Activating mutations in JAK2, a tyrosine kinase that serves as a critical signaling protein for multiple cytokines, are associated with BCR-ABL1–negative myeloproliferative neoplasms. In this issue of Blood, Zheng and colleagues report that the constitutively activated kinase CK2 binds to JAK2 and is required for JAK-Stat signaling, suggesting that CK2 inhibitors may provide a second, complimentary therapeutic for diseases with elevated JAK2 signaling, such as myeloproliferative neoplasms.1
JAK family tyrosine kinases (JAK1, JAK2, JAK3, Tyk2) bind to cytokine receptors and are activated on cytokine binding. The activated JAKs phosphorylate tyrosines in themselves and in the associated cytokine receptors. The resulting phosphotyrosines form binding sites for multiple signaling proteins, including the Stat transcription factors that mediate many of the actions of the activating cytokines.2 JAK2 is activated by more than 15 different cytokines including erythropoietin, oncostatin M (OSM), GM-SCF, thrombopoietin, some interleukins, leukemia inhibitory factor, and IFN-γ3 as well as by some G protein–coupled receptors.4 Activating mutations in JAK2 have been found in patients with myeloproliferative neoplasms, with the most common mutation being V617F. This mutation is present in more than 95% of patients with polycythemia vera (PV), 32% to 57% of patients with essential thrombocythemia (ET), and 35% to 50% of patients with primary myelofibrosis (PMF).3,5 This has made JAK2 an attractive target for therapeutic intervention, and in fact, multiple JAK2 inhibitors are currently in phase 1, 2, and 3 trials.3 For these reasons, it is critical to identify the proteins that bind to JAK2 and understand how they regulate and/or mediate JAK2 function. Although quite a few proteins have been identified that are recruited to phosphorylated tyrosines in the cytokine receptors per se, including Stat proteins, very few proteins have been identified that regulate JAK2 signaling as a consequence of binding to JAK2.
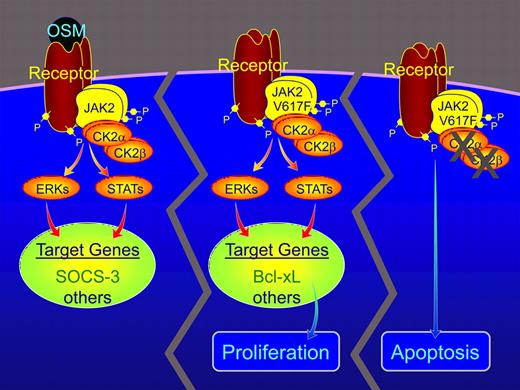
CK2 increases JAK2 activity and signaling. As reported by Zheng and colleagues, the binding of active CK2 to JAK2 appears to be essential for oncostatin M (OSM)–dependent activation of JAK2 and signaling molecules downstream of JAK2, including Stats, ERKs, and expression of target genes including the gene for suppressor of cytokine signaling-3 (SOCS-3).1 HEL cells and cells from patients with PV express the hyperactive JAK2 V617F. In these cells, the binding of CK2 to JAK2 V617F is essential for maximal JAK2 activity and the expression of the antiapoptotic protein Bcl-xL. Inhibition of CK2 activity results in a substantial decrease in JAK2, Stat3, and ERK activity, decreased Bcl-xL, and increased apoptosis.
CK2 increases JAK2 activity and signaling. As reported by Zheng and colleagues, the binding of active CK2 to JAK2 appears to be essential for oncostatin M (OSM)–dependent activation of JAK2 and signaling molecules downstream of JAK2, including Stats, ERKs, and expression of target genes including the gene for suppressor of cytokine signaling-3 (SOCS-3).1 HEL cells and cells from patients with PV express the hyperactive JAK2 V617F. In these cells, the binding of CK2 to JAK2 V617F is essential for maximal JAK2 activity and the expression of the antiapoptotic protein Bcl-xL. Inhibition of CK2 activity results in a substantial decrease in JAK2, Stat3, and ERK activity, decreased Bcl-xL, and increased apoptosis.
Zheng and colleagues provide the first evidence that the serine/threonine kinase CK2 binds to JAK2 and is critical for activation of the JAK2-Stat signaling pathway in response to ligand stimulation and for the constitutive activation of JAK2 in cells expressing JAK2 V617F.1 CK2 is a ubiquitously expressed kinase that exists primarily as a tetramer composed of 2 catalytic subunits (CK2α) and 2 regulatory subunits (CK2β). CK2 is found in multiple subcellular compartments, and has more than 300 known substrates, many associated with cell viability.6,7 Zheng et al show that depleting cells of CK2α and/or CK2β using siRNA greatly decreases the ability of the cytokine oncostatin M to stimulate tyrosyl phosphorylation of Stat3 and express SOCS3, a Stat3-dependent gene.1 Consistent with CK2 being required for ligand activation of JAK-Stat signaling, 2 inhibitors of CK2 (TBB and emodin) block oncostatin M-dependent tyrosyl phosphorylation of Stats 1, 3, and 5, and expression of SOCS3. Depletion of CK2 also decreases IFN-γ–stimulated tyrosyl phosphorylation of Stats1 and 3 and GH-stimulated phosphorylation of Stat5, providing further evidence that the stimulatory effect of CK2 on JAK-Stat signaling may be a direct effect of CK2 on JAK2. The authors report that CK2 binds to JAK2, that binding is independent of phosphotyrosines in JAK2 and increases with oncostatin M, and that CK2 can phosphorylate JAK2. Depletion of CK2β alone or with CK2α has a modest inhibitory effect on JAK2 autophosphorylation while CK2 inhibitors have a more robust inhibitory effect, suggesting that CK2 binding to JAK2 is required for maximal ligand activation of JAK2. However, the degree of JAK2 inhibition is significantly less than the inhibition of Stat signaling, raising the possibility that CK2 may have additional effects on Stat signaling.
Based on these findings, Zheng and colleagues hypothesize that CK2 inhibitors will be potent inhibitors of constitutively activated JAK2 V617F and pathways downstream of JAK2 V617F. They find in cells from PV patients and in cultured human erythroid leukemia (HEL) cells, both of which express endogenous JAK2 V617F, that indeed, JAK2 binds CK2 and that CK2 inhibitors significantly depress the amount of active JAK2, Stat3, Stat5, and ERK. More importantly, CK2 inhibitors decrease proliferation and induce apoptosis in cells expressing JAK2 V617F, assessed by proliferation assays, cell-cycle analysis, increased levels of annexin V and decreased levels of pro-caspase 8, pro-caspase 3, and/or Bcl-xL.
These studies raise a number of interesting questions related to both basic and clinical science realms. How does CK2 bind to JAK2 and is it regulated in any way that could be manipulated? Given the large number of CK2 substrates, manipulation of the JAK2/CK2 interaction would provide a more precise therapeutic intervention than simply inhibiting JAK2 or CK2. What sites in JAK2 does CK2 phosphorylate and what are the ramifications of those phosphorylations on JAK2 activity and binding partners? Is the effect of CK2 direct on JAK2 or on some other JAK2 binding partner? Interestingly, Zheng et al find CK2 also binds to JAK1, and that CK2 inhibitors decrease oncostatin M-induced JAK1 activation to a much greater extent than oncostatin M-induced JAK2 activation. Is it possible that CK2 acts primarily on JAK1 in the context of oncostatin M signaling? Finally, might CK2 inhibitors provide an additional therapeutic intervention for myeloproliferative neoplasms associated with constitutively activated JAK2 or for the variety of other diseases that are associated with elevated JAK-Stat signaling, such as solid tumors (eg, androgen-independent prostate cancer) and rheumatologic diseases3 ?
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal