In this issue of Blood, the functional attributes of human Vγ9Vδ2 T cells are firmly extended to the secretion of the proinflammatory cytokine IL-17.1
Over the past 5 years, immunology has been marked by intense investigation of the production of IL-17 by various populations of T cells, and their contributions to immunity to infection, and autoimmunity. While initially the focus had been on T helper 17 (Th17) CD4+ T cells, more recent studies in various experimental models demonstrated a critical role for IL-17–producing γδ T cells.2 However, because these experiments had been done invariably with mice, a critical question remained unanswered: Do γδ T cells make any relevant contribution to IL-17 production in humans? In this issue of Blood, Caccamo et al show they do.1
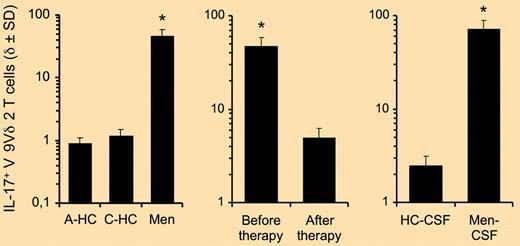
Various groups had hitherto consistently observed that the major subset of γδ T cells present in the human peripheral blood, characterized by the expression of a Vγ9Vδ2 T-cell receptor (TCR), contained very few (typically < 1%) IL-17 producers.3,4 While this still holds for healthy individuals, Caccamo and colleagues identified a dramatically different scenario in the blood and cerebrospinal fluid of children suffering from bacterial meningitis: 60%–70% of their Vγ9Vδ2 T cells were IL-17+ (see figure). Interestingly, this pattern was reversed after successful antibacterial therapy.1 Of note, Vγ9Vδ2 T cells are not the only human γδ T-cell subset capable of producing IL-17; Vδ1+ T cells were shown to make IL-17 specifically in the context of HIV-1 infection (in which they are markedly expanded).5
Frequencies of IL-17+ Vγ9Vδ2 T cells in control subjects and patients with bacterial meningitis. (A) Frequencies of IL-17–producing cells among Vγ9Vδ2 T cells from the peripheral blood of adult healthy donors (A-HC; n = 30), control children (C-HC; n = 8), and children affected by bacterial meningitis (Men; n = 12) on short-term stimulation with antigen (IPP). (B) The frequency of IL-17+ Vγ9Vδ2 T cells in the peripheral blood of children affected by bacterial meningitis (n = 12), before and after successful therapy, determined on antigen stimulation. (C) Frequencies of IL-17+ Vγ9Vδ2 T cells in the cerebrospinal fluid (CSF) of control children (HC-CSF; n = 8) and children affected by bacterial meningitis (Men-CSF; n = 12) on short-term stimulation with antigen (IPP). *P < .001 compared with all other groups.1
Frequencies of IL-17+ Vγ9Vδ2 T cells in control subjects and patients with bacterial meningitis. (A) Frequencies of IL-17–producing cells among Vγ9Vδ2 T cells from the peripheral blood of adult healthy donors (A-HC; n = 30), control children (C-HC; n = 8), and children affected by bacterial meningitis (Men; n = 12) on short-term stimulation with antigen (IPP). (B) The frequency of IL-17+ Vγ9Vδ2 T cells in the peripheral blood of children affected by bacterial meningitis (n = 12), before and after successful therapy, determined on antigen stimulation. (C) Frequencies of IL-17+ Vγ9Vδ2 T cells in the cerebrospinal fluid (CSF) of control children (HC-CSF; n = 8) and children affected by bacterial meningitis (Men-CSF; n = 12) on short-term stimulation with antigen (IPP). *P < .001 compared with all other groups.1
To further dissect the process of IL-17+ Vγ9Vδ2 T-cell differentiation, Caccamo and et al established an in vitro system, based on the combination of various cytokines, that generated 30%-40% IL-17+ Vγ9Vδ2 T cells with a phenotype matching that observed in vivo.1 Again, these percentages were much greater than ever reported before (using different in vitro settings).4 The trick used by Caccamo and colleagues was to extend the in vitro cultures—that typically involved 6 days under Th17 polarizing conditions (using TGF-β, IL-1β, IL-6, IL-23)—with an extra week of expansion in IL-2–rich medium.1 Under such conditions, IL-6 made an important contribution to the generation of IL-17+ γδ T cells, unlike what was previously reported for both human Vγ9Vδ24 and murine γδ T cells.6 Thus, the work by Caccamo and colleagues provides both physiologic importance (in vivo) and methodologic advance (in vitro) to the study of IL-17+ Vγ9Vδ2 T cells.
Although both processes of differentiation and expansion of IL-17+ γδ T cells may involve common mediators, such as IL-1β and IL-23,1,7 they must also display some key distinctive features. For example, while TGF-β is a potent antiproliferative cytokine for T cells, it is critically required for the differentiation of IL-17–producing T cells, particularly IL-17+ γδ T cells in both mice6 and humans.1,4 It will therefore be important to understand the components of the inflammatory niches where human IL-17+ Vγ9Vδ2 T cells accumulate in vivo.
A key unanswered question regarding human γδ T cells is whether they follow similar rules of developmental preprogramming as their mouse counterparts.2 Thus, work from Ribot et al6 and Chien's group8 demonstrated that murine γδ T cells acquire their capacity to produce IL-17 or IFN-γ in the thymus, and defined TCRγδ and CD27 signals as key determinants of this thymic functional/developmental choice.2 Interestingly, human cord blood and neonatal peripheral blood γδ T cells have been shown to promptly secrete IFN-γ.9 In healthy adults, 50%-80% of blood Vγ9Vδ2 T cells produce IFN-γ,9 but < 1% make IL-17.4 In this scenario, the abundance of IL-17+ Vγ9Vδ2 T cells in meningitis patients described by Caccamo and colleagues is likely because of functional reprogramming (in the particular proinflammatory milieu driven by the infection) rather than to the expansion of a precommitted IL-17+ subset as suggested in murine models of infection.7 Thus, the degree of developmental preprogramming versus functional plasticity of γδ T cells2 requires further investigation in mice and humans.
Beyond the basic immunology issues to be resolved, future research should aim at finding clinical-grade tools to manipulate human IL-17+ γδ T cells and, more generally, all IL-17+ T cells. An attractive prospect is to interfere with de novo differentiation of IL-17+ T cells. Importantly, all known IL-17+ T-cell subsets, including IL-17+ Vγ9Vδ2 T cells1 or IL-17+ Vδ1+ T cells,5 seem to depend on RORγt as a lineage-specifying transcription factor. Hence, the discovery and design of RORγt antagonists, as recently reported by Littman and colleagues,10 entail great promise for the therapeutic modulation of IL-17+ T cells in settings of chronic inflammation or autoimmunity.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal