL1 (clone 143211, IgG1; R&D Systems), anti-KIR3DL1 (clone DX9, IgG1; Miltenyi Biotec) and anti-NKG2A (clone Z199, IgG2b; Beckman Coulter) mouse antibodies. KIR2DL1 single-positive NK cells were identified with PE-conjugated anti-KIR2DL1 (clone 143211, IgG1; R&D Systems) in combination with a cocktail of FITC-conjugated anti-KIR3DL1 (clone DX9, IgG1; Miltenyi Biotec) and anti-KIR2DL2/3S2 (clone CH-L, IgG2b) and anti-NKG2A (clone Z199, IgG2b; Beckman Coulter) mouse antibodies developed with FITC-conjugated goat anti–mouse IgG2b antibodies (Southern Biotech).
NK-cell cloning and cytotoxicity assay
Donor alloreactive NK-cell repertoires were assessed once at the time of leukapheresis. Patient alloreactive NK-cell repertoires were assessed at days 3, 9, 12, 18, and 20 after NK-cell infusion. Large numbers of NK clones were generated by limiting dilution, and cytotoxicity assays against recipient target cells were used to detect the frequency of alloreactive NK clones. PBMCs depleted of T cells by negative anti-CD3 immunomagnetic selection (Miltenyi Biotec) were plated under limiting-dilution conditions, activated with phytohemagglutinin (PHA; Biochrom KG), and cultured with IL-2 (Chiron BV) and irradiated feeder cells. Feeder cells were obtained by pooling buffy coats from 5-9 healthy donors. Such donors were not typed because PHA plus IL-2 activation allows efficient NK-cell cloning regardless of feeder cell HLA type.9 Cloning efficiencies ranged from 1 in 5 to 1 in 10 plated NK cells. Cloned NK cells were screened for alloreactivity by standard 51Cr-release cytotoxicity at an effector-to-target ratio of 10:1 against patient KIR ligand–mismatched PHA lymphoblasts and against one recipient's leukemic cells. Approximately 100 NK clones from each person were screened. Clones exhibiting > 30% lysis were scored as alloreactive. The assay was considered positive when the frequency of lytic clones was > 1 in 50.9
Leukapheresis and positive selection of CD3−CD56+ NK cells
Standard volume leukaphereses were performed as already reported.18 A minimum of 5 × 109 total PBMCs were incubated with MACS colloidal superparamagnetic CD3 microbeads (Miltenyi Biotec), consisting of monoclonal mouse anti–human CD3 antibodies conjugated to microspheres. The CliniMACS device was used in 2 steps: (1) CD3+ T-cell depletion followed by (2) positive CD56+ NK-cell selection under good manufacturing product conditions. The target cell dose was considered to be 5 × 106 haploidentical NK cells/kg of body weight, and the minimum cell dose was 1 × 106 haploidentical NK cells/kg. A cell product sample was evaluated by flow cytometry to count T, B, and NK cells and monocytes before and after CliniMACS selection. Highly purified CD56+CD3− NK cells were cryopreserved. To reduce the risk of postinfusion GVHD, a maximun of 1 × 105 CD3+ T cells/kg was allowed in the NK graft. Fungal and bacterial sterility tests were performed on the final NK-cell product; viability and the nucleated cell dose were assessed. Extensive phenotyping determined purity and residual B and T cells and monocytes. Cytotoxicity was tested against NK-sensitive K562 cells.
Immunosoppressive chemotherapy and NK infusion
To favor haploidentical NK-cell engraftment, all patients received immunosuppressive chemotherapy (fludarabine [Flu] 25 mg/mq from day −7 to −3 and cyclophosphamide [Cy] 4 g/mq on day −2 [Flu/Cy]). Two days after Cy administration, patients received the NK-cell infusion (day 0), which was followed by subcutaneous administration of IL-2 (10 × 106 IU/d, 3 times weekly; Novartis) for 2 weeks (6 doses total). No GVHD prophylaxis was used because GVHD was not anticipated.
Chimerism assay
PB and BM chimerism was determined by variable number tandem repeat (VNTR) assay. Analysis was performed on whole blood. Nine loci were routinely analyzed (D3S1358, VWA, FGA, TH01, TPOX, CSF1PO, D5S818, D13S317, and D7S820) in addition to amelogenina that identifies XY chromosomes. Patient PBLs and clones were studied before the NK-cell infusion and at different time points afterward. Donor chimerism was expressed as the percentage of circulating donor cells.
Serum cytokine concentrations
Human IL-15 ELISA Kit (Tema Ricerca) was used to test IL-15 concentration in serum. Briefly, 0.1 mL per well of standard solutions or patients' serum was divided into aliquots in duplicate into a precoated 96-well plate. The plate was sealed and incubated at 37°C. After 90 minutes of incubation, the plate content was discarded, 0.1 mL of biotinylated anti–human IL-15 antibody working solution was added to each well, and the plate was incubated at 37°C for 60 minutes. The plate was then washed 3 times with 0.01M PBS. Prepared ABC working solution (0.1 mL) was added to each well, and the plate was incubated at 37°C for 30 minutes. After rinsing 5 times with 0.01M PBS, 90 μL of prepared TBS color developing agent was added to each well, and the plate was incubated at 37°C for 25 minutes. Prepared TMB stop solution (0.1 mL) was added to each well, and the plate was read at 450 nm in a microplate reader (Multiskan EX; M-Medical). The human IL-15 concentration was extrapolated from the standard curve.
Statistical analysis
The results are presented as median values and ranges or, when indicated, as mean ± SD. To investigate the relation between serum IL-15 levels (day 3) and PB donor chimerism (peak value) after infusion, Pearson correlation coefficient was calculated.
Results
Identification of donors able to mount donor versus recipient NK-cell alloreactivity
Haploidentical KIR ligand–mismatched donors were screened for 42 adults with AML, which was defined as high-risk because of poor prognostic features such as advanced age or resistant/relapsed disease at diagnosis. Eighteen patients had one suitable donor (42.8%). Thirteen patients entered a pilot study of adoptive NK-cell immunotherapy (see Table 1 for patients' characteristics). Among donor-recipient pairs, 6 or 13 (46%) were mismatched for HLA-C group 1, 4 of 13 (30.9%) were mismatched for HLA-C group 2, 1 of 13 (7.7%) was mismatched for HLA Bw4, 1 of 13 (7.7%) was mismatched for both HLA-C1 and Bw4, and 1 of 13 (7.7%) was mismatched for both HLA-C2 and Bw4.
Demographic, hematologic, and graft features of study patients with AML
| UPN . | Age, y . | Sex . | WBC count, × 109/L . | Karyotype . | Genotype . | FAB . | HLA typing (mismatch) . | Donor KIR-L mismatch . | Disease status before NK infusion . | Infused NK cells, ×106/kg) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | F | 6.2 | 46 XX | NE | M2 | -C group 2 | 2DL1 | Resistant | 3.16 |
| 2 | 66 | M | 0.4 | 46 XY | FLT3 WT | M1 | -C group 2 | 2DL1 | Resistant | 1.34 |
| NPM WT | ||||||||||
| 3 | 65 | M | 51.3 | 46 XY | NE | M1 | -C group 1 | 2DL2/3 | Resistant | 5.00 |
| 4 | 62 | F | 2.4 | 46 XX | FLT3 WT | M1 | -C group 1 | 2DL2/3 | Resistant | 5.00 |
| NPM1WT | ||||||||||
| 5 | 62 | M | 5.25 | Complex | FLT3 WT | sec | -C group 2 | 2DL1 3DL1 | Resistant | 1.11 |
| NPM WT | -Bw4 | |||||||||
| 6 | 59 | F | 4.32 | 46 XX | FLT3 WT | M1 | -C group 2 | 2DL1 | CR | 4.00 |
| NPM WT | ||||||||||
| 7 | 73 | M | 75.0 | 46 XY | FLT3 TKD | M5 | -C group 1 | 2DL2/3 3DL1 | CR | 5.00 |
| -Bw4 | ||||||||||
| 8 | 58 | M | 74.8 | 46 XY | FLT3 WT | M4 | -Bw4 | 3DL1 | CR | 1.81 |
| NPM WT | ||||||||||
| 9 | 69 | M | 25.0 | Complex | FLT3 WT | M1 | -C group 1 | 2DL2/3 | CR | 2.05 |
| NPM WT | ||||||||||
| 10 | 53 | F | 4.1 | 46 XX | NE | M1 | -C group 1 | 2DL2/3 3DL1 | Molecular relapse | 3.89 |
| +8−7 | -Bw4 | |||||||||
| 11 | 67 | M | 2.7 | NE | FLT3 WT | M0 | -C group 1 | 2DL2/3 | CR | 2.74 |
| NPM WT | ||||||||||
| 12 | 61 | M | 2.9 | 46 XX | FLT3 WT | sec | -C group 1 | 2DL2/3 | CR | 2.51 |
| NPM WT | ||||||||||
| 13 | 58 | F | 5.8 | inv(16) | CBFMYH11 | NE | -C group 2 | 2DL1 | Molecular relapse | 1.29 |
| UPN . | Age, y . | Sex . | WBC count, × 109/L . | Karyotype . | Genotype . | FAB . | HLA typing (mismatch) . | Donor KIR-L mismatch . | Disease status before NK infusion . | Infused NK cells, ×106/kg) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | F | 6.2 | 46 XX | NE | M2 | -C group 2 | 2DL1 | Resistant | 3.16 |
| 2 | 66 | M | 0.4 | 46 XY | FLT3 WT | M1 | -C group 2 | 2DL1 | Resistant | 1.34 |
| NPM WT | ||||||||||
| 3 | 65 | M | 51.3 | 46 XY | NE | M1 | -C group 1 | 2DL2/3 | Resistant | 5.00 |
| 4 | 62 | F | 2.4 | 46 XX | FLT3 WT | M1 | -C group 1 | 2DL2/3 | Resistant | 5.00 |
| NPM1WT | ||||||||||
| 5 | 62 | M | 5.25 | Complex | FLT3 WT | sec | -C group 2 | 2DL1 3DL1 | Resistant | 1.11 |
| NPM WT | -Bw4 | |||||||||
| 6 | 59 | F | 4.32 | 46 XX | FLT3 WT | M1 | -C group 2 | 2DL1 | CR | 4.00 |
| NPM WT | ||||||||||
| 7 | 73 | M | 75.0 | 46 XY | FLT3 TKD | M5 | -C group 1 | 2DL2/3 3DL1 | CR | 5.00 |
| -Bw4 | ||||||||||
| 8 | 58 | M | 74.8 | 46 XY | FLT3 WT | M4 | -Bw4 | 3DL1 | CR | 1.81 |
| NPM WT | ||||||||||
| 9 | 69 | M | 25.0 | Complex | FLT3 WT | M1 | -C group 1 | 2DL2/3 | CR | 2.05 |
| NPM WT | ||||||||||
| 10 | 53 | F | 4.1 | 46 XX | NE | M1 | -C group 1 | 2DL2/3 3DL1 | Molecular relapse | 3.89 |
| +8−7 | -Bw4 | |||||||||
| 11 | 67 | M | 2.7 | NE | FLT3 WT | M0 | -C group 1 | 2DL2/3 | CR | 2.74 |
| NPM WT | ||||||||||
| 12 | 61 | M | 2.9 | 46 XX | FLT3 WT | sec | -C group 1 | 2DL2/3 | CR | 2.51 |
| NPM WT | ||||||||||
| 13 | 58 | F | 5.8 | inv(16) | CBFMYH11 | NE | -C group 2 | 2DL1 | Molecular relapse | 1.29 |
UPN indicates unique patient number; WBC, white blood cell; FAB, French-American-British; KIR-L, killer Ig-like receptor ligand; NK, natural killer; NE, not evaluated; FLT3, FMS-like tyrosine kinase 3; NPM, nucleophosmin; WT, wild type; CR, complete remission; TKD, tyrosine kinase domain; and CBF, core binding factor.
The 13 mismatched donors all possessed the inhibitory KIR gene that recognized missing expression of its HLA class I ligand on recipient cells. The 7 HLA-C group 1 mismatched donors possessed KIR2DL2 and/or KIR2DL3, the 5 HLA-C group 2 mismatched donors possessed KIR2DL1, and the 1 HLA-Bw4 mismatched donor had KIR3DL1 (Table 1). Functional analyses showed the presence of alloreactive NK clones in all donors whose NK clones were tested against allogeneic targets which did not express their HLA-C group. Frequencies of donor-versus-recipient alloreactive NK clones were 9.5% ± 3.1% for HLA-C group 2 mismatched pairs and 7.5% ± 3% for HLA-C group 1 mismatched pairs, respectively. In contrast, the donor who was mismatched for Bw4 did not possess NK-alloreactive clones against HLA-Bw4–negative targets.
NK-cell purification
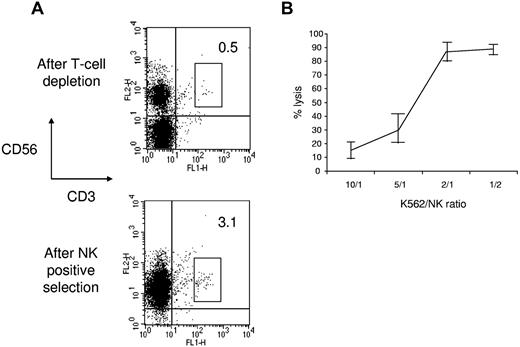
A median of 17.10 × 106 CD56+CD3− cells/kg (range, 3.80-42.50 × 106 CD56+CD3− cells/kg) was collected from PB in one leukapheresis. Immunomagnetic selection provided a cell population with a median purity of 93.5% (range, 66.4%-99.2%) and a median recovery of 53.05% (range, 30.9%-72.8%) for infusion (Table 1). Positive NK-cell selection resulted in a median of 3.03 log T-cell depletion (range, 2.1-4.5 log T-cell depletion). Each positive selection required 1 column and 1 CD56+ tubing set. Target NK-cell dose recovery (5 × 106/kg) was obtained in 10 of 13 patients (77%) and the minimum NK-cell dose (106/kg) in 13 of 13 patients. To prevent the GVH effect in weakly immunosuppressed patients, we established a maximum of 105 T cells/kg in the final cell product. Because CD56+ cell purification significantly enriched CD56+CD3+ double-positive T cells (Figure 1A), in most patients, we were not able to infuse the total number of collected CD56+CD3− NK cells. Thus, a median of 2.74 × 106 CD3−CD56+ cells/kg (range, 1.11-5 × 106 CD3−CD56+ cells/kg) were infused. NK-cell viability after purification was 95% (range, 92%-98%). Purified NK cells killed NK-sensitive K562 cells in a flow cytometry–based cytotoxicity assay (Figure 1B).
Donor NK-cell purification. (A) Flow cytometric analysis of NK cells after T-cell depletion and after CD56+ cell positive selection. The results are representative of the whole patient population. (B) NK cells obtained after purification were tested against K562 cells in a flow cytometric–based cytotoxicity test. The results represent the mean ± SD of 6 independent experiments.
Donor NK-cell purification. (A) Flow cytometric analysis of NK cells after T-cell depletion and after CD56+ cell positive selection. The results are representative of the whole patient population. (B) NK cells obtained after purification were tested against K562 cells in a flow cytometric–based cytotoxicity test. The results represent the mean ± SD of 6 independent experiments.
Safety of NK-cell therapy
After administration of Flu/Cy chemotherapy and infusion of highly purified NK cells, hematopoietic cell recovery was rapid and similar to what is observed after a standard chemotherapy cycle. The median times to absolute neutrophil count of 0.5 × 109/L and to platelet count of 20 × 109/L were 18 and 20 days, respectively. Few infections were documented, and hospital stays were short (data not shown). No clinical or laboratory signs of GVHD were observed. All patients received all IL-2 injections as scheduled. In some cases, local side effects, such as mild erythema, were observed at the injection site.
Analysis of postinfusion NK-cell repertoire
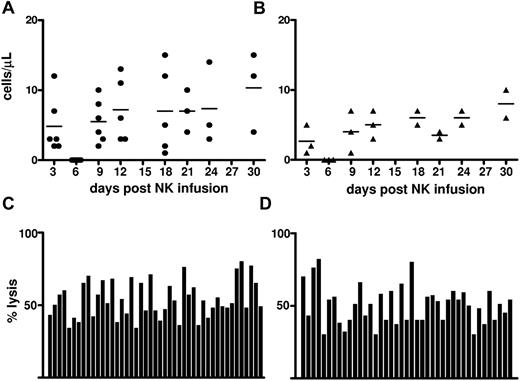
The Flu/Cy preparative regimen caused lymphopenia19 and homeostatic augmentation of endogenous IL-15 which is essential for in vivo expansion and survival of NK cells. In our patients, IL-15 concentration peaked on day 3 after NK-cell infusion (Figure 2A). Postinfusion NK-cell number kinetics in PB is reported in Figure 2B and shows that NK-cell count increased when IL-15 concentration decreased. The donor chimerism assay in PB and BM showed that donor-derived cells were present in PB and BM after NK-cell infusion, peaking on days 10 and 5, respectively (Figure 2C-D). Although a statistically significant correlation between serum IL-15 levels and PB donor chimerism was not found (r = 0.4, P = .1), in some cases, the rise in IL-15 serum level was followed by the increase in donor chimerism. Moreover, because several mechanisms may regulate NK-cell expansion/clearance, including alloimmunization of recipient's T cells against donor antigens,13 especially after repeated infusions, we specifically analyzed the results of the only patient who was treated with 2 subsequent NK-cell infusions, and we observed that IL-15 concentration and the percentage of donor chimerism were different after the first and the second infusion. In particular, NK cells of donor origin were shown up to day 17 after the first infusion, whereas donor NK cells were detected for only 5 days after the second infusion (data not shown). These data paralleled with higher and durable concentrations of serum IL-15 after the first than after the second infusion (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Increase in IL-15 concentration and donor chimerism in lymphoablated recipients after NK-cell infusion. (A) IL-15 concentration was evaluated in the serum of all patients at different time points after NK-cell infusion. Each curve corresponds to one different patient. (B) CD56+CD3− cells were enumerated in the PB of all patients by flow cytometry at different time points after NK-cell infusion. Baseline corresponds to the day before chemotherapy was started. The results are the mean ± SD of all patients. (C-D) The percentage of donor chimerism, as tested on whole blood, was evaluated in the PB (C) and BM (D) by VNTR analysis in all treated patients (n = 13). Ten of 13 patients were fully evaluable all over the different time points, whereas in the remaining patients, particularly during the neutropenic phase after chemotherapy, cell concentration did not allow a reliable VNTR analysis. Here are reported the results of all positive patients (n = 7 for PB and n = 4 for BM). In the remaining evaluable patients, no donor chimerism was observed.
Increase in IL-15 concentration and donor chimerism in lymphoablated recipients after NK-cell infusion. (A) IL-15 concentration was evaluated in the serum of all patients at different time points after NK-cell infusion. Each curve corresponds to one different patient. (B) CD56+CD3− cells were enumerated in the PB of all patients by flow cytometry at different time points after NK-cell infusion. Baseline corresponds to the day before chemotherapy was started. The results are the mean ± SD of all patients. (C-D) The percentage of donor chimerism, as tested on whole blood, was evaluated in the PB (C) and BM (D) by VNTR analysis in all treated patients (n = 13). Ten of 13 patients were fully evaluable all over the different time points, whereas in the remaining patients, particularly during the neutropenic phase after chemotherapy, cell concentration did not allow a reliable VNTR analysis. Here are reported the results of all positive patients (n = 7 for PB and n = 4 for BM). In the remaining evaluable patients, no donor chimerism was observed.
Alloreactivity of postinfusion NK cells
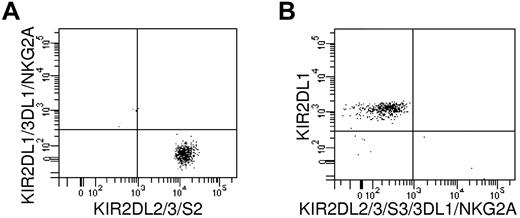
The kinetics of alloreactive NK-cell subsets was documented by flow cytometry and by limiting dilution cloning of circulating NK cells during the first month after the NK-cell infusion. Figure 3A shows the kinetics of KIR2DL2/3/S2+/NKG2A− cells from 7 HLA-C1 positive donors infused into HLA-C1–negative recipients. Such cells are potentially alloreactive because they contain only KIR2DL2/3+ NK cells that are alloreactive against targets from C1-negative recipients. Figure 3B shows the kinetics of KIR2DL1/S1+/NKG2A− cells from 5 HLA-C2–positive donors infused into HLA-C2–negative recipients. Such cells are potentially alloreactive because they contain only KIR2DL1+ NK cells that are alloreactive against targets from C2-negative recipients. Moreover, to directly document donor NK-cell function in vivo, NK-cell clones were obtained from patients who received an infusion of NK cells, and their alloreactivity against recipient PHA blasts was determined in a standard 51Cr-release assay. In Figure 3C and D, each bar represents the degree of alloreactivity (percentage of specific lysis against recipient PHA blasts) exerted by one individual clone. All alloreactive clones obtained from all patients are shown. In particular, Figure 3C shows alloreactive NK clones detected in 5 HLA-C1–negative recipients after NK-cell infusion from HLA-C1–positive donors. In agreement with the functional results, immunofluorescence analyses of KIR and NKG2A receptors in 2 randomly selected alloreactive NK-cell clones showed such clones express, as their only inhibitory receptor for self, the KIR for which there is no class I ligand in the recipient (Figure 4). They are representative of 20 tested that exhibited the same phenotype.
Detection of donor-versus-recipient alloreactive NK-cell repertoires after NK-cell infusion. (A) Time kinetics of KIR2DL2/3/S2+/NKG2A− NK cells from 7 HLA-C1–positive donors infused into HLA-C1–negative recipients. Such cells are potentially alloreactive because they contain KIR2DL2/3+ only NK cells (which are alloreactive against C1-negative recipients). (B) Time kinetics of KIR2DL1/S1+/NKG2A− NK cells from 5 HLA-C2–positive donors infused into HLA-C2–negative recipients. Such cells are potentially alloreactive because they contain KIR2DL1+ only NK cells (which are alloreactive against C2-negative recipients). (C-D) After the NK-cell infusion, NK-cell clones were obtained from patients who received an infusion of NK cells (see “Methods”), and their alloreactivity against recipient PHA blasts was determined in a 51Cr-release assay. Each bar represents the degree of alloreactivity (percentage of specific lysis against recipient PHA blasts) exerted by one individual clone. All alloreactive clones, exhibiting ≥ 30% specific lysis, obtained from all patients are shown. (C) NK clones detected in 5 HLA-C1–negative recipients after NK-cell infusion from HLA-C1–positive donors. (D) NK clones detected in 3 HLA-C2–negative recipients after NK-cell infusion from HLA-C2–positive donors.
Detection of donor-versus-recipient alloreactive NK-cell repertoires after NK-cell infusion. (A) Time kinetics of KIR2DL2/3/S2+/NKG2A− NK cells from 7 HLA-C1–positive donors infused into HLA-C1–negative recipients. Such cells are potentially alloreactive because they contain KIR2DL2/3+ only NK cells (which are alloreactive against C1-negative recipients). (B) Time kinetics of KIR2DL1/S1+/NKG2A− NK cells from 5 HLA-C2–positive donors infused into HLA-C2–negative recipients. Such cells are potentially alloreactive because they contain KIR2DL1+ only NK cells (which are alloreactive against C2-negative recipients). (C-D) After the NK-cell infusion, NK-cell clones were obtained from patients who received an infusion of NK cells (see “Methods”), and their alloreactivity against recipient PHA blasts was determined in a 51Cr-release assay. Each bar represents the degree of alloreactivity (percentage of specific lysis against recipient PHA blasts) exerted by one individual clone. All alloreactive clones, exhibiting ≥ 30% specific lysis, obtained from all patients are shown. (C) NK clones detected in 5 HLA-C1–negative recipients after NK-cell infusion from HLA-C1–positive donors. (D) NK clones detected in 3 HLA-C2–negative recipients after NK-cell infusion from HLA-C2–positive donors.
KIR and NKG2A receptor expression by alloreactive NK-cell clones. Immunofluorescence analyses of 2 representative alloreactive NK clones are shown. Such clones express, as their only inhibitory receptor for self, the KIR for which there is no class I ligand in the recipient. (A) An NK clone from HLA-C1–positive donor detected in HLA-C1–negative recipient is KIR2DL2/3/S2+ and KIR2DL1/3DL1/NKG2A−. (B) An NK clone from HLA-C2–positive donor detected in HLA-C2–negative recipient is KIR2DL1+ and KIR2DL2/3/S2/3DL1/NKG2A−. These 2 clones are representative of 20 tested that exhibited the same phenotype.
KIR and NKG2A receptor expression by alloreactive NK-cell clones. Immunofluorescence analyses of 2 representative alloreactive NK clones are shown. Such clones express, as their only inhibitory receptor for self, the KIR for which there is no class I ligand in the recipient. (A) An NK clone from HLA-C1–positive donor detected in HLA-C1–negative recipient is KIR2DL2/3/S2+ and KIR2DL1/3DL1/NKG2A−. (B) An NK clone from HLA-C2–positive donor detected in HLA-C2–negative recipient is KIR2DL1+ and KIR2DL2/3/S2/3DL1/NKG2A−. These 2 clones are representative of 20 tested that exhibited the same phenotype.
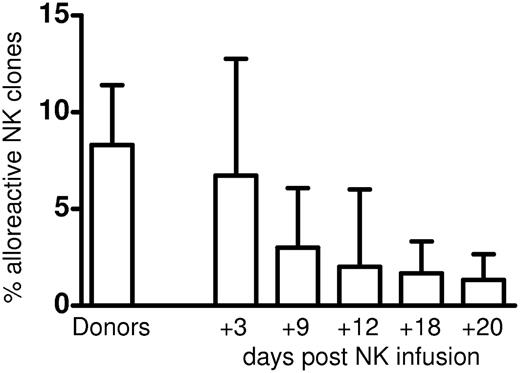
The frequencies of alloreactive NK clones detected in patients shortly after the NK-cell infusion tended to reproduce the frequency originally detected in the donors (Figure 5). As expected from the relatively modest immune-suppressive treatment delivered to the patients and the relatively small number of NK cells infused, the frequencies of alloreactive NK cells detected in patients decreased over time. Chimerism analyses performed in 5 randomly selected alloreactive NK clones showed they were of donor origin. No alloreactive NK clones were detected after infusing NK cells from the HLA-Bw4–mismatched donor who did not possess detectable frequencies of donor-versus-recipient alloreactive NK clones. Altogether, these data indicate there was no preferential outgrowth of recipient versus donor clones or that IL-2 treatment stimulated recipient rather than donor NK cells and boosted non–ligand-specific NK-cell cytotoxicity. Finally, alloreactive NK clones isolated from patient 8 (see Table 1) were tested for lysis of the patient's cryopreserved primary leukemic cells. All alloreactive clones killed the patient's leukemic cells.
Kinetics of donor-versus-recipient alloreactive NK-cell clones after NK-cell infusion. Each bar represents mean ± SD of frequencies of NK clones that killed recipient targets detected in donors and in recipients at days +3, +9, +12, +18, and +20, after NK-cell infusion.
Kinetics of donor-versus-recipient alloreactive NK-cell clones after NK-cell infusion. Each bar represents mean ± SD of frequencies of NK clones that killed recipient targets detected in donors and in recipients at days +3, +9, +12, +18, and +20, after NK-cell infusion.
Clinical outcome
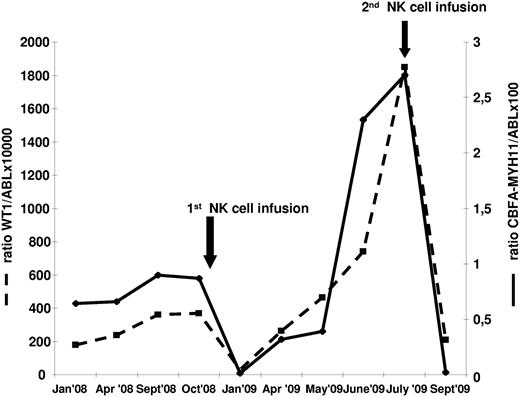
One of the 5 patients with active/progressive disease obtained a CR, which lasted for 6 months. Disease persisted in the other4 patients who ultimately died. Both patients in molecular relapse, as evaluated by increasing levels of leukemia-associated (Wilms tumor 1 gene, WT1) and leukemia-specific (CBF-MYH11) transcripts, achieved CR, which lasted 9 and 4 months, respectively. Because donor NK cells were available, one patient received an additional cycle of Flu/Cy therapy followed by NK-cell infusion at the time of second molecular relapse, which again resulted in molecular CR (Figure 6). Three of the 6 patients who were treated in CR are disease free after 34, 32, and 18 months, respectively. The other 3 patients relapsed soon after NK-cell infusion.
Clinical outcome after NK-cell infusion of 1 patient with AML harboring inv(16) in early molecular relapse. Because adequate numbers of donor NK cells were available, a second NK-cell infusion, after immunosuppressive chemotherapy, was performed at the time of the second relapse. The figure shows the value of both WT1 and CBF molecular transcript at different time points.
Clinical outcome after NK-cell infusion of 1 patient with AML harboring inv(16) in early molecular relapse. Because adequate numbers of donor NK cells were available, a second NK-cell infusion, after immunosuppressive chemotherapy, was performed at the time of the second relapse. The figure shows the value of both WT1 and CBF molecular transcript at different time points.
Discussion
The present study, involving elderly patients with high-risk AML, reports the biologic and clinical results of KIR ligand–mismatched NK-cell adoptive immunotherapy after immunosuppressive chemotherapy. Major endpoints were evaluation of feasibility and safety as well as the procedure's antileukemia efficacy and potential. Miller et al were the first to infuse NK cells in adult patients with AML with active/progressive disease.12 Although clinical responses were observed in some cases, the allogeneic grafts included enriched NK cells and a mixed population of different cell subsets, including T cells, B cells and monocytes, which could have played an additional role in the therapeutic effect of cellular infusion. Thus, the contribution of NK cells was not fully elucidated. Furthermore, NK-cell donors were not selected according to KIR ligand mismatches, although retrospectively best responses were observed in KIR ligand–mismatched patients.
Our results show that the whole procedure is feasible and safe in elderly patients with AML. Because ∼ 40% of screened patients had KIR ligand–mismatched donors, KIR-ligand–mismatched NK-cell therapy can be applied to a significant fraction of patients with AML. NK cells engrafted and hematologic toxicity were tolerable, certainly not worse than it might be expected after an immunosuppressive/myelotoxic chemotherapy regimen that included Flu and Cy. Indeed, the NK-cell infusion was not associated with any additional toxicity because neutropenia was acceptable and not complicated by significant infections, and, importantly, no signs of GVHD were documented in any patient. After infusion of haploidentical KIR ligand–mismatched NK cells, donor NK cells were detected in PB and BM and functional alloreactive NK clones were found in patients' blood during the first month after NK-cell infusion.
Although this pilot study was designed to assess the feasibility and toxicity of cellular adoptive immunotherapy, our clinical results suggest NK cell–based therapy has a potential clinical benefit for patients with poor prognosis AML. As expected, patients who seemed to benefit from the procedure were treated in CR or very early in molecular relapse or both. Indeed, after 34, 32, and 18 months of follow-up, 3 of 6 patients who were infused in CR are leukemia free. The disappearance of the leukemia-associated transcript in patients in molecular relapse suggests that immunosuppressive chemotherapy followed by NK cells exerted an antileukemic effect.
In a pediatric cohort of patients with AML, who underwent NK-cell therapy after an immunosuppressive regimen, the 2-year event-free survival was 100%. Notably, unlike our elderly patients with a median age of 62 years who were at high risk of relapse, all the children were considered at low risk of relapse, with a significant fraction harboring good-prognosis cytogenetics.14 Furthermore, because children weigh less than adults, the median number of infused NK cells was significantly higher than in the present trial, although the separation procedure was the same. These differences may partially explain the discrepancy in clinical results and suggest that in adult patients the clinical effect of NK-cell therapy may be implemented by increasing the number of infused NK cells.
In conclusion, further studies are highly warranted to specifically assess the role of NK-cell therapy in the management after remission of adult patients with AML because the present results show that adoptive immunotherapy with haploidentical NK cells obtained from KIR ligand–mismatched donors is feasible and safe in elderly patients with high-risk AML.
Furthermore, this is the first study to document the emergence and persistence over time of functional donor-versus-recipient NK-cell alloreactivity after NK-cell infusion. Because toxicity appears similar to a standard chemotherapy-based consolidation cycle, NK-cell therapy may be a promising strategy for consolidating CR in high-risk elderly patients who are not candidates for stem cell transplantation. Indeed, a phase 2 study with a greater NK-cell dose and the option of multiple NK-cell infusions is currently being conducted at our institutions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Valeria Martelli for phenotypic analysis of NK cells.
This work was supported by the Italian Ministry of Health (Progetto Strategico Oncologia, 2006), Regione Emilia-Romagna (Progetto di Ricerca Università-Regione Emilia Romagna, 2007-2009, “Hematopoietic stem cell transplant in the elderly,” no. 1412), Italian Association Against Leukemia, Bologna (BolognAIL), MIUR (PRIN 2006), a translational research grant from the Leukemia and Lymphoma Society, Italian Association for Cancer Research, the Italian Ministry of Research, and the Italian Ministry of Health. L.R. is a Leukemia and Lymphoma Society Special Scholar in Clinical Research. Miltenyi Biotec provided some separation kits for NK cells.
Authorship
Contribution: A.C. contributed to study design, was responsible for inpatient and outpatient care, and wrote the paper; L.R. was responsible for NK immunologic tests and contributed to writing the paper; E.U. and S.T. helped with immunologic tests; A.D., S.P., A.I., and S.P. contributed to inpatient and outpatient care and helped with data collection; A.B. and F.F. contributed to genetic analyses; E.D. and M.R.M. contributed to NK-cell purification; V.G. contributed to cell collection; G.B., G.M., and M.B. helped with inpatient care and manuscript revision; A.V. contributed to study design and to writing the paper; and R.M.L. was the principal investigator of the clinical trial, contributed to study design, helped with patient care and with manuscript revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Curti, Department of Hematology and Oncological Sciences “L. and A. Seràgnoli,” Via Massarenti, 9, 40138, Bologna, Italy; e-mail: antonio.curti2@unibo.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal