Abstract
Immune responses require spatial and temporal coordinated interactions between different cell types within distinct microenvironments. This dynamic interplay depends on the competency of the involved cells, predominantly leukocytes, to actively migrate to defined sites of cellular encounters in various tissues. Because of their unique capacity to transport antigen from the periphery to secondary lymphoid tissues for the activation of naive T cells, dendritic cells (DCs) play a key role in the initiation and orchestration of adaptive immune responses. Therefore, pathogen-mediated interference with this process is a very effective way of immune evasion. CYTIP (cytohesin-interacting protein) is a key regulator of DC motility. It has previously been described to control LFA-1 deactivation and to regulate DC adherence. CYTIP expression is up-regulated during DC maturation, enabling their transition from the sessile to the motile state. Here, we demonstrate that on infection of human monocyte-derived DCs with herpes simplex virus type 1 (HSV-1), CYTIP is rapidly degraded and as a consequence β-2 integrins, predominantly LFA-1, are activated. Furthermore, we show that the impairment of migration in HSV-1-infected DCs is in part the result of this increased integrin-mediated adhesion. Thus, we propose a new mechanism of pathogen-interference with central aspects of leukocyte biology.
Introduction
Rapid migration of immune cells is pivotal for the initiation of innate and adaptive immune responses. Leukocytes circulate through the whole body, reaching and guarding all tissues. Therefore, the ability to infiltrate any type of tissue is crucial for their function. The mechanisms of leukocyte migration are fundamentally different from those of other cell types.1 While on 2-dimensional (2D) surfaces their locomotion is strictly dependent on integrin-mediated anchoring to the substratum, in 3-dimensional (3D) environments, such as the interstitium, integrins can be used but are not a prerequisite.2-4
Dendritic cells (DCs) are prototypic examples of leukocytes migrating through miscellaneous 3D environments in an adhesion-independent manner.5 Because of their surpassing qualification to acquire, process, and present antigens, DCs play a key role in the initiation and orchestration of immune responses. This ability depends on the dynamic interaction with other immune cells, primarily T cells, in secondary lymphatic organs and tissues. On activation by danger signals of pathogenic origin and proinflammatory cytokines, DCs mature, become motile, and start to migrate chemotactically to draining lymph nodes. Rather than solely following preformed tracks formed by integrin ligands, which would restrict their mobility, DCs are able to flexibly adapt their migration from integrin-dependent to integrin-independent modes.1 This plasticity allows them to directly follow chemokine gradients, making their locomotion fast and versatile.5
However, although adhesion is not required for leukocyte migration in 3D, a surplus restrains their locomotion. Strong activation of adhesion receptors immobilizes leukocytes, causing adhesive arrest.6 Therefore, integrin-mediated adhesion can be a determinant of leukocyte mobility and has to be tightly controlled. The individual integrin repertoire of a given cell determines its behavior in different microenvironments.7 Integrins of the β2 family, consisting of the β-subunit CD18 that combines with the 4 different α-chains αL (CD11a), αM (CD11b), αX (CD11c), and αD (CD11d), are found exclusively on leukocytes7 where they play crucial roles in the attachment to endothelia, diapedesis, and T-cell effector functions.8 The integrin αLβ2 (CD11a/CD18 or LFA-1) is expressed constitutively on all leukocytes, whereas all other β2-integrins are restricted to various subsets.
Importantly, integrins are not constitutively active7 ; instead, their ligand binding capacity is regulated by integration of intracellular and extracellular signals through so-called inside-out signaling.9 Cytohesin-1 is expressed mainly in hematopoietic cells10 and was shown to be an intracellular interaction partner of CD18.11 The binding of cytohesin-1 to the intracellular tail of CD18 results in increased β2-integrin affinity.10-13 This cytohesin-1-mediated LFA-1 activation is regulated by the cytohesin-interacting protein (CYTIP), which is exclusively expressed in cells of the hematopoietic lineage and is up-regulated during DC maturation.14 CYTIP is recruited to the cell cortex by integrin signaling and interacts with cytohesin-1. Subsequently, the complex translocates from the plasma membrane to the cytosol, thereby interrupting the activation of LFA-1.14 Recently, it was shown that CYTIP regulates leukocyte trafficking15 and the adherence of DCs to fibronectin16 as well as T-cell detachment and T-cell priming.16,17
Herpes simplex virus type 1 (HSV-1) is the prototypic member of the large and diverse family of herpesviridae, including several human pathogens.18 HSV-1 infections affect the majority of the adult population worldwide and are responsible for a variety of diseases, ranging from mild localized infections to life-threatening variants in newborns or immune-compromised persons.19 Moreover, HSV infections are related to HIV transmission, and coinfected patients have a higher plasma viral load. Thus, understanding the viral immune-evasion mechanisms is of great importance for the development of new therapies. Because DCs are the most important stimulators of antiviral immune responses by activating cytotoxic T lymphocytes, HSV-1 has evolved multiple strategies to inhibit normal DC function.20
In the present study, we provide evidence that by rapid degradation of CYTIP in DCs, HSV-1 activates β2-integrins, predominantly LFA-1, thereby enhancing integrin-mediated adhesion and impeding DC migration. This hijacking of the CD18 activation pathway interferes with central aspects of DC biology, such as homing to the T-cell areas of lymph nodes and T-cell priming. Therefore, our findings suggest a novel mechanism of viral immune-escape strategies.
Methods
Antibodies
The following monoclonal antibodies against the human antigens were used: CD83, CD86, HLA-DR, CCR7, CD11a, CD11b, CD18 (BD Biosciences); rat anti-CYTIP clone 2F9 and rat anti–cytohesin-1 clone 7H2 (kind gift from E. Kremmer, Helmholtz Center Munich, Germany); anti-activated CD11/CD18 clone mAB24 (Hycult Biotech); anti-activated CD11b clone CBRM1/5 (BioLegend); anti–β-actin (Sigma-Aldrich); blocking antibodies against integrin αL (NKI-L15) and αM (Bear-1; C.G. Figdor); β2-integrin activating KIM185 (kindly provided by M. Robinson, Celltech); horseradish peroxidase–conjugated anti–rat, anti–mouse, and anti–rabbit antibodies (Cell Signaling Technology); and isotype controls and phycoerythrin-labeled secondary antibodies (BD Biosciences).
Viruses
HSV-1 strain 17+ (HSV-1) was the wild-type laboratory strain used, from which the strain HSV-1/17+/CMV-EGFP/UL43 (HSV-1 EGFP), the ICP0 deletion mutant dl1403 (HSV-1 ΔICP0), and the vhs deletion mutant pR20.5/vhs (HSV-1 Δvhs) were derived. Ultraviolet (UV)-inactivated virus (HSV-1 UV) was generated by irradiation of HSV-1 virions with UV light (1.200 J cm−2). Virus stocks were prepared, and virus-titer was determined as previously described.21 All strains were obtained from BioVex.
Generation of DCs
DCs were generated as described previously.22
HSV-1 infection of DCs
Mature DCs were harvested and adjusted to a concentration of 107 cells/mL in RPMI 1640 (Lonza) supplemented with 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Lonza). Virus was added with a multiplicity of infection (MOI) of 1 for 1 hour at 37°C. Subsequently, DCs were cultured in DC medium until further use. Where indicated, dimethyl sulfoxide (Sigma-Aldrich) or MG-132 (Merck) was added for the rest of the culture time. MnCl2, EDTA (Sigma-Aldrich), or blocking/activating antibodies (20 μg/mL) were added at the indicated time points after infection in the respective experiments.
Expression analysis using Affymetrix gene chips
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometric analyses
See supplemental Methods.
Western blot analyses
See supplemental Methods.
Fibronectin adhesion assays
The 96-well maxisorb plates (Nunc) were coated with 20 μg/mL fibronectin (Sigma-Aldrich) in PBS at 4°C overnight and blocked with 0.01% gelatin (Sigma-Aldrich) in PBS for 2 hours at room temperature. DCs were harvested at the respective time points after infection, washed, and adjusted to 4 × 105 cells/mL in RPMI 1640. Subsequently, wells were washed and 100 μL of the DC suspension was added. Cells were allowed to adhere for 45 minutes at 37°C. After adhesion, wells were washed and the number of adherent cells was determined by measuring β-glucuronidase activity as described previously.22
ICAM adhesion assay
The 96-well maxisorb plates were coated with 4 μg/mL goat anti–human IgG, Fc specific (Jackson ImmunoResearch Laboratories) in PBS overnight. Wells were blocked with 1% BSA in PBS for 30 minutes at 37°C. After washing, 100 μL of 500 ng/mL ICAM-1-Fc in PBS was added for 1 hour at 37°C. Adherence and quantification of DCs were performed as described for the fibronectin adhesion assay.
siRNA transfections
See supplemental Methods.23
Transwell migration assays
See supplemental Methods.
3D migration assay
DCs were embedded into a collagen matrix (PureCol, Inamed BioMaterials, final concentration 1.7 mg/mL; 0.45 × 106 cells/mL), which remained uncoated, or contained fibronectin (Roche Diagnostics, final concentration 20 μg/mL) or Dynabeads Protein G (Invitrogen, final concentration in matrix 75 μg/mL) coated with ICAM-1 Fc (20 μg/mL) in custom-made migration chambers. Polymerized gels were overlaid with medium containing 2 μg/mL CCL19 (R&D Systems). Migration of DCs was recorded by bright-field time-lapse videomicroscopy at 37°C using inverted DM IL microscopes (Leica Microsystems) fitted with 10×/0.22 HI Plan objectives and STC-405 cameras (Sentech), beginning 90 minutes after infection. Cells were imaged at a frame rate of 2 minutes up to 180 frames, controlled by custom-written software. Computer-assisted cell tracking was performed as described previously24 with custom-written software. The average speed was calculated as the step length per minute, including nonmoving periods of 30 randomly selected cells in every single experiment.
T-cell-adhesion assay
See supplemental Methods.
Statistical analysis
Data were analyzed using 2-tailed Student t test and analysis of variance with the GraphPad Instat software Version 3.10 (GraphPad). Differences were considered as significant at P values < .05.
Results
HSV-1 infection of mature DCs leads to increased integrin-mediated adhesion to fibronectin
Previously, we have demonstrated that the chemotaxis of HSV-1–infected human monocyte-derived DCs toward CCL19 is drastically reduced.22 Because increased adhesion leads to adhesive arrest and thereby impairs leukocyte migration, we addressed the question whether HSV-1 infection of DCs alters their adherence to proteins of the extracellular matrix. We chose fibronectin as a prototype ligand for numerous cell adhesion molecules with wide distribution in vertebrate tissues.25
To determine the efficiency of infection, mature DCs were infected with HSV-1 virions expressing enhanced green fluorescent protein (EGFP) and analyzed by FACS at the indicated time points. As shown in supplemental Figure 1, DCs are efficiently infected even at early time points.
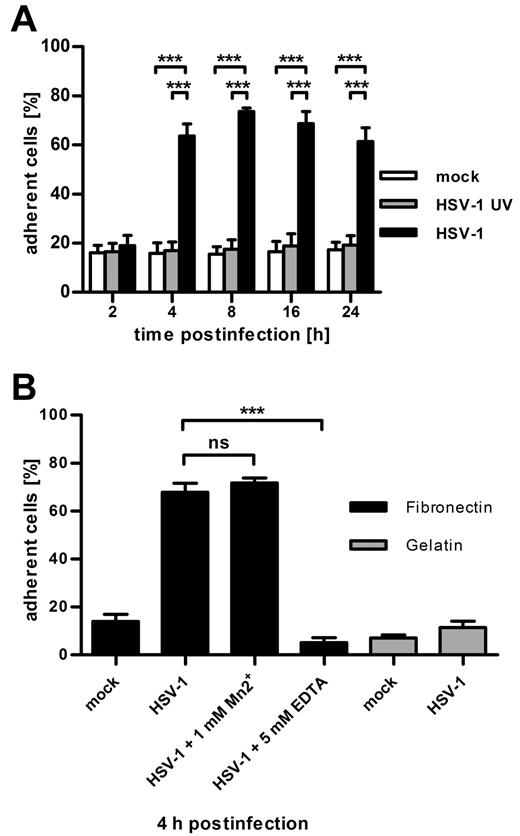
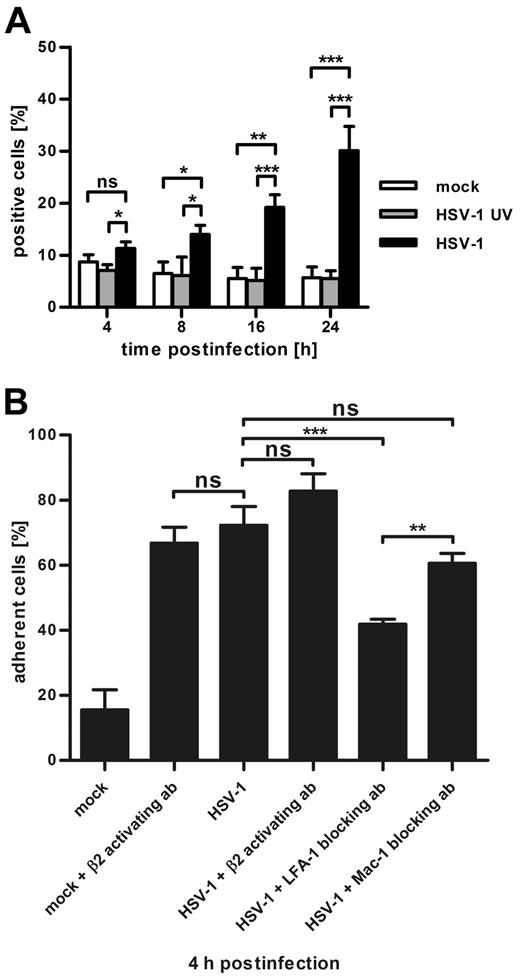
To investigate the effect of HSV-1 infection on their adhesion, DCs were either infected with HSV-1 wild-type virions, UV-inactivated HSV-1 virus particles, or treated with equivalent amounts of virus dilution buffer (mock). UV-inactivated virus particles are taken up into the cell, but expression of viral genes is averted (as assayed by FACS for GFP expression, data not shown). At the indicated time points after infection, fibronectin adhesion assays were performed (Figure 1A). Two hours after infection, there was no difference in adherence to fibronectin between all 3 conditions detectable, whereas at all later time points the HSV-1–infected DCs showed a significant increase in their adhesion compared with mock-infected as well as to HSV-1 UV-infected DCs.
DCs infected with herpes simplex virus type 1 adhere to fibronectin and down-regulate CYTIP expression. (A) Quantification of adherent cells shows that, as early as 4 hours after infection, HSV-1 strongly increases the fibronectin adherence of infected DCs. Mature DCs were mock-infected (white columns) or infected with UV-inactivated virus (gray columns) or HSV-1 (black columns) at a MOI of 1. At the indicated time points after infection, cells were allowed to adhere to fibronectin-coated 96-well plates. Adherence was quantified by measuring β-glucuronidase activity of adherent cells. (B) Fibronectin adhesion of HSV-1-infected DCs (MOI 1) is mediated by integrins and is specific for fibronectin. Four hours after infection, DCs were treated with 1mM Mn2+ or 5mM EDTA and were allowed to adhere to fibronectin-coated (black columns) or gelatin-coated (gray columns) 96-wells. Adherence was quantified by measuring β-glucuronidase activity. Mn2+ has no significant effect on the adhesion of infected DCs, whereas ethylenediaminetetraacetic acid completely blocks adhesion. Neither mock-infected nor HSV-1-infected DCs adhere to gelatin. Error bars represent ± SD. ***P < .001. ns indicates not significant. Experiments were repeated at least 3 times independently. Each single experiment was performed in quadruplicate.
DCs infected with herpes simplex virus type 1 adhere to fibronectin and down-regulate CYTIP expression. (A) Quantification of adherent cells shows that, as early as 4 hours after infection, HSV-1 strongly increases the fibronectin adherence of infected DCs. Mature DCs were mock-infected (white columns) or infected with UV-inactivated virus (gray columns) or HSV-1 (black columns) at a MOI of 1. At the indicated time points after infection, cells were allowed to adhere to fibronectin-coated 96-well plates. Adherence was quantified by measuring β-glucuronidase activity of adherent cells. (B) Fibronectin adhesion of HSV-1-infected DCs (MOI 1) is mediated by integrins and is specific for fibronectin. Four hours after infection, DCs were treated with 1mM Mn2+ or 5mM EDTA and were allowed to adhere to fibronectin-coated (black columns) or gelatin-coated (gray columns) 96-wells. Adherence was quantified by measuring β-glucuronidase activity. Mn2+ has no significant effect on the adhesion of infected DCs, whereas ethylenediaminetetraacetic acid completely blocks adhesion. Neither mock-infected nor HSV-1-infected DCs adhere to gelatin. Error bars represent ± SD. ***P < .001. ns indicates not significant. Experiments were repeated at least 3 times independently. Each single experiment was performed in quadruplicate.
As a tool to investigate whether this augmented adherence is the result of integrin-mediated binding to fibronectin, manganese (Mn2+), or EDTA were added, respectively (Figure 1B). Mn2+ is a potent stimulator of β1 and β2-integrin affinity maturation,26 whereas EDTA blocks binding of integrins to their ligands by chelation of divalent cations. Whereas the addition of Mn2+ to HSV-1-infected DCs 4 hours after infection had no effect, EDTA was able to completely abolish the adhesion (Figure 1B). To ensure that the adhesion to fibronectin is specific and not the result of a general increase in adhesiveness, we allowed DCs to adhere to gelatin-coated wells as a control. Quantification of adherent cells revealed that adhesion of infected DCs to gelatin is not strengthened (Figure 1B). These data demonstrate that HSV-1 gene products actively influence the adhesion of DCs to extracellular matrix proteins and that the increase in fibronectin adherence is specific and mediated by integrins.
The expression profile of CYTIP in mature DCs is strongly reduced by HSV-1 infection
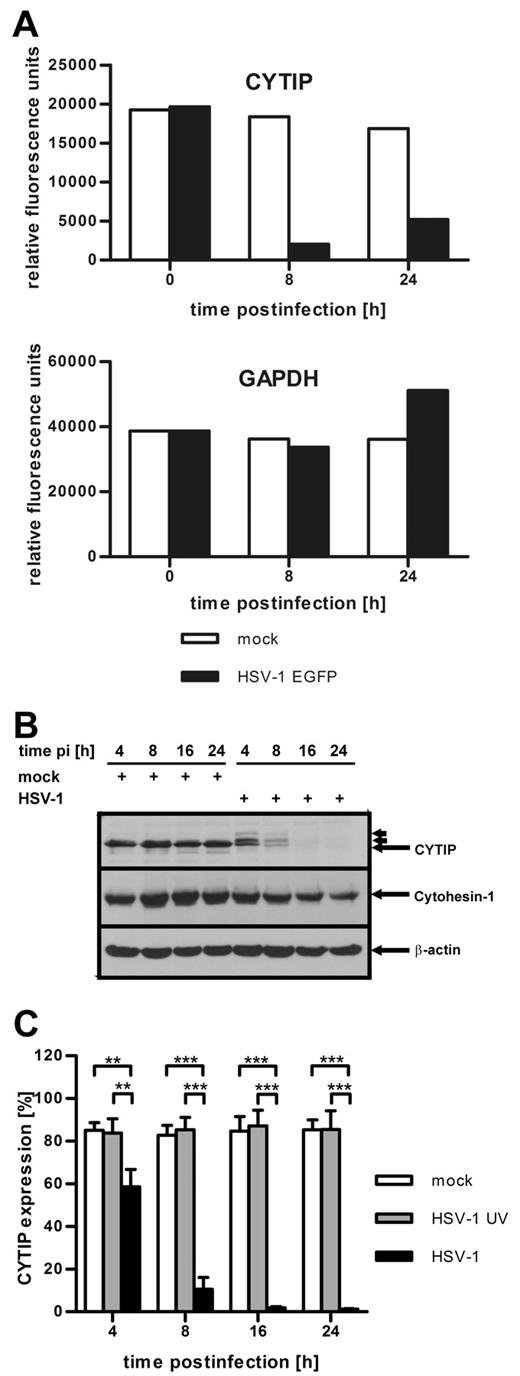
Recently, it has been shown that CYTIP regulates the adhesion of DCs to fibronectin and that the loss of fibronectin adherence of mature DCs depends on increased CYTIP expression.16 To obtain initial information regarding the consequences of HSV-1 infection of mature DCs on their gene expression, Affymetrix gene-chip analyses were performed (Figure 2A). At the indicated time points, total RNA was extracted from HSV-1 EGFP and mock-infected DCs and profiled on Affymetrix Human Genome U133A arrays. Interestingly, CYTIP-specific mRNA was among the most prominently reduced mRNA species in HSV-1–infected DCs. As early as 8 hours after infection, a dramatic reduction of CYTIP mRNA could be observed (Figure 2A top panel) compared with the mRNA expression level in uninfected DCs. Other expression profiles, such as the mRNA levels of GAPDH, were not significantly influenced by HSV-1 infection and remained almost constant both in HSV-1– and mock-infected cells (Figure 2A bottom panel). Thus, CYTIP expression is specifically down-regulated in HSV-1–infected DCs.
CYTIP is rapidly down-regulated in HSV-1-infected DCs. (A) Total mRNA was isolated from HSV-1 EGFP-infected (MOI of 1, black columns) and mock-infected (white columns) DCs at the indicated time points. The RNA was labeled and hybridized to Affymetrix Human Genome U133A arrays. The expression profile of CYTIP (top graph) shows a dramatic down-regulation 8 and 24 hours after infection compared with mock-infected cells. The expression profile of GAPDH (bottom graph) is shown as control; no significant influence on gene expression could be observed. (B) DCs were mock-infected or infected with HSV-1 at a MOI of 1 and harvested at the indicated time points after infection (pi). Western blot analyses with antibodies against CYTIP (top panel), cytohesin-1 (middle panel), and β-actin as loading control (bottom panel) show that CYTIP is rapidly down-regulated in HSV-1-infected DCs, whereas cytohesin-1 is only slightly influenced by infection. Small arrows indicate the appearance of additional CYTIP bands with higher molecular masses. (C) Exact quantification of CYTIP expression in mock-infected DCs (white columns), cells treated with UV-inactivated HSV-1 virions (gray columns), and HSV-1-infected DCs (MOI of 1, black columns) by intracellular flow cytometry illustrates that CYTIP is down-regulated with fast kinetics only after infection with HSV-1. Error bars represent ± SD. ***P < .001. **P < .01. Gene profiling by Affymetrix analysis (A) was performed once. Experiments B and C were repeated at least 3 times independently. (B) One representative experiment is shown.
CYTIP is rapidly down-regulated in HSV-1-infected DCs. (A) Total mRNA was isolated from HSV-1 EGFP-infected (MOI of 1, black columns) and mock-infected (white columns) DCs at the indicated time points. The RNA was labeled and hybridized to Affymetrix Human Genome U133A arrays. The expression profile of CYTIP (top graph) shows a dramatic down-regulation 8 and 24 hours after infection compared with mock-infected cells. The expression profile of GAPDH (bottom graph) is shown as control; no significant influence on gene expression could be observed. (B) DCs were mock-infected or infected with HSV-1 at a MOI of 1 and harvested at the indicated time points after infection (pi). Western blot analyses with antibodies against CYTIP (top panel), cytohesin-1 (middle panel), and β-actin as loading control (bottom panel) show that CYTIP is rapidly down-regulated in HSV-1-infected DCs, whereas cytohesin-1 is only slightly influenced by infection. Small arrows indicate the appearance of additional CYTIP bands with higher molecular masses. (C) Exact quantification of CYTIP expression in mock-infected DCs (white columns), cells treated with UV-inactivated HSV-1 virions (gray columns), and HSV-1-infected DCs (MOI of 1, black columns) by intracellular flow cytometry illustrates that CYTIP is down-regulated with fast kinetics only after infection with HSV-1. Error bars represent ± SD. ***P < .001. **P < .01. Gene profiling by Affymetrix analysis (A) was performed once. Experiments B and C were repeated at least 3 times independently. (B) One representative experiment is shown.
Infection of mature DCs with HSV-1 leads to rapid down-regulation of CYTIP
To evaluate the information obtained from the gene profiling analysis, we tested whether CYTIP protein levels are affected by HSV-1 infection. From previous studies, it is well established that CYTIP indirectly regulates the adhesiveness of lymphocytes by translocating the β2-integrin-binding protein cytohesin-1 from the membrane to the cytosol.14 When located at the plasma membrane, cytohesin-1 interacts with the cytoplasmic domain of CD18, thereby activating LFA-1.10-12 Because of the importance of cytohesin-1 for the induction of β2-mediated adhesion, we also examined its expression by Western blot analysis. Figure 2B shows CYTIP–, cytohesin-1–, and β-actin–specific immunoblots of HSV-1-infected and mock-infected DCs. CYTIP was rapidly reduced during the course of infection, whereas cytohesin-1 expression was only slightly diminished at later time points. β-actin protein levels served as loading control. Interestingly, HSV-1 leads to the formation of bands with altered migration in SDS-PAGE (indicated by small arrows in Figures 2B and 3B). Those additional bands might represent phosphorylated and/or ubiquitinated forms of CYTIP.
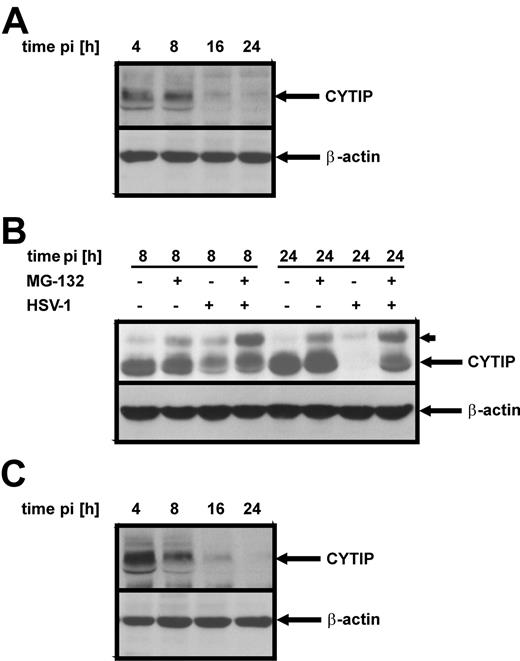
CYTIP is degraded via the proteasome, and vhs and ICP0 do not contribute to its degradation. Western blot analyses were performed with an antibody specific for CYTIP. Infection with either a vhs deletion mutant virus (A) or an ICP0 deletion mutant (C) leads to degradation of CYTIP with kinetics similar to that of HSV-1 wild-type infection. DCs were infected at a MOI of 1 and harvested at the indicated time points after infection (pi). (B) Inhibition of the proteasome by the addition of 10 μM MG-132 beginning 1 hour after infection completely blocks the loss of CYTIP. Notably, an additional band with higher molecular weight accumulates in MG-132-treated samples. The enrichment of this band in HSV-1–infected DCs indicates a modification that is marked for degradation. Experiments were performed at least 3 times independently, and 1 representative experiment is shown.
CYTIP is degraded via the proteasome, and vhs and ICP0 do not contribute to its degradation. Western blot analyses were performed with an antibody specific for CYTIP. Infection with either a vhs deletion mutant virus (A) or an ICP0 deletion mutant (C) leads to degradation of CYTIP with kinetics similar to that of HSV-1 wild-type infection. DCs were infected at a MOI of 1 and harvested at the indicated time points after infection (pi). (B) Inhibition of the proteasome by the addition of 10 μM MG-132 beginning 1 hour after infection completely blocks the loss of CYTIP. Notably, an additional band with higher molecular weight accumulates in MG-132-treated samples. The enrichment of this band in HSV-1–infected DCs indicates a modification that is marked for degradation. Experiments were performed at least 3 times independently, and 1 representative experiment is shown.
To precisely quantify the loss of CYTIP, we performed intracellular FACS staining in mock-infected, HSV-1 UV-infected, and HSV-1–infected DCs (Figure 2C). As early as 4 hours after infection, HSV-1–infected DCs already showed a highly significant reduction of CYTIP expression compared with both HSV-1 UV-infected as well as mock-infected DCs. Eight hours after infection and at later time points, CYTIP was detectable only in a small proportion of infected DCs. These data demonstrate that HSV-1 infection of mature DCs leads to a fast and complete reduction of CYTIP, but not of cytohesin-1.
CYTIP is degraded via the proteasome
Considering the early onset of CYTIP reduction on protein and mRNA levels after HSV-1 infection, this effect is most probably mediated either by immediate-early viral gene products or by tegument components, which are delivered directly with the infection into the cell. The virion host shutoff protein (vhs), a viral mRNA-specific RNase that induces rapid shutoff of host protein synthesis and degradation of cellular mRNAs, is contained in the viral tegument and has been postulated to contribute to HSV-1 immune evasion.27 Therefore, we examined its effect on CYTIP using a vhs deletion mutant virus (HSV-1 Δvhs). Immunoblot analysis revealed that, even without vhs, CYTIP levels were reduced with a kinetic similar to that of wild-type virus infection (Figure 3A). To determine whether the loss of CYTIP is the result of proteasomal degradation of the protein, we used the proteasome inhibitor MG-132. Immunoblot analyses 8 and 24 hours after infection demonstrated that treatment of HSV-1–infected DCs with 10 μM MG-132 is sufficient to completely prevent the reduction of CYTIP (Figure 3B). Notably, inhibition of the proteasome leads to enrichment of an additional band with higher molecular weight (marked by small arrow in Figure 3B) both in mock- as well as in HSV-1–infected DCs, indicating that this modified form of CYTIP is degraded by the proteasome. Importantly, its proportion is greater in infected DCs and is even detectable without proteasomal inhibition 8 hours after infection, demonstrating that CYTIP degradation is increased after HSV-1 infection. In this respect, we hypothesized that the HSV-1 immediate-early protein ICP0 might be involved. ICP0 is a ubiquitin E3 ligase and induces the proteasome-dependent degradation of several cellular proteins.28 Immunoblot analysis of DCs infected with an ICP0 deletion mutant virus (HSV-1 ΔICP0) demonstrated that also this mutant led to a fast and complete degradation of CYTIP, comparable with the kinetics of wild-type infection (Figure 3C). Thus, HSV-1 targets CYTIP to proteasomal degradation via an unknown, ICP0-independent mechanism.
Infection of mature DCs with HSV-1 induces the activation of β2-integrins
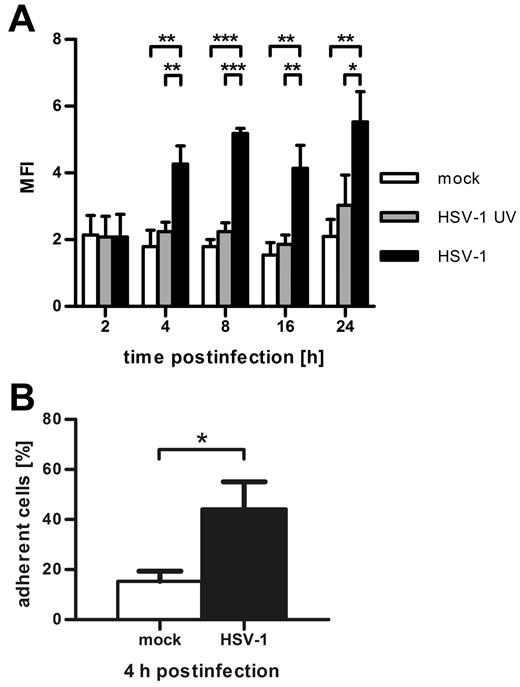
Because CYTIP regulates the activation of β2-integrins, we investigated whether infected DCs show an increase in β2-integrin affinity. Therefore, we analyzed CD11/CD18 affinity by FACS with the activation-specific monoclonal antibody mAB24 (Figure 4A). Two hours after infection, no difference in β2-integrin affinity was detectable between HSV-1–infected, HSV-1 UV-infected, and mock-infected DCs. As early as 4 hours after infection, a significant increase could be observed only in HSV-1–infected DCs, which remained constant up to 24 hours after infection. To assess whether HSV-1–infected DCs also show increased adhesion to typical β2-integrin ligands and not only to fibronectin, we performed ICAM-1–adhesion assays 4 hours after infection. As shown in Figure 4B, infected DCs indeed adhere significantly stronger to ICAM-1 than the mock control. Hence, HSV-1 activates β2-integrins on the DCs, leading to increased ICAM-1–adhesion.
HSV-1 infection of DCs leads to CD18 activation and increased adhesion to ICAM-1. (A) At the indicated time points after infection, flow cytometric analyses of mock-infected DCs (white columns), HSV-1 UV-infected (gray columns), and HSV-1–infected DCs (black columns) were performed with the CD11/CD18 activation-specific antibody mAB24. Concomitantly with CYTIP down-regulation, β2-integrins undergo affinity maturation in HSV-1–infected DCs. Shown is the mean fluorescence intensity as calculated by substraction of isotype fluorescence from mAB24 fluorescence. (B) Four hours after infection, DCs were allowed to adhere to ICAM-1–Fc-coated 96-wells for 45 minutes. After gentle washing, adherence was quantified by measuring the β-glucuronidase activity of the cells. HSV-1–infected cells adhere significantly stronger compared with mock-infected control cells. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. Experiments were repeated at least 3 times independently. ICAM-1–adhesion experiments were performed in quadruplicate.
HSV-1 infection of DCs leads to CD18 activation and increased adhesion to ICAM-1. (A) At the indicated time points after infection, flow cytometric analyses of mock-infected DCs (white columns), HSV-1 UV-infected (gray columns), and HSV-1–infected DCs (black columns) were performed with the CD11/CD18 activation-specific antibody mAB24. Concomitantly with CYTIP down-regulation, β2-integrins undergo affinity maturation in HSV-1–infected DCs. Shown is the mean fluorescence intensity as calculated by substraction of isotype fluorescence from mAB24 fluorescence. (B) Four hours after infection, DCs were allowed to adhere to ICAM-1–Fc-coated 96-wells for 45 minutes. After gentle washing, adherence was quantified by measuring the β-glucuronidase activity of the cells. HSV-1–infected cells adhere significantly stronger compared with mock-infected control cells. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. Experiments were repeated at least 3 times independently. ICAM-1–adhesion experiments were performed in quadruplicate.
Inhibition of LFA-1 activation in HSV-1-infected DCs strongly reduces their adhesion to fibronectin
Although the canonical cellular receptors for fibronectin are β1 and β3 integrins, β2-integrins have been described to bind fibronectin as well.29-31 It has been shown that αMβ2 (CD11b/CD18 or Mac-1) binding promiscuity accounts to a large extend for this binding.32 To investigate in more detail which β2-integrins are responsible for the increase of fibronectin adhesion after HSV-1 infection, we analyzed the expression of CD11a, CD11b, and CD18 by FACS. The surface expression of those integrins was only marginally altered by HSV-1 infection and remained at constant high levels of > 90% positive cells up to 24 hours after infection (data not shown). Using a CD11b activation-specific antibody, we assayed Mac-1 activation by FACS. Importantly, although 4 hours after infection HSV-1–infected DCs already strongly adhere to fibronectin (Figure 1A), at this time point Mac-1 is not significantly activated compared with mock-infected DCs (Figure 5A). During the course of infection, CD11b activity increases, reaching a maximum 24 hours after infection.
Activated LFA-1, not Mac-1, determines the fibronectin adhesion of HSV-1-infected DCs. (A) Flow cytometric analyses of activated CD11b were performed in mock-infected (white columns), HSV-1–UV-infected (gray columns), and HSV-1–infected (black columns) DCs at the indicated time points after infection. Although Mac-1 is only marginally activated at early time points after infection, its activation increases at later time points. (B) Four hours after infection, fibronectin adhesion assays were performed using 20 μg/mL of the β2 activatory antibody KIM185, the LFA-1 blocking antibody NKI-L15, or the Mac-1 blocking antibody Bear-1, respectively. Activation of β2-integrins on mock-infected DCs renders them highly adhesive, although it has no effect on HSV-1–infected DCs. Blocking of Mac-1 on infected cells has no significant effect on DC adhesion to fibronectin, whereas it is strongly reduced by the inhibition of LFA-1. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant. The experiments were repeated at least 3 times independently, and each single adhesion experiment was performed in quadruplicate.
Activated LFA-1, not Mac-1, determines the fibronectin adhesion of HSV-1-infected DCs. (A) Flow cytometric analyses of activated CD11b were performed in mock-infected (white columns), HSV-1–UV-infected (gray columns), and HSV-1–infected (black columns) DCs at the indicated time points after infection. Although Mac-1 is only marginally activated at early time points after infection, its activation increases at later time points. (B) Four hours after infection, fibronectin adhesion assays were performed using 20 μg/mL of the β2 activatory antibody KIM185, the LFA-1 blocking antibody NKI-L15, or the Mac-1 blocking antibody Bear-1, respectively. Activation of β2-integrins on mock-infected DCs renders them highly adhesive, although it has no effect on HSV-1–infected DCs. Blocking of Mac-1 on infected cells has no significant effect on DC adhesion to fibronectin, whereas it is strongly reduced by the inhibition of LFA-1. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. ns indicates not significant. The experiments were repeated at least 3 times independently, and each single adhesion experiment was performed in quadruplicate.
To further assess the specific relevance of LFA-1 and Mac-1, we performed fibronectin adhesion assays with the β2-activating antibody KIM185, the LFA-1 blocking antibody NKI-L15, and the Mac-1 blocking antibody Bear-1. Four hours after infection, the respective antibodies were added at a concentration of 20 μg/mL (Figure 5B). The activation of β2-integrins in mock-infected DCs increased their adhesion to levels comparable with HSV-1–infected DCs, demonstrating that indeed β2-integrins mediate the adherence. Notably, the adhesion of HSV-1–infected DCs could not be further increased by the addition of KIM185, showing that in this case β2-integrins are already active. Most important, the inhibition of Mac-1 did not significantly decrease the fibronectin adhesion of infected DCs, whereas blockade of LFA-1 strongly reduced it. Accordingly, LFA-1, and not Mac-1, is primarily responsible for the augmented fibronectin adhesion of HSV-1–infected DCs.
Enhanced adhesion of HSV-1–infected DCs correlates with diminished locomotion
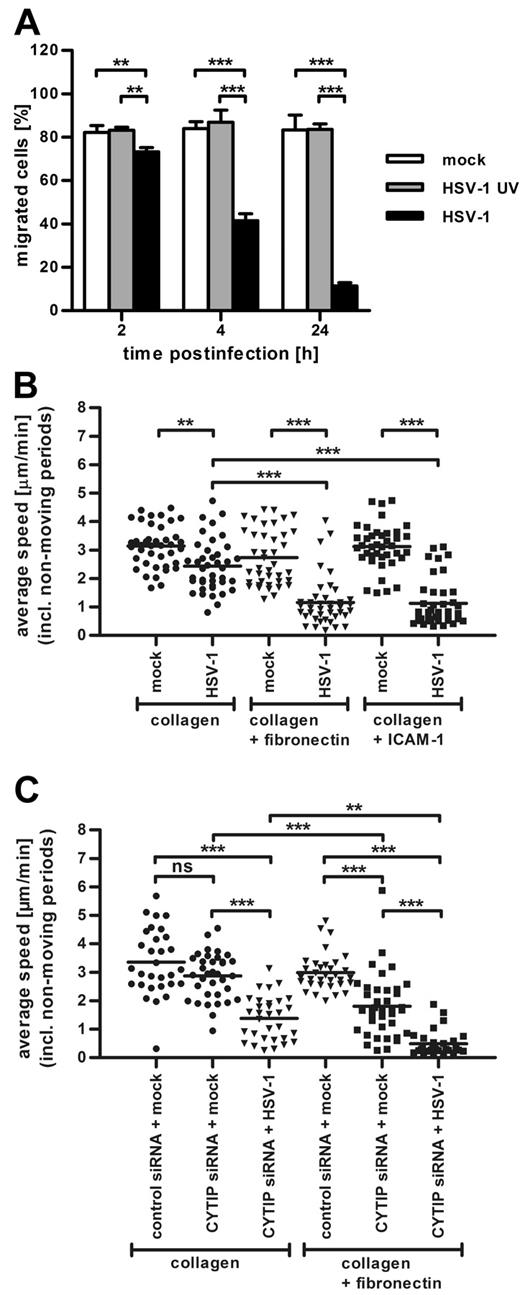
Our finding that HSV-1 infection drastically augments DC adhesion prompted us to assess whether the kinetic of this increase correlates with the previously described22 decrease of migration. To this end, we performed transwell migration assays with fibronectin-coated polycarbonate filters (Figure 6A). Although neither mock infection nor the treatment with UV-inactivated virions had an effect on DC migration, the infection with HSV-1 rapidly decreased their chemotaxis. Two hours after infection, the migration of infected DCs was only slightly diminished. Strikingly, already 4 hours after infection, infected DCs migrated approximately 50% less compared with their uninfected counterparts, indicating that the increase in adhesion correlates with the inhibition of migration.
Chemotaxis of mature DCs is impaired after HSV-1 infection and CYTIP knockdown. (A) Transwell migration assays on fibronectin-coated transwells of mock-infected (white columns), HSV-1 UV-infected (gray columns), and HSV-1-infected (black columns) DCs harvested at the indicated time points after infection show that the infection with HSV-1 dramatically reduces DC chemotaxis toward CCL19. (B) The chemotaxis of HSV-1–infected DCs toward CCL19 in a 3D-collagen matrix or in collagen matrices containing 20 μg/mL fibronectin or 75 μg/mL ICAM-1 Fc-coated beads is decreased compared with mock-infected cells in all conditions. The presence of immobilized integrin ligands in the gels further reduces migration of HSV-1–infected DCs but has no influence on mock-infected DCs. (C) A total of 4 × 106 immature DCs were electroporated with 10 μg of a nontargeting control siRNA or with 10 μg siRNA targeting CYTIP. Four hours later, maturation cocktail was added and DCs were matured for 48 hours. Where indicated, mature CYTIP-knockdown DCs were additionally infected with HSV-1. Subsequently, DCs were used in 3D migration assays in pure collagen gels or in gels containing 20 μg/mL fibronectin. Silencing of CYTIP strongly impairs migration of DCs in collagen gels containing fibronectin. The infection of CYTIP-silenced DCs further reduces chemotaxis in both conditions but has a more prominent effect in fibronectin-coated gels. DC migration toward a CCL19 gradient was monitored by bright-field time-lapse videomicroscopy beginning 90 minutes after infection. Cells were imaged at a frame rate of 2 minutes. The average speed of a cell was calculated as the step length per minute, including nonmoving periods and displayed in micrometers per minute. Average speed was quantified from 30 cells for each condition over 180 frames (B-C). Error bars represent ± SD. ***P < .001. **P < .01. ns indicates not significant. One representative experiment of 3 is shown.
Chemotaxis of mature DCs is impaired after HSV-1 infection and CYTIP knockdown. (A) Transwell migration assays on fibronectin-coated transwells of mock-infected (white columns), HSV-1 UV-infected (gray columns), and HSV-1-infected (black columns) DCs harvested at the indicated time points after infection show that the infection with HSV-1 dramatically reduces DC chemotaxis toward CCL19. (B) The chemotaxis of HSV-1–infected DCs toward CCL19 in a 3D-collagen matrix or in collagen matrices containing 20 μg/mL fibronectin or 75 μg/mL ICAM-1 Fc-coated beads is decreased compared with mock-infected cells in all conditions. The presence of immobilized integrin ligands in the gels further reduces migration of HSV-1–infected DCs but has no influence on mock-infected DCs. (C) A total of 4 × 106 immature DCs were electroporated with 10 μg of a nontargeting control siRNA or with 10 μg siRNA targeting CYTIP. Four hours later, maturation cocktail was added and DCs were matured for 48 hours. Where indicated, mature CYTIP-knockdown DCs were additionally infected with HSV-1. Subsequently, DCs were used in 3D migration assays in pure collagen gels or in gels containing 20 μg/mL fibronectin. Silencing of CYTIP strongly impairs migration of DCs in collagen gels containing fibronectin. The infection of CYTIP-silenced DCs further reduces chemotaxis in both conditions but has a more prominent effect in fibronectin-coated gels. DC migration toward a CCL19 gradient was monitored by bright-field time-lapse videomicroscopy beginning 90 minutes after infection. Cells were imaged at a frame rate of 2 minutes. The average speed of a cell was calculated as the step length per minute, including nonmoving periods and displayed in micrometers per minute. Average speed was quantified from 30 cells for each condition over 180 frames (B-C). Error bars represent ± SD. ***P < .001. **P < .01. ns indicates not significant. One representative experiment of 3 is shown.
Infection with HSV-1 rapidly inhibits DC migration in 3D collagen gels
Because the mechanisms of DC migration on 2D and in 3D environments are fundamentally different,3 live cell videomicroscopy in 3D collagen matrices was performed. In addition, we investigated the effect of integrin ligands embedded in the gels, thereby emulating more physiologic conditions. As readout, we analyzed the average speed of cells and visualized individual tracks of DCs.
The migration of mock-infected DCs was not significantly altered by addition of the integrin ligands fibronectin and ICAM-1 to the collagen gels (Figure 6B; supplemental Figure 2). In sharp contrast, HSV-1–infected DCs showed lower average speed compared with mock-infected DCs in all conditions (Figure 6B; supplemental Figure 2; supplemental Videos 1-2). However, chemotaxis of infected DCs was significantly more reduced in the presence of immobilized integrin ligands, indicating that enhanced adhesion to integrin ligands reciprocally correlates with leukocyte migration.
Silencing of CYTIP increases DC adhesion and impairs their migration
To investigate the direct effect of CYTIP down-regulation on migration, we silenced CYTIP in DCs by RNAi and again performed 3D migration assays. CYTIP knockdown did not interfere with normal DC maturation but led to a drastic reduction of CYTIP expression (supplemental Figure 3) and enhanced the adhesion of DCs to fibronectin (supplemental Figure 4), confirming data by Hofer et al.16 As shown in Figure 6C, supplemental Figure 5, and supplemental Video 1, RNAi of CYTIP had already some effect on the chemotaxis in pure collagen compared with DCs electroporated with a nontargeting negative control siRNA. However, a strong reduction of migration was observed in fibronectin-coated collagen gels (Figure 6C; supplemental Figure 5; supplemental Video 2), demonstrating that enhanced binding to integrin ligands impairs DC migration. HSV-1 infection of CYTIP-silenced DCs led to further reduction of migration in both conditions (Figure 6C). Moreover, the inhibition of migration in infected CYTIP-silenced DCs was markedly stronger in fibronectin-coated gels compared with pure collagen, again showing that increased anchoring to integrin ligands retards DC locomotion.
LFA-1, not Mac-1, controls the 3D migration of HSV-1–infected DCs
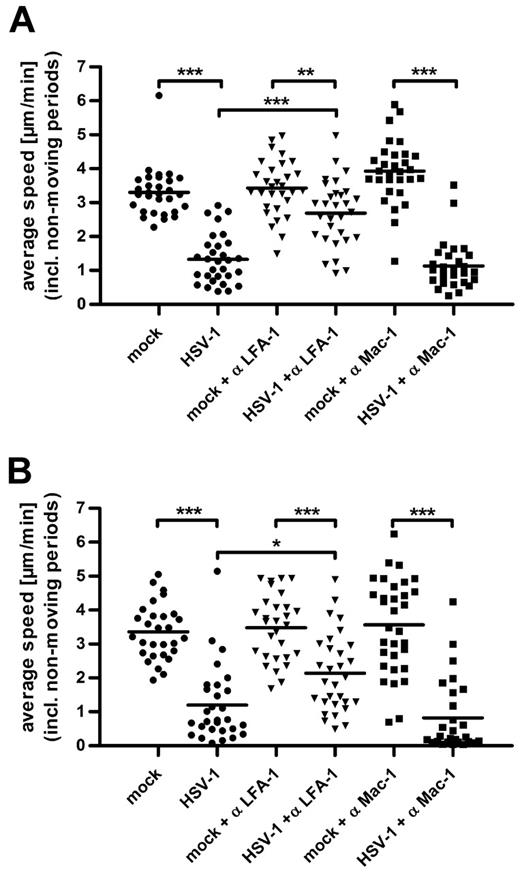
To assess the impact of LFA-1 and Mac-1 on the reduction of chemotaxis in HSV-1–infected DCs, we used the respective blocking antibodies in fibronectin-coated (Figure 7A) and ICAM-1–containing (Figure 7B) collagen gels. In both conditions, the migration of mock-infected DCs was not influenced by blocking LFA-1 or Mac-1 (Figure 7A-B; supplemental Figure 6A-B). In sharp contrast, blocking LFA-1 strongly enhanced the migration of HSV-1–infected DCs both in gels containing fibronectin (Figure 7A; supplemental Figure 6A) and ICAM-1 (Figure 7B; supplemental Figure 6B). The addition of Mac-1 blocking antibodies had no significant effect on the average speed of infected DCs, again indicating that Mac-1 plays a secondary role in HSV-1–induced adhesion to fibronectin (Figure 7A) and ICAM-1 (Figure 7B). Thus, in 3D migration of HSV-1–infected DCs, activated LFA-1 is the main player responsible for the reduced chemotaxis.
LFA-1 activation in HSV-1–infected DCs strongly reduces their chemotaxis. The specific influence of LFA-1 and Mac-1 on the adhesion of HSV-1–infected DCs was determined by adding the anti-LFA-1 and anti-Mac-1 blocking antibodies NKI-L15 and Bear-1, respectively (both 20 μg/mL) in collagen matrices coated with fibronectin (A) or containing immobilized ICAM-1 Fc (B). In both conditions, the migration of mock-infected DCs was not significantly influenced either by LFA-1 or Mac-1 blocking antibodies. In contrast, the inhibition of LFA-1 significantly increased migration of infected DCs, whereas blocking of Mac-1 had no effect. Chemotaxis toward a CCL19 gradient was monitored by bright-field time-lapse videomicroscopy beginning 90 minutes after infection at a frame rate of 2 minutes. The average speed from 30 cells for each condition over 180 frames was calculated as the step length per minute, including nonmoving periods and displayed in micrometers per minute. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. One representative experiment of 3 is shown.
LFA-1 activation in HSV-1–infected DCs strongly reduces their chemotaxis. The specific influence of LFA-1 and Mac-1 on the adhesion of HSV-1–infected DCs was determined by adding the anti-LFA-1 and anti-Mac-1 blocking antibodies NKI-L15 and Bear-1, respectively (both 20 μg/mL) in collagen matrices coated with fibronectin (A) or containing immobilized ICAM-1 Fc (B). In both conditions, the migration of mock-infected DCs was not significantly influenced either by LFA-1 or Mac-1 blocking antibodies. In contrast, the inhibition of LFA-1 significantly increased migration of infected DCs, whereas blocking of Mac-1 had no effect. Chemotaxis toward a CCL19 gradient was monitored by bright-field time-lapse videomicroscopy beginning 90 minutes after infection at a frame rate of 2 minutes. The average speed from 30 cells for each condition over 180 frames was calculated as the step length per minute, including nonmoving periods and displayed in micrometers per minute. Error bars represent ± SD. ***P < .001. **P < .01. *P < .05. One representative experiment of 3 is shown.
HSV-1–infected DCs show defective T-cell detachment
Hofer et al16 described CYTIP not only to be an important modulator of DC adhesion to fibronectin, but also as a regulator of T-cell detachment. CYTIP silencing in mature DCs resulted in prolonged adherence of T cells to DCs and thus impaired their T-cell priming capacity. Therefore, we examined the interaction of T cells with mock-infected and HSV-1–infected DCs. CFSE stained bulk T cells were allowed to bind to immobilized DCs. After washing, bound T cells were quantified by spectrophotometry. As shown in supplemental Figure 7, HSV-1 infection of DCs significantly enhanced the binding of T cells. Hence, HSV-1 interferes with the detachment of T cells from infected DCs.
Taken together, our data demonstrate that HSV-1 induces rapid degradation of CYTIP and constant activation of LFA-1 in infected DCs. The resulting LFA-1–mediated adhesion strongly impairs migration and the detachment of T cells, thereby influencing 2 hallmarks of DC biology.
Discussion
In the present study, we describe a new mechanism of HSV-1 to evade adaptive immune responses by manipulation of pivotal DC functions. HSV-1 hijacks the LFA-1 activation pathway in DCs and converts it to be constitutively active, thereby conferring increased adhesiveness for extracellular matrix proteins to DCs. Cells migrating in an amoeboid manner, such as DCs,3 normally form only short-lived contacts to the substrate, thereby allowing rapid locomotion. By enhancing the adherence of DCs to integrin ligands, HSV-1 thus impairs their chemotaxis. Furthermore, we show that this aberrant adhesion also inhibits T-cell detachment. The mechanism responsible for the modulation of LFA-1 affinity has also been investigated: during the course of infection CYTIP is rapidly degraded by the proteasome, resulting in high affinity LFA-1.
DCs play the major role in the establishment of epidermal viral immunity against HSV-1,33 and initial delay in the generation of immune responses is mediated by interference with normal DC functions.34 The only way to prevent the virus from entering a latent state is a rapid response of the DC network to activate CD4+ and CD8+ T cells as well as B cells,34 leading to elimination of infected cells35 and inactivation of virus particles by neutralizing antibodies before neurons are affected. Hence, the migration of antigen-presenting DCs from the sites of infection to secondary lymphoid tissues is imperative for the induction of antiviral immune responses,36 and inhibition of DC migration and thereby T-cell priming is a powerful way for herpes viruses to evade or at least to delay immune responses until latency is established. Eidsmo et al showed that HSV-infected DCs indeed are compromised in their trafficking capabilities and are largely absent from the migrating population in vivo.37
Recent publications demonstrate that adhesion is not a prerequisite for leukocyte migration in confined environments, such as the interstitium.1-3 Nevertheless, because strong integrin-mediated anchoring of leukocytes overrides promigratory signals and leads to adhesive arrest,6 the interaction with the environment can be a determinant for their locomotion. Therefore, it is apparent that the affinity status of integrins has to be tightly controlled to ensure appropriate functioning of the immune system.
The positioning of monocyte-derived cells is mainly controlled by members of the β2-integrin family, and DCs are much more restricted in β2-integrin activation than other leukocytes.13 Previous studies implicated cytohesin-110-12 and CYTIP14 in the regulation of β2-integrin affinity and integrin-mediated leukocyte adherence. CYTIP acts as a negative regulator of LFA-1 activation by interrupting the interaction with its activator cytohesin-1. Hence, the loss of CYTIP results in increased LFA-1 affinity. For DCs, it could be shown that CYTIP controls their adhesion to fibronectin and T-cell detachment.16 Here we demonstrate that, in HSV-1–infected DCs, cytohesin-1 expression is only slightly altered, whereas CYTIP expression is rapidly down-regulated and concomitantly β2-integrins are activated. The kinetics of adherence to fibronectin also reflects this finding, indicating that increased adhesion is linked to elevated β2-integrin activation. Two hours after infection, without β2-integrin activation, infected DCs show only basal adhesion. During the course of infection, CYTIP is reduced and the adhesion is drastically increased. Silencing of CYTIP by RNAi revealed that indeed the loss of CYTIP alone results already in augmented adhesion. The inhibition of LFA-1 and Mac-1 demonstrated the dominant role of LFA-1 for the fibronectin adhesion of HSV-1–infected DCs. This is a novel finding because it is generally acknowledged that Mac-1, which shows great binding promiscuity, is mainly responsible for the β2-mediated adherence to fibronectin.32
Consistent with the data obtained from the adhesion assays, HSV-1–infected mature DCs showed a strong inhibition of migration, which is increased by immobilized integrin ligands and with further CYTIP reduction during the course of infection. The importance of CYTIP for the regulation of DC motility is reflected by the fact that CYTIP expression is drastically up-regulated during DC maturation (ie, the transition from their sessile to their motile state). Notably, LFA-1 normally is inactive on mature DCs.38 Using mutant mice with constitutively active LFA-1, Semmrich et al demonstrated that indeed strongly increased LFA-1–mediated adhesion reciprocally correlates with mobility.39 Accordingly, treatment of infected DCs with LFA-1 blocking antibodies increased their migration, whereas blocking of Mac-1 had no effect. Thus, LFA-1–mediated adhesion impairs migration and, at least at early time points, Mac-1 is not the determinative receptor for the integrin-mediated inhibition of chemotaxis.
In the presence of integrin ligands, silencing of CYTIP resulted in a significant reduction of migration, demonstrating that CYTIP controls DC migration via regulation of integrin-mediated adhesion. HSV-1 infection of CYTIP-knockdown DCs led to further inhibition of chemotaxis, indicating that the virus might also target additional pathways important for DC migration. Indeed, DCs infected with HSV-1 show increased activation of PI3K (data not shown), which is implicated in cytohesin-1–mediated, LFA-1–dependent adherence of monocytes40 and was shown to activate β2-mediated integrin adhesion by inducing membrane recruitment of cytohesin-1.41,42 At later time points, the up-regulation of Mac-1 activity as well as additional viral mechanisms, such as the down-regulation of CCR7 from the surface,22 might also target DC chemotaxis, thereby resulting in further reduction of migration. A direct influence of herpes viruses on the LFA-1–activating molecule cytohesin-1 has already been described. The human herpesvirus 8 protein kaposin A recruits cytohesin-1 to the plasma membrane, thereby activating CD1843 and inducing strong adhesion. In HSV-1 no kaposin A homolog exists, but influencing cell adhesion seems to be a common herpes virus strategy.
The exact viral mechanisms of CYTIP down-regulation remain to be elucidated. Because of the rapid kinetic of CYTIP down-regulation, it is verisimilar that viral immediate-early gene products or components of the viral tegument, which are directly delivered into the cytoplasm of the host cell during infection, are involved. Because DC migration to lymph nodes is fast (3-24 hours), this early inhibition is mandatory for an efficient immune escape. The fact that UV-inactivated virus had no effect on DC adhesion, CYTIP-down-regulation and the affinity status of β2-integrins indicates that viral gene expression is necessary. Infection with a mutant viruses lacking the viral RNase vhs, which induces rapid shutoff of host protein synthesis and is described to play a role in HSV immune evasion,27 or ICP0, a HSV-1 immediate-early proteins with ubiquitin E3 ligase activity,28 revealed that neither vhs nor ICP0 are involved in CYTIP down-regulation. However, inhibition of the proteasome completely prevented the loss of CYTIP, demonstrating that it is degraded via the proteasomal pathway. HSV-1 infection of DCs leads to CYTIP double bands, which are enriched by MG-132 treatment, indicating that the additional bands are rapidly degraded by the proteasome. Many proteins are phosphorylated before ubiquitination44 ; therefore, it could be speculated that the bands with higher molecular weight represent phosphorylated and/or ubiquitinated CYTIP.
HSV-1 not only represses migration, thereby indirectly the priming of antiviral T cells, but also directly inhibits this process. T cells scan antigen-major histocompatibility complex complexes on DCs by establishing short-lived and highly dynamic cell-cell contacts provided by cell adhesion molecules, such as LFA-1 and ICAM-1.45 If specific antigen recognition takes place, the contact is prolonged by strengthening of DC-T-cell adhesion, leading to formation of the immunologic synapse and ultimately the activation of T cells.46 In either case, T cells need to detach from DCs to scan further DCs or to clonally expand, respectively. It could be shown that the inhibition of detachment after silencing of CYTIP impairs the expansion of antigen-specific T cells16 and that activated LFA-1 inhibits full T-cell activation.17,39 In concordance with those findings, here we demonstrate that indeed the detachment of T cells from HSV-1–infected DCs is reduced. This is consentient with previous studies from our group, showing that the T-cell-stimulatory capacity of HSV-1–infected DCs is reduced.47
In conclusion, the data presented here provide evidence that HSV-1 influences central aspects of DC biology. By manipulating their interaction with components of the interstitial space as well as T cells, HSV-1 targets 2 of the most critical aspects in the induction of adaptive immune responses (ie, the migration of DCs to secondary lymphoid tissues and the efficient priming and activation of T cells). Understanding this new viral immune-evasion mechanism might help to develop new antiviral therapies in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Elisabeth Kremmer (Helmholtz Center Munich, Munich, Germany) for supply with anti–cytohesin-1 and anti-CYTIP antibodies, Christine Heufler (Department of Dermatology, Medical University of Innsbruck, Innsbruck, Austria) for discussion and advice, Professor Peter Friedl for providing the equipment to perform the 3D migration assays, and Bettina Weigelin for help with the experimental design.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 796, project B2; A.S.), the European Union (grant Immunanomap MRTNCT-2006-035946; C.G.F.), and joint European Union-Mexico project FONCICYT (Fondo de Cooperación Internacional para el tomento de la investigación cientifica y technológica, c0002-2008-1-ALA/2006/18149; C.G.F.). A.A.T. was supported by the Deutsche Forschungsgemeinschaft (stipend, research training grant GK592).
Authorship
Contribution: A.A.T. and C.E. carried out the experiments; A.A.T. wrote the paper; C.E. and C.G.F. supplied antibodies and soluble ICAM-1-Fc and contributed to discussion; A.A.T. and A.S. designed the study; and A.S. contributed to discussion and acquired research funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Steinkasserer, Department of Immune Modulation at the Department of Dermatology, University Hospital Erlangen, Hartmannstrasse 14, 91052 Erlangen, Germany; e-mail: alexander.steinkasserer@uk-erlangen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal