Abstract
Immune responses lead to expression of immunoregulatory molecules on T cells, including natural killer (NK) receptors, such as CD94/NKG2A on CD8+ T cells; these receptors restrain CD8+ responses, thereby preventing T-cell exhaustion in chronic infections and limiting immunopathology. Here, we examined the requirements for inducing CD94/NKG2A on T cells responding to antigen. In vitro, moderate induction of CD94/NKG2A expression occurred after exposure of naive CD8+ (but not CD4+) cells to CD3 ligation or specific peptide. Surprisingly, expression was inhibited by CD28/B7 costimulation. Such inhibition applied only to CD94/NKG2A and not other NK receptors (NKG2D) and was mediated by IL-2. Inhibition by IL-2 occurred via a NFAT cell-independent component of the calcineurin pathway, and CD94/NKG2A induction was markedly enhanced in the presence of calcineurin blockers, such as FK506 or using calcineurin-deficient T cells, both in vitro and in vivo. In addition to CD28-dependent inhibition by IL-2, CD94/NKG2A expression was impaired by several other cytokines (IL-4, IL-23, and transforming growth factor-β) but enhanced by others (IL-6, IL-10, and IL-21). The complex interplay between these various stimuli may account for the variable expression of CD94/NKG2A during responses to different pathogens in vivo.
Introduction
CD94 is a member of the lectin-like type II transmembrane protein family and forms heterodimers with NKG2A, NKG2B (a splice variant of the NKG2A gene), NKG2C, or NKG2E.1 These 4 NKG2 proteins have high (90%) homology in their amino acid sequence of the extracellular carbohydrate domains, whereas NKG2D is a functionally distinct type of receptor that does not pair with CD94.2 For heterodimers between CD94 and NKG2A, B, C, or E, CD94/NKG2A is the predominant receptor on lymphoid cells and has inhibitory function, whereas CD94/NKG2C and CD94/NKG2E are stimulatory receptors.3 All of the CD94/NKG2 receptors recognize a peptide derived from the cleaved signal sequences of a subset of classic major histocompatibility complex class Ia molecules that are bound to nonclassic class Ib molecules, HLA-E in humans and Qa-1 in mice.4,5
CD94/NKG2A receptors are expressed mainly on natural killer (NK) cells and NK1.1+ T cells and are also found on a subset of CD8+ T cells with an activated/memory (CD44hi) phenotype.6,7 In addition, naive CD8+ T cells can express CD94/NKG2A receptors after TCR engagement8-11 ; these receptors in mice are generally not expressed on normal CD4+ T cells.11,12 In both NK cells and NK1.1+ T cells, CD94/NKG2A expression can be modulated by certain cytokines; thus, incubation with IL-21 led to CD94/NKG2A up-regulation on these cells, whereas IL-4 caused receptor down-regulation.13,14
For CD8+ T cells, CD94/NKG2A expression is induced on the majority of antigen-specific naive cells after stimulation with certain viral (lymphocytic choriomeningitis virus [LCMV]) or bacterial (Listeria monocytogenes) pathogens.8 Nevertheless, CD94/NKG2A up-regulation is not a general consequence of TCR ligation because exposure to certain pathogens, notably polyoma virus and influenza virus, causes only slow onset of CD94/NKG2A expression despite extensive T-cell proliferation.9,11 Hence, synthesis of this receptor may involve complex control mechanisms.
The functional roles of CD94/NKG2A receptors are still unclear. For instance, CD94/NKG2A on CD8+ T cells after LCMV infection was not inhibitory, whereas these same receptors induced by polyoma virus or influenza virus were clearly inhibitory, as measured by cytotoxic activity or cytokine production of antigen-specific CD8+ T cells.9,11,15 Although the reason for this discrepancy is unknown, it is notable that high CD94/NKG2A expression on CD8+ T cells is associated with severe pathogenesis during influenza infection in mice and disease progression for HIV infection in humans11,16,17 ; high receptor expression is also seen on CD8+ T cells responding to various cancers, both in humans and in mice.9,18,19 These studies defy simple interpretation.
To shed light on this issue, we have sought detailed information on the factors controlling the induction of CD94/NKG2A expression on naive CD8+ T cells responding to antigen. We report here that TCR-induced CD94/NKG2A expression is highly complex and subject to strong negative regulation by CD28 costimulation and the calcineurin pathway.
Methods
Mice
C57BL/6 (B6), mice strain and B10.D2 mice were purchased from the Animal Resources Center. Sources of OT-II, OT-I, 2C, 2C.IL2−/−, and 2C.IL2Rβ−/− TCR transgenic mice were described previously.20,21 STAT6−/− mice22 and CnAβ−/− mice23 were provided by M. B. Oldstone and J. Kaye and crossed with 2C or OT-I mice to generate 2C.CnAβ−/− and OT-I.CnAβ−/− mice, respectively. All animal experiments were performed according to protocols approved by the Animal Experimental and Ethic Committee at the Garvan Institute.
Reagents, antibodies, and flow cytometry
All reagents and antibodies used for fluorescence-activated cell sorter analysis were described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
T-cell preparation and in vitro stimulation
FACS-sorted purified naive CD4+ and CD8+ T cells were subjected to the various indicated polyclonal or antigenic stimulation as described in supplemental Methods.
Adoptive transfer and in vivo stimulation
Purified naive 2C or OT-I CD8+ T cells indicated were adoptively transferred into normal B6 mice and immunized with the various indicated conditions as described in supplemental Methods.
Statistical analysis
Unpaired 2-tailed Student t test was used to determine statistically significant differences. P values of < .05 were considered statistically significant.
Results
TCR ligation induces CD94/NKG2A expression
In previous studies, up-regulation of CD94/NKG2A on naive CD8+ T cells after stimulation with specific antigen in vitro was found to be quite weak (10%-20% positive cells) in primary cultures and only rose to high levels after repeated stimulation.8
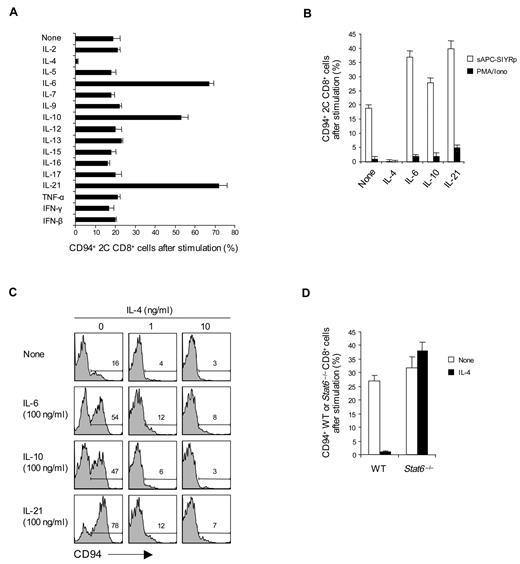
To examine the requirements for inducing CD94 and NKG2A expression on T cells in primary culture, purified naive (CD44lo) subsets of CD8+ T cells prepared from C57BL/6 (B6) or 2C TCR transgenic (Tg) mice24 were cultured in vitro with cross-linked anti-CD3 monoclonal antibody (without costimulation). FACS analysis showed that subjecting either B6 or 2C CD8+ T cells to CD3 ligation for 2 to 3 days induced strong expression of CD94 and NKG2A, as well as NKG2D and CD44 (Figure 1A,D); except for CD44, these markers were not up-regulated by phorbol myristate acetate (PMA) and ionomycin. For NKG2A, strong staining occurred with a monoclonal antibody specific for NKG2A as well as with a monoclonal antibody that cross-reacts with NKG2A, NKG2C, and NKG2E (NKG2ACE) but not NKG2D. The level of staining for CD94, NKG2A, and NKG2ACE was almost indistinguishable, indicating that expression was restricted to CD94/NKG2A heterodimers. For simplicity, we henceforth refer to staining for “CD94/NKG2A”; the particular monoclonal antibodies used for staining are shown in the figures.
Expression of NK receptors in naive CD8+, but not CD4+, T cells after TCR stimulation. (A-D) A total of 0.2 to 1 × 105 unlabeled (A-B,D) or CFSE-labeled (C) purified naive CD8+ (A-D) or CD4+ (D) T cells purified from 2C TCR Tg (A-C) and B6 (D) mice were stimulated with either plate-bound anti-CD3 monoclonal antibody (1-10 μg/mL; A-D) or PMA (50 ng/mL) plus ionomycin (500 ng/mL) (PMA/Iono; A,D) and analyzed on day 3 (A-B), days 1 to 3 (C), and day 2 (D) for the indicated activation markers (CD44 and CD25; A,C) and NK-cell receptors (CD94, NKG2A, NKG2ACE, and NKG2D; A-D) by flow cytometry. The percentages of cells in the respective regions (A,C) are indicated. (A-D) Data are representative of at least 3 independent experiments (B,D; mean ± SD).
Expression of NK receptors in naive CD8+, but not CD4+, T cells after TCR stimulation. (A-D) A total of 0.2 to 1 × 105 unlabeled (A-B,D) or CFSE-labeled (C) purified naive CD8+ (A-D) or CD4+ (D) T cells purified from 2C TCR Tg (A-C) and B6 (D) mice were stimulated with either plate-bound anti-CD3 monoclonal antibody (1-10 μg/mL; A-D) or PMA (50 ng/mL) plus ionomycin (500 ng/mL) (PMA/Iono; A,D) and analyzed on day 3 (A-B), days 1 to 3 (C), and day 2 (D) for the indicated activation markers (CD44 and CD25; A,C) and NK-cell receptors (CD94, NKG2A, NKG2ACE, and NKG2D; A-D) by flow cytometry. The percentages of cells in the respective regions (A,C) are indicated. (A-D) Data are representative of at least 3 independent experiments (B,D; mean ± SD).
Induction of CD94/NKG2A expression on CD8+ T cells correlated with the concentration of anti-CD3 monoclonal antibody used and reached a maximum of 50% to 60% staining of cells with 5 μg/mL of anti-CD3 monoclonal antibody (Figure 1B). Expression of these markers occurred slowly and reached a peak on day 3 of culture (Figure 1C); by contrast, up-regulation of other markers, such as CD25 and CD44, was apparent by day 1.
These findings applied to naive CD8+ T cells. For CD4+ T cells, by contrast, CD3 ligation failed to cause expression of CD94 or NKG2A (Figure 1D); there was also no up-regulation of NKG2D on CD4+ T cells (data not shown).
Influence of cytokines
To examine the effects of cytokines on CD94/NKG2A expression, naive CD8+ T cells were cultured with an intermediate dose of anti-CD3 monoclonal antibody (2.5 μg/mL) plus 1 of 18 different cytokines. Three of these cytokines, namely, IL-6, IL-10, and IL-21, caused a marked increase in the expression of CD94/NKG2A (Figure 2A). By contrast, IL-4, IL-23, and TGF-β had the opposite effect and abolished or markedly reduced CD94/NKG2A expression (Figure 2A; supplemental Figure 1A). A similar enhancing effect by IL-6, IL-10, and IL-21 and blocking by IL-4 applied when 2C CD8+ T cells were cultured with specific H-2Kb-restricted SIYR peptide presented by B6 (H-2b) spleen cells (sAPC-SIYRp) (Figure 2B); after CD3 ligation, these cytokines also had a comparable, although less prominent, effect on expression of NKG2D expression (supplemental Figure 1B). It should be noted that the capacity of cytokines to modulate CD94/NKG2A expression also applied at the level of mRNA synthesis (supplemental Figure 1E). None of the cytokines induced CD94/NKG2A expression without CD3/TCR ligation (data not shown). With CD3/TCR ligation, there was no induction of Ly49/C/I/F/G or KLRG1 (supplemental Figure 1F).
Effect of cytokines on CD94/NKG2A expression. (A-C) A total of 0.5 to 1 × 105 naive CD8+ T cells purified from 2C TCR Tg mice (A-C) or from WT (Balb/c) and STAT6−/− mice (D) were stimulated with either plate-bound anti-CD3 monoclonal antibody (A, 2.5 μg/mL; C-D, 5 μg/mL), PMA plus ionomycin (PMA/Iono; B), or 5 × 105 irradiated T-depleted B6 (H-2b) spleen cells pulsed with 10μM SIYR peptide (sAPC-SIYRp; B) in the presence or absence of the indicated cytokines at various concentrations (A-B,D, all 50 ng/mL except IFN-β used at 2 × 102 units/mL; C, 0-100 ng/mL). On day 2 after culture, cells were analyzed for CD94 expression by flow cytometry. The percentages of cells in the respective regions (C) are indicated. Data (A-D) are representative of 3 or 4 independent experiments (A-B,D; mean ± SD).
Effect of cytokines on CD94/NKG2A expression. (A-C) A total of 0.5 to 1 × 105 naive CD8+ T cells purified from 2C TCR Tg mice (A-C) or from WT (Balb/c) and STAT6−/− mice (D) were stimulated with either plate-bound anti-CD3 monoclonal antibody (A, 2.5 μg/mL; C-D, 5 μg/mL), PMA plus ionomycin (PMA/Iono; B), or 5 × 105 irradiated T-depleted B6 (H-2b) spleen cells pulsed with 10μM SIYR peptide (sAPC-SIYRp; B) in the presence or absence of the indicated cytokines at various concentrations (A-B,D, all 50 ng/mL except IFN-β used at 2 × 102 units/mL; C, 0-100 ng/mL). On day 2 after culture, cells were analyzed for CD94 expression by flow cytometry. The percentages of cells in the respective regions (C) are indicated. Data (A-D) are representative of 3 or 4 independent experiments (A-B,D; mean ± SD).
The inhibitory influence of IL-4 was conspicuous. Thus, with CD3 ligation, low concentrations of IL-4 abolished CD94/NKG2A expression, even with addition of high concentrations of IL-6, IL-10, or IL-21 (Figure 2C); Gata-3 was not involved because the inhibition induced by IL-4 plus IL-12 did not cause Gata-3 up-regulation (supplemental Figure 1C-D). IL-4 failed to impair CD94/NKG2A expression by Stat6−/− CD8+ T cells, indicating that inhibition was Stat6 dependent (Figure 2D).
Inhibition by costimulation
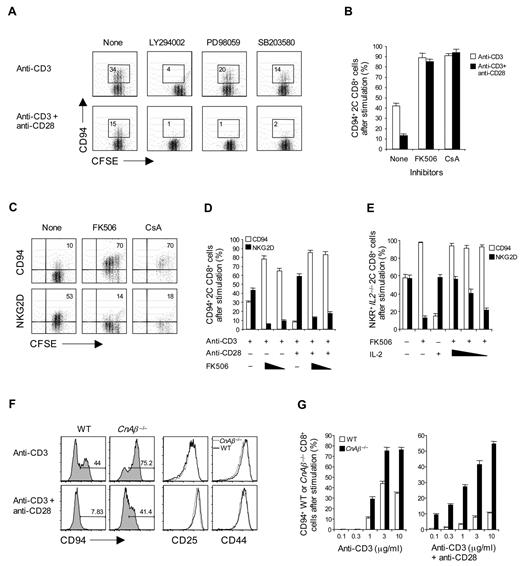
As expected, costimulation with anti-CD28 monoclonal antibody plus anti-CD3 monoclonal antibody augmented the expression of NKG2D (Figure 3A). Surprisingly, this was not the case for CD94/NKG2A expression. Thus, up-regulation of CD94/NKG2A expression on 2C CD8+ T cells was considerably lower when cells were subjected to combined CD3/CD28 ligation than to CD3 ligation alone (Figure 3A-B). This inhibitory effect of CD28 costimulation was most pronounced with low doses of anti-CD3 monoclonal antibody and high doses of anti-CD28 monoclonal antibody (Figure 3B-C). The negative effect of costimulation also applied when 2C CD8+ T cells were cultured with 2 closely related specific peptides, p2Ca and QL9, presented by H-2Ld (Ld) molecules (note that QL9 is a stronger peptide than p2Ca; Figure 3D-F). For these peptides, we first used Drosophila cell lines expressing combinations of Ld, B7, and/or intercellular adhesion molecule-1 (ICAM-1) molecules.25 With these cells, expression of B7 by the Drosophila antigen-presenting cell (APC) was clearly inhibitory. Thus, when naive 2C CD8+ T cells were cultured with QL9 peptide presented by Drosophila cells expressing Ld plus ICAM-1 (flyLd.ICAM; ie, B7-negative APC), the 2C CD8+ T cells divided rapidly as indicated by CFSE dilution and showed prominent up-regulation of CD94/NKG2A (Figure 3D). In marked contrast, despite intense proliferation, 2C CD8+ T cells showed only minimal expression of CD94/NKG2A when the cells were cultured with peptide presented by either flyLd.B7 or flyLd.B7.ICAM APC. With these B7+ APCs, the failure to induce CD94/NKG2A expression was overcome by the addition of anti–B7-1 monoclonal antibody but not by anti-ICAM-1 monoclonal antibody (Figure 3E).
Influence of CD28 costimulation on CD94/NKG2A expression. (A-F) A total of 0.5 to 1 × 105 unlabeled (A-C,E-F) or CFSE-labeled (D) purified naive 2C CD8+ T cells were stimulated with either (A-C) plate-bound anti-CD3 monoclonal antibody (A,C; 2.5 μg/mL; B, 1-10 μg/mL) with or without anti-CD28 monoclonal antibody (A-B, 10 μg/mL; C, 0-10 μg/mL). (D-E) A total of 1 to 2 × 105 transfected fly cells as APCs (flyLd.ICAM, flyLd.B7 and flyLd.B7.ICAM) pulsed with 10μM QL9 peptide (E; with or without 10 μg/mL anti–ICAM-1 or anti-B7–1 monoclonal antibody), or (F) 5 × 105 irradiated T-depleted B10.D2 (H-2d) spleen cells (B10.D2 sAPCs) pulsed with 10μM p2Ca or QL9 peptide with or without 10 μg/mL CTLA4Ig or anti–IL-2 monoclonal antibody; note that fly APCs die within day 1 when cultured at 37 and were therefore added either once (at day 0; E) or daily for 3 days (at days 0-2; D). On day 3 after culture, cells were analyzed for the indicated NK receptors by flow cytometry. The percentages of cells in the respective regions (A,D) are indicated. (A-F) Data are representative of at least 4 independent experiments (B-C,E-F; mean ± SD). *P < .05. **P < .005.
Influence of CD28 costimulation on CD94/NKG2A expression. (A-F) A total of 0.5 to 1 × 105 unlabeled (A-C,E-F) or CFSE-labeled (D) purified naive 2C CD8+ T cells were stimulated with either (A-C) plate-bound anti-CD3 monoclonal antibody (A,C; 2.5 μg/mL; B, 1-10 μg/mL) with or without anti-CD28 monoclonal antibody (A-B, 10 μg/mL; C, 0-10 μg/mL). (D-E) A total of 1 to 2 × 105 transfected fly cells as APCs (flyLd.ICAM, flyLd.B7 and flyLd.B7.ICAM) pulsed with 10μM QL9 peptide (E; with or without 10 μg/mL anti–ICAM-1 or anti-B7–1 monoclonal antibody), or (F) 5 × 105 irradiated T-depleted B10.D2 (H-2d) spleen cells (B10.D2 sAPCs) pulsed with 10μM p2Ca or QL9 peptide with or without 10 μg/mL CTLA4Ig or anti–IL-2 monoclonal antibody; note that fly APCs die within day 1 when cultured at 37 and were therefore added either once (at day 0; E) or daily for 3 days (at days 0-2; D). On day 3 after culture, cells were analyzed for the indicated NK receptors by flow cytometry. The percentages of cells in the respective regions (A,D) are indicated. (A-F) Data are representative of at least 4 independent experiments (B-C,E-F; mean ± SD). *P < .05. **P < .005.
These findings clearly implicated B7 as having a negative effect on CD94/NKG2A expression, presumably via B7 interaction with CD28. Further support for this conclusion came from findings with Ld+ B10.D2 spleen cells as APC for p2Ca and QL9 peptides. Here, up-regulation of CD94/NKG2A expression on 2C CD8+ T cells was considerably increased by addition of CTLA4Ig to block CD28/B7 interaction (Figure 3F).
CD28 costimulation is known to augment production of various cytokines, especially IL-2.26 Therefore, in considering various possibilities to explain the negative influence of CD28 costimulation on CD94/NKG2A expression, we tested the effects of blocking the activity of IL-2. Here, for 2C CD8+ T cells responding to QL9 and p2Ca presented by B10.D2 spleen APC, the striking finding was that addition of anti–IL-2 monoclonal antibody to the cultures was even more effective than CTLA4Ig at increasing CD94/NKG2A expression (Figure 3F). Hence, the negative influence of CD28 costimulation seemed to be IL-2 dependent.
Inhibition by IL-2
At face value, an inhibitory role for IL-2 on CD94/NKG2A expression seemed unlikely because, as mentioned earlier, adding IL-2 had no effect (either positive or negative) on up-regulation of CD94/NKG2A induced by CD3 ligation alone (Figure 2A). Likewise, up-regulation of CD94/NKG2A on 2C CD8+ T cells after CD3 ligation alone was the same for IL2−/− and wild-type (WT) 2C CD8+ T cells and was not altered by the addition of either exogenous IL-2 or anti–IL-2 monoclonal antibody during culture (Figure 4A-B).
Effect of IL-2 on the inhibition of CD94/NKG2A expression by CD28 costimulation. (A-D) A total of 0.5 to 1 × 105 naive IL2+/+ and IL2−/− 2C CD8+ T cells purified from WT and IL2−/− 2C TCR Tg mice were stimulated either with (A-B) plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) with or without anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of 50 ng/mL IL-2 or 10 μg/mL anti–IL-2 monoclonal antibody, (C) 1 × 105 flyLd.B7.ICAM (added once at day 0) or 2 × 105 irradiated T-depleted B10.D2 sAPCs pulsed with 10μM QL9 peptide with or without 50 ng/mL IL-2, or (D) 1 × 105 flyLd.B7.ICAM (added daily at days 0-2) pulsed with 10μM p2Ca or QL9 peptide. (E-H) A total of 0.5 × 105 naive IL2−/− 2C CD8+ T cells were stimulated either with (E-G) plate-bound anti-CD3 monoclonal antibody (E-F, 2.5 μg/mL; G, 2 and 20 μg/mL) plus 10 μg/mL anti-CD28 monoclonal antibody or (H) 1 × 105 flyLd.B7.ICAM (added once at day 0) pulsed with the indicated doses of QL9 peptide (10−4 to 10μM). (E-H) Cultures were supplemented with or without either (E) the indicated amounts of IL-2 (0-100 ng/mL), (F) the various common γ-chain (γc) family cytokines (all 50 ng/mL), or (G-H) the indicated concentrations of IL-2 (G, 0-50 ng/mL; H, 0-100 ng/mL). On day 3 after culture, cells were analyzed for CD94 expression by flow cytometry. The percentages of cells in the respective regions (A,D,G) are indicated. (A-H) Data are representative of 3 or 4 independent experiments (B-C,E-F,H; mean ± SD); the inhibition of CD94 expression induced by IL-2 (H) was calculated by the formula: % inhibition = 100 − (100 × % CD94-positive cells treated with IL-2/% CD94-positive cells untreated).
Effect of IL-2 on the inhibition of CD94/NKG2A expression by CD28 costimulation. (A-D) A total of 0.5 to 1 × 105 naive IL2+/+ and IL2−/− 2C CD8+ T cells purified from WT and IL2−/− 2C TCR Tg mice were stimulated either with (A-B) plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) with or without anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of 50 ng/mL IL-2 or 10 μg/mL anti–IL-2 monoclonal antibody, (C) 1 × 105 flyLd.B7.ICAM (added once at day 0) or 2 × 105 irradiated T-depleted B10.D2 sAPCs pulsed with 10μM QL9 peptide with or without 50 ng/mL IL-2, or (D) 1 × 105 flyLd.B7.ICAM (added daily at days 0-2) pulsed with 10μM p2Ca or QL9 peptide. (E-H) A total of 0.5 × 105 naive IL2−/− 2C CD8+ T cells were stimulated either with (E-G) plate-bound anti-CD3 monoclonal antibody (E-F, 2.5 μg/mL; G, 2 and 20 μg/mL) plus 10 μg/mL anti-CD28 monoclonal antibody or (H) 1 × 105 flyLd.B7.ICAM (added once at day 0) pulsed with the indicated doses of QL9 peptide (10−4 to 10μM). (E-H) Cultures were supplemented with or without either (E) the indicated amounts of IL-2 (0-100 ng/mL), (F) the various common γ-chain (γc) family cytokines (all 50 ng/mL), or (G-H) the indicated concentrations of IL-2 (G, 0-50 ng/mL; H, 0-100 ng/mL). On day 3 after culture, cells were analyzed for CD94 expression by flow cytometry. The percentages of cells in the respective regions (A,D,G) are indicated. (A-H) Data are representative of 3 or 4 independent experiments (B-C,E-F,H; mean ± SD); the inhibition of CD94 expression induced by IL-2 (H) was calculated by the formula: % inhibition = 100 − (100 × % CD94-positive cells treated with IL-2/% CD94-positive cells untreated).
These findings applied with CD3 ligation alone, and quite different results occurred with combined CD3/CD28 ligation. Here, IL-2 had a marked negative effect on CD94/NKG2A expression. Thus, with IL2−/− 2C CD8+ T cells, up-regulation of CD94/NKG2A expression was clearly higher after combined CD3/CD28 ligation than after CD3 ligation alone (Figure 4A-B), indicative of classic costimulation by CD28 with these IL-2-deficient CD8+ T cells. This was a marked contrast to WT 2C CD8+ T cells where CD94NKG2A expression was substantially lower with CD3/CD28 ligation than with CD3 ligation alone, presumably because of inhibition by the high levels of IL-2 generated after CD3/CD28 ligation. Further support for inhibition by IL-2 came from the finding that the high induction of CD94/NKG2A induced by exposing IL2−/− 2C CD8+ T cells to CD3/CD28 ligation was markedly reduced by adding IL-2 to the cultures (Figure 4B). Conversely, adding anti–IL-2 monoclonal antibody enhanced CD94/NKG2A expression when WT 2C CD8+ T cells were exposed to CD3/CD28 ligation (Figure 4B). Additional evidence for inhibition by IL-2 came from the finding that, whereas CTLA4Ig enhanced CD94/NKG2A induction by WT 2C CD8+ T cells, CTLA4Ig had the opposite effect on IL-2-unresponsive IL-2Rβ−/− 2C CD8+ T cells and caused reduced expression on these cells (supplemental Figure 2A).
These findings indicated that the negative effect of CD28 costimulation on the expression of CD94/NKG2A on CD8+ T cells reflected an inhibitory influence by the high levels of IL-2 in the cultures, with IL-2 synthesis being much higher after combined CD3/CD28 ligation than with CD3 ligation alone. It should be stressed again, however, that IL-2 was only inhibitory with joint CD3/CD28 ligation and not with CD3 ligation alone (Figure 4A-B). Hence, inhibition by IL-2 was critically dependent on CD28 costimulation.
Inhibition of CD94/NKG2A expression by IL-2 was also observed when 2C CD8+ T cells were exposed to specific peptide presented by B7+ APC. Thus, with both Ld.B7.ICAM fly cells and normal B10.D2 spleen cells as APCs for p2Ca and QL9 peptides, IL2−/− 2C CD8+ T cells showed much higher CD94/NKG2A up-regulation than WT 2C CD8+ T cells (Figure 4C-D). For IL2−/− 2C CD8+ T cells, CD94/NKG2A expression was inhibited by adding IL-2 to the cultures. With these cells, inhibition required approximately 10 ng/mL of IL-2 (Figure 4E) and was also observed to a lesser extent with a related cytokine, IL-15, but not with IL-7 or IL-21 (Figure 4F).
It should be noted that the inhibitory effect of IL-2 on CD94/NKG2A expression was most prominent with relatively weak TCR signaling, both for CD3/CD28 ligation (Figure 4G) and stimulation of 2C CD8+ T cells with specific peptide (Figure 4H); in both situations, inhibition by IL-2 was much less evident with strong TCR signaling.
Inhibition by IL-2 applied only to CD94/NKG2A and not NKG2D expression. Indeed, IL-2 enhanced the expression of NKG2D. Thus, with IL2−/− 2C CD8+ T cells, NKG2D expression was low after CD3 ligation alone but high after combined IL-2 plus CD3 ligation (supplemental Figure 2B).
Role of the calcineurin pathway
To seek information on how IL-2 and CD28 prevent the expression of CD94/NKG2A, CD8+ T cells were cultured in the presence of various inhibitors of intracellular signaling. With both CD3 ligation and CD3/CD28 ligation, CD94/NKG2A up-regulation was abolished by addition of LY294002, indicating strong dependence on phosphatidylinositol 3-kinase (Figure 5A). Partial inhibition was seen with PD98059 and SB203580, implicating the mitogen-activated protein kinase pathways. There was also strong inhibition by rapamycin, indicating a requirement for mammalian target of rapamycin (mTOR; data not shown). With these inhibitors, similar inhibition was observed on NKG2D expression (data not shown).
Negative signaling for CD94/NKG2A expression by calcineurin pathway. (A-C) A total of 0.5 to 1 × 105 unlabeled (B) or CFSE-labeled (A,C) purified naive 2C CD8+ T cells were stimulated either with (A-B) plate-bound anti-CD3 monoclonal antibody alone (2.5 μg/mL) or (A-C) anti-CD3 (2.5 μg/mL) plus anti-CD28 monoclonal antibodies (10 μg/mL) in the presence or absence of (A) the indicated phosphatidylinositol 3-kinase and mitogen-activated protein kinase inhibitors, 5μM LY294002, 10μM PD98059, or 10μM SB203580, or (B,C) calcineurin inhibitors, FK506 (B, 0.2nM; B,C, 5nM) or CsA (B, 50 ng/mL; B,C, 200 ng/mL). (D) A total of 1 × 105 purified naive 2C CD8+ T cells were stimulated with plate-bound anti-CD3 monoclonal antibody alone (2.5 μg/mL) with or without anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of FK506 (0.1 and 0.5nM for anti-CD3; 1 and 5nM for anti-CD3 plus anti-CD28). (E) A total of 1 × 105 purified naive IL2−/− 2C CD8+ T cells were stimulated with plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) and anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of IL-2 (100 ng/mL), FK506 (5nM), or both (5nM for FK506; 1, 10, and 100 ng/mL for IL-2). (F-G) A total of 0.5 × 105 naive WT and CnAβ−/− CD8+ T cells purified from WT (from CnAβ+/+ littermate) and CnAβ−/− mice were stimulated with plate-bound anti-CD3 monoclonal antibody (F, 2.5 μg/mL; G, 0.1-10 μg/mL) with or without 10 μg/mL anti-CD28 monoclonal antibody. On day 3 after culture, cells were analyzed for expression of either the indicated NK receptors (A-G, CD94; C-E, NKG2D) or the activation markers (F; CD25 and CD44) by flow cytometry. The percentages of cells in the respective regions (A,C,F) are indicated. (A-G) Data are representative of more than 3 independent experiments (B,D-E,G; mean ± SD).
Negative signaling for CD94/NKG2A expression by calcineurin pathway. (A-C) A total of 0.5 to 1 × 105 unlabeled (B) or CFSE-labeled (A,C) purified naive 2C CD8+ T cells were stimulated either with (A-B) plate-bound anti-CD3 monoclonal antibody alone (2.5 μg/mL) or (A-C) anti-CD3 (2.5 μg/mL) plus anti-CD28 monoclonal antibodies (10 μg/mL) in the presence or absence of (A) the indicated phosphatidylinositol 3-kinase and mitogen-activated protein kinase inhibitors, 5μM LY294002, 10μM PD98059, or 10μM SB203580, or (B,C) calcineurin inhibitors, FK506 (B, 0.2nM; B,C, 5nM) or CsA (B, 50 ng/mL; B,C, 200 ng/mL). (D) A total of 1 × 105 purified naive 2C CD8+ T cells were stimulated with plate-bound anti-CD3 monoclonal antibody alone (2.5 μg/mL) with or without anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of FK506 (0.1 and 0.5nM for anti-CD3; 1 and 5nM for anti-CD3 plus anti-CD28). (E) A total of 1 × 105 purified naive IL2−/− 2C CD8+ T cells were stimulated with plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) and anti-CD28 monoclonal antibody (10 μg/mL) in the presence or absence of IL-2 (100 ng/mL), FK506 (5nM), or both (5nM for FK506; 1, 10, and 100 ng/mL for IL-2). (F-G) A total of 0.5 × 105 naive WT and CnAβ−/− CD8+ T cells purified from WT (from CnAβ+/+ littermate) and CnAβ−/− mice were stimulated with plate-bound anti-CD3 monoclonal antibody (F, 2.5 μg/mL; G, 0.1-10 μg/mL) with or without 10 μg/mL anti-CD28 monoclonal antibody. On day 3 after culture, cells were analyzed for expression of either the indicated NK receptors (A-G, CD94; C-E, NKG2D) or the activation markers (F; CD25 and CD44) by flow cytometry. The percentages of cells in the respective regions (A,C,F) are indicated. (A-G) Data are representative of more than 3 independent experiments (B,D-E,G; mean ± SD).
The unexpected finding was that addition of inhibitors of the calcineurin pathway (ie, FK506 or cyclosporin A [CsA]), caused a dramatic increase in the expression of CD94/NKG2A, both with CD3 ligation and CD3/CD28 ligation (Figure 5B-D). The enhancing effect of these drugs did not simply reflect inhibition of IL-2 synthesis because the drugs also enhanced CD94/NKG2A expression by IL2−/− 2C CD8+ T cells (Figure 5E). Moreover, the drugs totally abolished the capacity of exogenous IL-2 to inhibit CD94/NKG2A expression (Figure 5E), even with weak CD3/CD28 ligation (supplemental Figure 3A). By contrast, FK506 and CsA both markedly reduced the induction of NKG2D expression (Figure 5C-E).
These findings indicated that the calcineurin signaling pathway had a strong negative effect on CD94/NKG2A expression; the elevated CD94/NKG2A induction by the calcineurin signaling blockade was prevented by coaddition of LY294002, indicating that the negative effect of the calcineurin pathway is countered by the activity of phosphatidylinositol 3-kinase (supplemental Figure 3B). Further support for inhibition via calcineurin signaling came from studies on mice that lacked calcineurin Aβ (CnAβ), the predominant calcineurin isoform found in T cells.27 Naive CD8+ T cells from these mice showed much stronger up-regulation of CD94/NKG2A than WT CD8+ T cells, both for CD3 ligation and CD3/CD28 ligation (Figure 5F-G). Reflecting residual calcineurin signaling,23 CnAβ−/− CD8+ T cells were able to proliferate and produced low but significant amounts of IL-2. Inhibition by the latter accounted for the lower CD94/NKG2A expression with CD3/CD28 ligation than with CD3 ligation alone (data not shown).
FK506 and CsA are known to prevent calcineurin from dephosphorylating nuclear factor of activated T cells (NFAT) in the cytoplasm, thereby inhibiting nuclear factor of activated T-cell translocation to the nucleus and preventing gene transcription.28 It seemed likely, therefore, that the enhancing activity of these drugs on CD94/NKG2A expression was NFAT dependent. This notion was tested with the aid of VIVIT, a drug that selectively blocks calcineurin interaction with NFAT but preserves its phosphatase activity.29 The unexpected finding was that, unlike FK506 and CsA, addition of VIVIT failed to enhance CD94/NKG2A expression (Figure 6A-B). Instead, VIVIT inhibited CD94/NKG2A expression; this applied both to stimulation of 2C CD8+ T cells with specific SIYR peptide (Figure 6A-B) and CD3/CD28 ligation (data not shown). VIVIT also inhibited NKG2D expression (Figure 6C). Except at high concentrations (> 10μM), VIVIT was not toxic and failed to impede proliferation or up-regulation of CD25 and CD44 (supplemental Figure 3C).
NFAT-independent negative signaling by calcineurin and inhibitory function of CD94/NKG2A. (A) A total of 2 × 105 total spleen cells from 2C TCR Tg mice were stimulated with 10μM SIYR peptide with or without calcineurin and NFAT inhibitor, FK506 and VIVIT, respectively, at the indicated concentrations (0.3-20nM for FK506 and 0.3-20μM for VIVIT). (B-C) A total of 1 × 105 naive WT and IL2−/− 2C CD8+ T cells were stimulated with 2 × 105 irradiated T-depleted B6 spleen cells pulsed with 10μM SIYR peptide (sAPC-SIYRp) with or without 5nM FK506 or 5μM VIVIT. On day 3 after culture, cells were analyzed for expression of the indicated NK receptors (A, CD94; B, NKG2ACE; C, NKG2D) by flow cytometry. (D) Schematic diagram for signaling pathways regulating CD94/NKG2A expression. TCR ligation induces both positive and negative signaling for CD94/NKG2A expression via PI3K and MAPK and through the calcineurin pathway, respectively. Negative signaling through the calcineurin pathway is NFAT independent and is further potentiated by B7/CD28 interaction, although only with concomitant interaction with IL-2; in the absence of IL-2, CD28/B7 interaction enhances positive signaling, thereby resulting in increased CD94/NKG2A expression compared with CD3 ligation alone. Thus, under defined conditions, the NFAT-dependent classic calcineurin pathway does serve to induce positive signaling for CD94/NKG2A expression, together with the PI3K and MAPK pathways; these pathways may act as potential targets for the positive and negative regulation of the various cytokines indicated. (E-G) A total of 4 × 104 NKG2ACE+ CD44hi OT-I CD8+ T cells purified on day 3 after primary (1°) stimulation of naive OT-I cells with plate-bound anti-CD3 monoclonal antibody (5 μg/mL) in the presence or absence of cytokines IL-6, IL-10, or IL-21 (50 ng/mL) were restimulated with plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) plus either isotype control or anti-NKG2A monoclonal antibody (10 μg/mL). Proliferation (E), IFN-γ production (F), and apoptotic cell death (G) were analyzed on the indicated time points after secondary (2°) culture. The percentages of cells in the respective regions (B-C,F) are indicated. (A-C,E-G) Data are representative of 2 or 3 independent experiments (A,E,G; mean ± SD).
NFAT-independent negative signaling by calcineurin and inhibitory function of CD94/NKG2A. (A) A total of 2 × 105 total spleen cells from 2C TCR Tg mice were stimulated with 10μM SIYR peptide with or without calcineurin and NFAT inhibitor, FK506 and VIVIT, respectively, at the indicated concentrations (0.3-20nM for FK506 and 0.3-20μM for VIVIT). (B-C) A total of 1 × 105 naive WT and IL2−/− 2C CD8+ T cells were stimulated with 2 × 105 irradiated T-depleted B6 spleen cells pulsed with 10μM SIYR peptide (sAPC-SIYRp) with or without 5nM FK506 or 5μM VIVIT. On day 3 after culture, cells were analyzed for expression of the indicated NK receptors (A, CD94; B, NKG2ACE; C, NKG2D) by flow cytometry. (D) Schematic diagram for signaling pathways regulating CD94/NKG2A expression. TCR ligation induces both positive and negative signaling for CD94/NKG2A expression via PI3K and MAPK and through the calcineurin pathway, respectively. Negative signaling through the calcineurin pathway is NFAT independent and is further potentiated by B7/CD28 interaction, although only with concomitant interaction with IL-2; in the absence of IL-2, CD28/B7 interaction enhances positive signaling, thereby resulting in increased CD94/NKG2A expression compared with CD3 ligation alone. Thus, under defined conditions, the NFAT-dependent classic calcineurin pathway does serve to induce positive signaling for CD94/NKG2A expression, together with the PI3K and MAPK pathways; these pathways may act as potential targets for the positive and negative regulation of the various cytokines indicated. (E-G) A total of 4 × 104 NKG2ACE+ CD44hi OT-I CD8+ T cells purified on day 3 after primary (1°) stimulation of naive OT-I cells with plate-bound anti-CD3 monoclonal antibody (5 μg/mL) in the presence or absence of cytokines IL-6, IL-10, or IL-21 (50 ng/mL) were restimulated with plate-bound anti-CD3 monoclonal antibody (2.5 μg/mL) plus either isotype control or anti-NKG2A monoclonal antibody (10 μg/mL). Proliferation (E), IFN-γ production (F), and apoptotic cell death (G) were analyzed on the indicated time points after secondary (2°) culture. The percentages of cells in the respective regions (B-C,F) are indicated. (A-C,E-G) Data are representative of 2 or 3 independent experiments (A,E,G; mean ± SD).
These findings indicated that the calcineurin signaling pathway had both negative and positive effects on CD94/NKG2A expression. The data with VIVIT suggested that, as for NKG2D expression, classic NFAT-dependent calcineurin signaling potentiated up-regulation of CD94/NKG2A expression. By contrast, the enhancing effect of FK506 and CsA indicated the existence of a NFAT-independent component of calcineurin signaling that inhibited CD94/NKG2A expression; this pathway did not apply to NKG2D expression. Because IL-2 was unable to block the enhancing effect of FK506 (Figure 5E; supplemental Figure 3A), the inhibitory influence of IL-2 on CD94/NKG2A induction appeared to be mediated entirely via NFAT-independent calcineurin signaling. The same seemed to apply to TGF-β and IL-23 because, like IL-2, these cytokines had little or no effect on preventing the enhancing effect of FK506 (supplemental Figure 4A-C). By contrast, IL-4 did block the enhancing effect of FK506, although not with Stat6−/− CD8+ T cells (supplemental Figure 4D).
A summary of the various factors exerting positive and negative effects on CD94/NKG2A induction in vitro is shown in Figure 6D.
Negative signaling via CD94/NKG2A
To examine the functional consequences of CD94/NKG2A expression, naive CD8+ T cells were subjected to CD3 ligation in the absence or presence of cytokines (IL-6, IL-10, or IL-21) to prepare FACS-purified CD94/NKG2A+ cells. These cells responded well as measured by proliferation, IFN-γ, and granzyme B synthesis when reexposed to CD3 ligation in vitro (Figure 6E-F; supplemental Figure 5A-C). Significantly, however, these responses were greatly reduced by coaddition of anti-NKG2A (or anti-CD94) monoclonal antibody, and the cells rapidly underwent apoptosis (Figure 6G; supplemental Figure 5D). These findings are in line with the view that CD94/NKG2A has a negative influence on intracellular signaling.30 It should be mentioned that, without NKG2A ligation, TCR/CD3 responses of purified CD8+ cells that did or did not express CD94/NKG2A were indistinguishable (data not shown).
Regulation of CD94/NKG2A expression in vivo
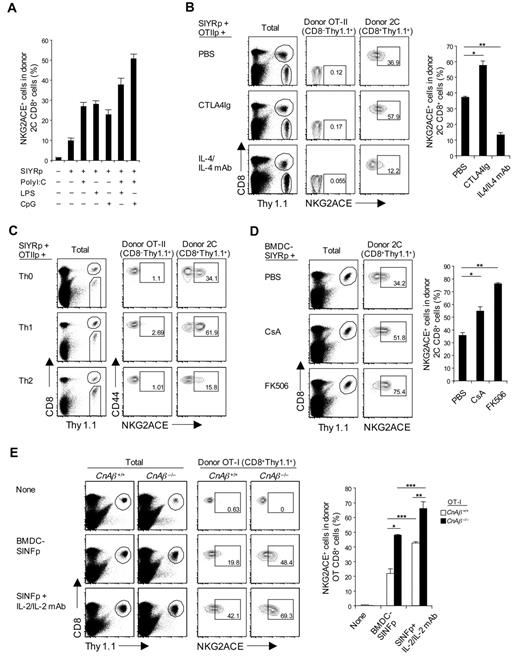
Induction of CD94/NKG2A expression in vivo was examined by adoptively transferring naive 2C CD8+ T cells plus SIYR peptide to normal B6 mice. In the absence of adjuvant, up-regulation of CD94/NKG2A expression on the donor 2C CD8+ T cells was minimal (Figure 7A), despite vigorous division of these cells (data not shown). CD94/NKG2A up-regulation increased with addition of adjuvants and was most prominent with a combination of CpG Oligodeoxynucleotide (ODN) and polyriboinosinic acid:polyribocytidylic acid (poly I:C; Figure 7A), presumably reflecting production of cytokines, such as IL-6 and IL-10 by APC,31 and also IL-10 from Toll-like receptor-stimulated B cells.32
Regulation of CD94/NKG2A expression in vivo. (A) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intraperitoneally with 5μM SIYR peptide with or without poly I:C (10 μg), LPS (5 μg), or CpG ODN (1 μg) or a combination of both poly I:C plus either LPS or CpG ODN. (B) A mixture of purified naive 2C CD8+ and OT-II CD4+ T cells (2 × 105 for 2C and 1 × 106 for OT-II; all Thy1.1) was transferred intravenously into B6 mice; and 1 day later, the mice were injected intraperitoneally with SIYR (5μM) plus ovalbumin323–339 peptide (OT-IIp; 10μM) with or without CTLA4Ig (50 μg) or IL-4 (1 μg)/anti-IL-4 monoclonal antibody (20 μg) complexes (injected daily for 2 consecutive days). *P < .01. **P < .005. (C) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were cotransferred intravenously with in vitro-stimulated differentiated OT-II CD4+ T helper subsets, Th0, Th1, and Th2 (1 × 106; Thy1.1) into B6 mice; on the same day, the mice were injected intraperitoneally with 5μM SIYR and 10μM ovalbumin323–339 peptide. (D) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intravenously with 2 × 106 LPS-matured bone marrow–derived DC pulsed with 10μM SIYR peptide (BMDC-SIYRp) with or without 2 μg CsA or 0.2μM FK506 (injected daily for 3 consecutive days). *P < .05. **P < .005. (E) A total of 5 × 105 purified naive WT or CnAβ−/− OT-I CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intravenously with 2 × 106 BMDCs pulsed with 10μM SINF peptide (BMDC-SINFp) either with or without intraperitoneal injection of IL-2 (0.5 μg)/anti-IL-2 monoclonal antibody (10 μg) complexes. *P < .005. **P < .05. ***P < .05. (A-E) On day 4 after antigenic stimulation in vivo, pooled spleen and lymph node from the recipient mice were analyzed for expression of NKG2ACE on donor 2C (A-D), OT-II (B-C), and OT-I (E) cells by flow cytometry. The percentages of cells in the respective regions (B-E) are indicated. (A-E) Data are representative of 2 or 3 independent experiments (A-B,D-E; mean ± SD from 4 or 5 mice per group).
Regulation of CD94/NKG2A expression in vivo. (A) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intraperitoneally with 5μM SIYR peptide with or without poly I:C (10 μg), LPS (5 μg), or CpG ODN (1 μg) or a combination of both poly I:C plus either LPS or CpG ODN. (B) A mixture of purified naive 2C CD8+ and OT-II CD4+ T cells (2 × 105 for 2C and 1 × 106 for OT-II; all Thy1.1) was transferred intravenously into B6 mice; and 1 day later, the mice were injected intraperitoneally with SIYR (5μM) plus ovalbumin323–339 peptide (OT-IIp; 10μM) with or without CTLA4Ig (50 μg) or IL-4 (1 μg)/anti-IL-4 monoclonal antibody (20 μg) complexes (injected daily for 2 consecutive days). *P < .01. **P < .005. (C) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were cotransferred intravenously with in vitro-stimulated differentiated OT-II CD4+ T helper subsets, Th0, Th1, and Th2 (1 × 106; Thy1.1) into B6 mice; on the same day, the mice were injected intraperitoneally with 5μM SIYR and 10μM ovalbumin323–339 peptide. (D) A total of 5 × 105 purified naive 2C CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intravenously with 2 × 106 LPS-matured bone marrow–derived DC pulsed with 10μM SIYR peptide (BMDC-SIYRp) with or without 2 μg CsA or 0.2μM FK506 (injected daily for 3 consecutive days). *P < .05. **P < .005. (E) A total of 5 × 105 purified naive WT or CnAβ−/− OT-I CD8+ T cells (Thy1.1) were transferred intravenously into B6 mice; and 1 day later, the mice were injected intravenously with 2 × 106 BMDCs pulsed with 10μM SINF peptide (BMDC-SINFp) either with or without intraperitoneal injection of IL-2 (0.5 μg)/anti-IL-2 monoclonal antibody (10 μg) complexes. *P < .005. **P < .05. ***P < .05. (A-E) On day 4 after antigenic stimulation in vivo, pooled spleen and lymph node from the recipient mice were analyzed for expression of NKG2ACE on donor 2C (A-D), OT-II (B-C), and OT-I (E) cells by flow cytometry. The percentages of cells in the respective regions (B-E) are indicated. (A-E) Data are representative of 2 or 3 independent experiments (A-B,D-E; mean ± SD from 4 or 5 mice per group).
To seek direct support for the influence of cytokines, SIINFEKL (SINF) peptide-reactive OT-I TCR Tg33 CD8+ T cells were adoptively transferred together with SINF peptide (without adjuvant), either alone or with IL-6 or a mixture of IL-6 and IL-4. As in vitro (Figure 2A), up-regulation of CD94/NKG2A was augmented by IL-6 (supplemental Figure 6); conversely, the enhancing effect of IL-6 was blocked by coaddition of IL-4. Likewise, the inhibitory influence of CD28 costimulation seen in vitro also applied in vivo. Thus, CD94/NKG2A up-regulation induced by 2C CD8+ T-cell stimulation with SIYR peptide in vivo was enhanced by coinjection of CTLA4Ig but blocked by IL-4 (Figure 7B); here, the donor CD8+ cells were supplemented with T-cell help by coinjecting OT-II CD4+ T cells plus ovalbumin323–339 peptide.34
Further evidence on the influence of cytokines and CD4+ T helper cells on CD94/NKG2A expression was examined by preparing polarized Th0, Th1, and Th2 OT-II CD4+ T cells under standard conditions in vitro (supplemental Figure 7A) and then adoptively transferring the in vitro-differentiated CD4+ T helper subsets together with 2C CD8+ T cells plus a mixture of SIYR and ovalbumin323–339 peptides in vivo. These 3 types of helper T cells had quite different effects on CD94/NKG2A expression on the 2C CD8+ T cells. Thus, CD94/NKG2A induction was moderate with Th0 cells, high with Th1 cells but low with Th2 cells (Figure 7C; supplemental Figure 7C). For Th2 cells, impaired induction of CD94/NKG2A correlated with marked secretion of IL-4 by these cells (supplemental Figure 7B). The strong induction of CD94/NKG2A by the Th1 cells presumably reflected release of stimulatory cytokines, although which particular cytokines were involved here was not established; the Th1 cells secreted large amounts of IFN-γ but, by itself, this cytokine had no obvious effect on CD94/NKG2A induction in vitro (Figure 2A).
As in vitro, blocking signaling through the calcineurin pathway with CsA or FK506 in vivo substantially increased CD94/NKG2A induction on 2C CD8+ T cells responding to SIYR peptide (Figure 7D); this effect was most marked with FK506. Likewise, studies with OT-I CD8+ T cells showed that SINF peptide-induced CD94/NKG2A induction on these cells in vivo was much higher with CnAβ−/− OT-I CD8+ T cells than with WT OT-I CD8+ T cells (Figure 7E); similar findings applied to 2C CD8+ T cells (supplemental Figure 7D).
By the aforementioned parameters, the factors controlling CD94/NKG2A expression in vivo correlated well with the in vitro data. As discussed below, however, there was one major difference between the in vivo and in vitro findings, namely, the influence of IL-2.
It was mentioned earlier that the inhibitory influence of IL-2 on CD94/NKG2A in vitro was prominent with weak TCR signaling but minimal with strong TCR signaling. Hence, it was of interest to examine whether IL-2 would be inhibitory under in vivo conditions (ie, where typical immune responses presumably reflect strong TCR signaling). Indeed, for the response of OT-1 CD8+ T cells to the strong agonist SINF peptide, coinjection of IL-2 enhanced (rather than reduced) up-regulation of CD94/NKG2A (Figure 7E; supplemental Figure 8). In addition, under in vivo conditions the enhancing effect of CTLA4Ig on CD94/NKG2A expression on 2C CD8+ T cells was as marked with IL-2-unresponsive (IL2Rβ−/−) 2C CD8+ T cells as with WT 2C CD8+ T cells (supplemental Figure 9). Hence, the enhancing effect of CTLA4Ig treatment in vivo could not be attributed simply to blocking IL-2 production. Our interpretation of these findings is that, under in vivo conditions, the inhibitory influence of IL-2 on CD94/NKG2A expression is countered by coproduction of stimulatory factors (“Discussion”).
Discussion
Typical in vivo responses of naive CD8+ T cells to antigen involve up-regulation of a wide variety of cell-surface molecules, including cytokine and chemokine receptors as well as markers found on NK cells, such as NKG2D and CD94/NKG2A. For most of these markers, it is well accepted that their up-regulation during the immune response is TCR dependent and amplified by CD28-dependent costimulation and contact with growth factors, especially IL-2. As shown here, however, the requirements for inducing CD94/NKG2A expression are quite different. In particular, CD28 costimulation and IL-2 were found to have a marked negative effect on CD94/NKG2A expression. Surprisingly, this paradoxical finding was easily apparent in vitro but largely obscured in vivo.
Under in vitro conditions, induction of CD94/NKG2A expression clearly required a TCR signal because induction was marked after anti-CD3 monoclonal antibody ligation but undetectable after culture with PMA plus ionomycin. CD94/NKG2A induction correlated directly with the intensity of TCR/CD3 signaling and was apparent only on CD8+ and not CD4+ T cells. By these parameters, the requirements for inducing CD94/NKG2A expression were the same as for NKG2D expression. With CD28 costimulation, however, the results were opposite. Thus, when combined with CD3 ligation, CD28 ligation potentiated the induction of NKG2D but inhibited expression of CD94/NKG2A. Similar findings applied to stimulation with specific antigen. Thus, when naive 2C CD8+ T cells were exposed to peptide presented by transfected Drosophila cells, induction of CD94/NKG2A was high with peptide-loaded fly APCs coexpressing ICAM-1 but very low with fly APC coexpressing B7-1 or a mixture of B7-1 and ICAM-1. With the latter, induction of CD94/NKG2A rose sharply with anti–B7-1 monoclonal antibody blockade but not with ICAM-1 blockade; B7 blockade also augmented CD94/NKG2A expression induced by normal spleen APCs. Collectively, these findings indicated that inhibition of CD94/NKG2A expression by costimulation was controlled solely by CD28/B7 interaction and did not appear to involve other forms of costimulation.
Inhibition by CD28 costimulation was found to be a consequence of IL-2 released by the responding T cells. Thus, CD28-dependent inhibition of CD94/NKG2A expression was abrogated by suppression of IL-2 production (ie, by adding anti–IL-2 monoclonal antibody to the cultures or using IL-2-unresponsive or IL2−/− CD8+ T cells). However, the inhibition was not mediated by IL-2 alone because no inhibition occurred in the absence of CD28 costimulation. Instead, IL-2 appeared to activate a negative signaling pathway involving the calcineurin pathway. This was apparent from the unexpected finding that calcineurin antagonists augmented CD94/NKG2A expression and that such expression could not be blocked by IL-2.
How calcineurin antagonists potentiated CD94/NKG2A expression is still unclear. Because these antagonists are known to block nuclear translocation of NFAT, we expected similar augmentation of CD94/NKG2A expression by VIVIT, a drug that prevents calcineurin interaction with NFAT but does not impair calcineurin phosphatase activity.35 Instead, VIVIT impaired CD94/NKG2A expression. This finding indicated that suppression of CD94/NKG2A expression by calcineurin involved a NFAT-independent signaling pathway. In this respect, it is of interest that calcineurin antagonists, such as CsA and FK506, are reported to enhance IL-12–dependent production of IFN-γ by T cells, apparently by increasing expression of transcription factors, c-fos and AP-1.36 Whether such expression is affected by VIVIT is unknown, nor is it clear whether these transcription factors are involved in CD94/NKG2A expression, although putative AP-1-binding sites in the CD94 promoter regions have been identified.37,38
Although a full appreciation of the signaling pathways involved in CD94/NKG2A expression will have to await further study, it is notable that expression was influenced by a number of cytokines. Some cytokines, namely, IL-6, IL-10, and IL-21, augmented CD94/NKG2A expression, whereas IL-4, IL-23, and TGF-β were strongly inhibitory. Unlike IL-2, the capacity of IL-4, IL-23, and TGF-β to inhibit CD94/NKG2A expression was CD28 independent. However, calcineurin antagonists blocked inhibition by IL-23 and TGF-β but did not affect inhibition by IL-4. Hence, these 4 cytokines showed distinct differences in their mode of CD94/NKG2A inhibition, and future studies will be needed to explain these differences. The marked inhibitions seen with TGF-β were surprising because it was reported previously that TGF-β caused an increase in CD94/NKG2A induction39 ; the reason for this discrepancy is unknown.
Seeking detailed information on the factors influencing CD94/NKG2A expression under in vivo conditions was impractical, but in a number of respects our in vivo findings correlated well with the in vitro data. Thus, as in vitro, CD94/NKG2A induction in response to specific peptide in vivo was augmented by IL-6, and also by IL-6– and IL-10–inducing adjuvants, such as CpG ODN, lipopolysaccharide (LPS), and poly I:C. Conversely, induction was inhibited by IL-4 and IL-4–producing Th2 cells. Likewise, as in vitro, CD94/NKG2A induction was augmented by CTLA4Ig and calcineurin antagonists. The one discordant finding was in the effects of IL-2. Thus, in marked contrast to the inhibition seen by this cytokine in vitro, IL-2 had a prominent potentiating effect on CD94/NKG2A induction in vivo. Because inhibition by IL-2 in culture was only marked with weak TCR ligation, we speculate that the contrary findings with IL-2 in vivo may reflect a combination of strong TCR signaling plus IL-2-dependent production of stimulatory cytokines under in vivo conditions. Further studies will be required to resolve this issue.
As mentioned earlier, most reports have shown that CD94/NKG2A induction in response to antigen in vivo is quite high, which contrasts with relatively low expression under standard conditions in vitro. Strong expression in vivo presumably reflects high exposure to various stimulatory cytokines released by adjuvant-activated APC and also by activated T cells, including CD4+ T helper cells. Here, it is notable that CD94/NKG2A expression induced by exposing CD8+ T cells to specific peptide in vivo was considerably enhanced by the coinjection of Th1 cells as CD4+ T helper cells. This helper activity presumably reflected synthesis of stimulatory cytokines, such as IL-2 and/or IL-21 by the Th1 cells, although this was not studied directly. These strongly stimulatory conditions apply during typical acute responses to viruses, such as LCMV strain Armstrong, which is rapidly eliminated.8,40 For this virus, CD94/NKG2A expression on specific CD8+ T cells was found to decrease several weeks after infection, implying that expression requires constant exposure to antigen.7 In support of this notion, chronic viral infections, such as LCMV clone 13 and polyoma virus, cause prolonged high expression of CD94/NKG2A on the responding CD8+ T cells.8,9,41 Expression of these immunoreceptor tyrosine-based inhibitory motif-associated inhibitory receptors is thought to reduce the intensity of the CD8+ T cell response, thereby preventing cell exhaustion and limiting immunopathology.9,11,42
Although up-regulation of CD94/NKG2A and other NK receptors on CD8+ T cells is a general feature of the immune response, the extent of up-regulation is variable. Thus, CD94/NKG2A induction during the initial stages of infection appears to be appreciably lower during polyoma virus than LCMV infection8,9 and is also low during influenza virus infection.11 Based on the data presented here, this variability may reflect that the immune response to individual pathogens is unique, with each pathogen eliciting a complex variety of factors that can either potentiate or retard the immune response, including the expression of receptors, such as CD94/NKG2A. Such regulation optimizes the response to the pathogen while minimizing immunopathology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Hong for mice genotyping, C. Brownlee and N. Alling for cell sorting, and the Biologic Testing Facility for animal care.
This work was supported by the National Health and Medical Research Council Program Grant and Project Grant funded by Australian Government and the World Class University (WCU) Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-10105).
Authorship
Contribution: J.-H.C. and H.-O.K. designed and performed experiments; K.W., M.P., B.H., K.-S.K., C.K., and S.G.T. provided supporting information; and J.-H.C., H.-O.K., and J.S. analyzed and interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan Sprent, Immunology Research Program, Garvan Institute of Medical Research, 384 Victoria St, Darlinghurst, NSW 2010, Australia; e-mail: j.sprent@garvan.org.au.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal