Abstract
Joint arthropathy secondary to recurrent hemarthroses remains a debilitating complication of hemophilia despite the use of prophylactic factor concentrates. Increased vascularity and neoangiogenesis have been implicated in the progression of musculoskeletal disorders and tumor growth. We hypothesized that de novo blood vessel formation could play a major role in the pathogenesis of hemophilic joint disease (HJD). We observed a 4-fold elevation in proangiogenic factors (vascular endothelial growth factor-A [VEGF-A], stromal cell–derived factor-1, and matrix metalloprotease-9) and proangiogenic macrophage/monocyte cells (VEGF+/CD68+ and VEGFR1+/CD11b+) in the synovium and peripheral blood of HJD subjects along with significantly increased numbers of VEGFR2+/AC133+ endothelial progenitor cells and CD34+/VEGFR1+ hematopoietic progenitor cells. Sera from HJD subjects induced an angiogenic response in endothelial cells that was abrogated by blocking VEGF, whereas peripheral blood mononuclear cells from HJD subjects stimulated synovial cell proliferation, which was blocked by a humanized anti-VEGF antibody (bevacizumab). Human synovial cells, when incubated with HJD sera, could elicit up-regulation of HIF-1α mRNA with HIF-1α expression in the synovium of HJD subjects, implicating hypoxia in the neoangiogenesis process. Our results provide evidence of local and systemic angiogenic response in hemophilic subjects with recurrent hemarthroses suggesting a potential to develop surrogate biologic markers to identify the onset and progression of hemophilic synovitis.
Introduction
Hemophilic joint disease (HJD) secondary to recurrent hemarthroses is one of the most disabling and expensive complications of severe hemophilia A or B (X-linked recessive disorders with < 1% factor VIII/IX [FVIII/FIX] activity).1,2 Clinical and subclinical hemarthroses during childhood can result in the development of synovitis, which is characterized by villous formation, increased vascularity, and chronic inflammatory cells, resulting in hypertrophied synovium,2,3 resultant joint arthropathy, and destructive arthritis.4 Although synovitis and joint arthropathy can be minimized by the prophylactic infusion of factor concentrates, which is the standard of care in the developed world, prophylaxis is unaffordable in the developing world. Moreover, the dose, timing, schedule, and duration of prophylaxis are topics of ongoing debate.5 In the presence of active synovitis, prophylaxis may not stop further joint deterioration, necessitating the use of procedures, such as isotopic and arthroscopic synovectomy.6,7 Alternatively, the selective implementation of these strategies would require a more sensitive tool for detecting synovitis than is currently possible with clinical surveillance or plain radiographs. Magnetic resonance imaging (MRI) can detect both synovial and cartilage changes resulting from recurrent hemarthroses,8 unlike plain radiographs, which detect only advanced bony changes associated with joint arthropathy.2 However, MRI is expensive and requires sedation in younger children, limiting its utility for routine monitoring of synovitis. A better understanding of the pathogenesis of HJD might make it possible to identify surrogate biologic markers to indicate the onset of synovitis to aid in treatment decisions, such as prophylaxis and synovectomy.
The pathogenesis of HJD is not well defined. Villous formation after a single hemarthrosis resulting from acid phosphatase and cathepsin D-induced synovial inflammation,2 cartilage damage from long-lasting inhibition of proteoglycan synthesis,9 and abrogated synovial apoptosis via iron-dependent increase in MDM2 expression and MYC-C amplification have been reported.10-12 Neoangiogenesis is a critical factor in processes, such as tumor growth and inflammatory arthritis.13 Vascular endothelial growth factor (VEGF), the principal signaling molecule in angiogenesis, can be induced by hypoxia and certain cytokines through interaction with its receptors, VEGFR1 and VEGFR2.14-16 The synovitic pannus in other joint diseases that share histologic similarities with HJD have enhanced oxygen demand and show evidence of de novo blood vessel formation, including endothelialization of the synovium.2 Endothelialization may occur as a result of mature endothelial cell migration or through the recruitment of bone marrow (BM)–derived endothelial progenitor cells (EPCs) and hematopoietic progenitor cells (HPCs) from the peripheral circulation.17 Importantly, proliferating synovium can secrete chemocytokines, such as VEGF, that might promote recruitment of endothelial cells (ECs) to sites of active angiogenesis.18 In other joint diseases, such as rheumatoid arthritis and osteoarthritis, VEGF expression in the serum has been correlated with disease activity.18 Colocalization of HIF-1α and VEGF emphasizes the role of hypoxia in the up-regulation of angiogenesis in rheumatoid joint diseases.19 Because of the observed vascularity in human HJD2,3 and experimental murine models of hemophilic synovitis,20 we hypothesized that neoangiogenesis could play a major role in the development of synovitis secondary to recurrent hemarthroses. We observed potent proangiogenic mediators, circulating HPCs and EPCs in HJD synovium, and peripheral blood of HJD subjects. Collectively, our data suggest that cells of the early and late myeloid lineage can induce proangiogenic mediators contributing to neoangiogenesis associated with hemophilic synovitis.
Methods
Subjects and samples
This is a single-institution study where plasma samples were prospectively collected after Institutional Review Board approval from Weill Cornell Medical College, with informed consent obtained in compliance with the Declaration of Helsinki. Subjects with severe (FVIII/IXc: 0.01%), moderate (FVIII/ IXc: 2%-5%), and mild (FVIII/ IXc: 5%-25%) factor activity and a history of more than 2 hemarthroses in a joint composed the prospectively studied cohort of experimental subjects. A retrospective cohort of subjects who had MRIs of joints performed to determine eligibility for isotopic synovectomy also had plasma analyzed for VEGF-A levels. Early joint disease in this retrospective cohort was defined as less than or equal to the median of 20 lifetime hemarthroses, no x-ray evidence of joint arthropathy, but with MRI evidence of synovitis. Advanced joint disease was defined as more than 20 lifetime hemarthroses and x-ray evidence of joint arthropathy. All subjects had baseline x-rays 6 months to 2 years from enrollment on the study, depending on their bleeding history. Two control groups were established. Group A, the bleeding disorders control group, was composed of subjects with FVIII/ IX, XI activity levels between 0.05 and 0.25 U/dL, and rarer homozygous factor I, V, X, and XIII deficiencies who had a history of non-joint-related bleeding but no hemarthroses. Group B was composed of normal healthy persons without a history of joint bleeds. Experimental subjects were screened to ensure that they had not had a joint bleed less than 4 weeks before blood sampling to maximize bleed resolution potential, and they had last infused with factor concentrates more than 72 hours before blood draw to mitigate effects of factor concentrates on the relevant studies. Both subjects and guardians signed informed consent and assent forms. Peripheral blood samples were collected in ethylenediaminetetraacetic acid, serum separator tubes, and PAXgene Blood RNA tubes (PreAnalytiX/QIAGEN). Within 2 hours of collection, ethylenediaminetetraacetic acid and serum separator tube samples were centrifuged at 1000g for 15 minutes. Platelet-poor plasma aliquots were then stored at −80°C. Samples were analyzed within 4 to 8 weeks of collection. A random subset of samples was rerun for sample storage and interassay control, which varied for VEGF-A by 15.4 pg/mL (1.16 ± 0.08 seconds). Plasma samples from rheumatoid arthritis subjects were used as positive controls to determine assay sensitivity.
Cell culture
Primary cultures of human synovial fibroblast cells were a generous gift from Dr Katalin Mikecz (Rush University) and were cultured as described.11 Functionally validated primary ECs (BD Biosciences) were cultured with Clonetics EGM-2 endothelial growth medium (Cambrex). All experiments with ECs (second to fourth passages) and synovial cells (fourth to 15th passages) did not display discernable morphologic changes before each assay. Testing for bacterial, fungal, and mycoplasma contamination was done routinely.
Functional assays
Morphologic assay.
ECs were incubated with plasma from HJD subjects and controls for 22 hours, and morphology was assessed. This time point was used based on a time course experiment (t = 2, 6, 12, 22, 32, and 40 hours) in which t = 22 hours morphologically acceptable “tube formation” was observed with recombinant VEGF.
Matrigel tube assay and effect of inhibitors.
ECs were preincubated with or without VEGF blocking peptide in Dulbecco modified Eagle medium (DMEM; 5 μg/mL, 1 hour, 37°C), washed twice with phosphate-buffered saline (PBS) or ECs in DMEM + 10% fetal bovine serum (FBS) or DMEM + 2.5% serum from HJD were seeded into Matrigel-coated 96-well plates (Angiogenesis System Endothelial Tubule formation plates, BD Biosciences) at a density of 48 000 cells/well (12 hours, 37°C). At the end of the incubation period, ECs were observed for any morphologic changes at 10×, 20×, and 40× magnification, and images were taken using Roper Scientific CoolSnap Camera. Morphologic changes were quantified using MetaMorph Imaging Systems where analysis of total tube length, mean tube length, and the total number of branch points was performed.21
Migration assays.
ECs (1 × 105) were serum-starved for 12 hours and placed into 8-μm pore size Transwell inserts, which were then placed into 24-well plates and allowed to migrate for 4 hours. Plasma from HJD subjects, DMEM alone, VEGF (1 μg/mL), anti-VEGF antibody (5 μg/mL), or anti-stromal cell–derived factor-1 (SDF-1) antibody (10 μg/mL) was placed into the lower chamber of a modified Boyden chamber as described.22 Cells in the lower chamber and the under surface of the membrane were trypsinized and counted in a blinded fashion by 2 independent observers.
Proliferation assays.
Peripheral blood mononuclear cells (PBMCs) were isolated from subjects with HJD and controls using Ficoll-Paque, washed twice with 1× PBS, and frozen (−80°C, up to 3 days). Synovial cells were seeded (10 000 cells/well) into a 24-well plate and incubated with proliferation media for 24 hours. For the experiment, PBMCs and THP-1 (macrophage cell lines used as a positive control) were concentrated by spinning down and removing the freezing media, washed with 1× PBS, and resuspended in 1 mL of DMEM with or without 1% FBS. To determine whether PBMCs from the HJD subjects and controls had an effect on synovial proliferation, PBMCs or THP-1 (10 000 cells/well) was added to synovial cells and incubated for 48 hours and counted in a blinded fashion by 2 independent observers. For proliferation blocking experiments, synovial cells were treated with bevacuzimab (Avastin); VEGF inhibitor (0.25 mg/mL in DMEM with or without 1% FBS; Genentech) was a generous gift from Dr S. Modak (Memorial Sloan-Kettering Cancer Center, New York, NY) followed by PBMCs and THP-1 cells and incubated for 48 hours. Cells were washed twice with 1× PBS, trypsinized, and counted in a blinded fashion using a hemocytometer. This concentration of bevacuzimab was determined by a dose inhibition experiment using elimination by Trypan blue.
Immunohistochemistry
Histologic sections of hemophilic synovium obtained after synovectomy/loose body removal/replacement (experimental), and surgically obtained synovium without a specific pathology (control) were a generous gift from Dr E. DiCarlo (Hospital for Special Surgery, New York, NY). Paraffin sections of synovium were processed using avidin/biotin enzyme complex system (Vector Laboratories) as described.23 The following primary antibodies were used: c-Kit (clone YB5.B8), CD11b (clone CBRM1/5), and VEGF-A (polyclonal) (eBioscience); VEGFR1/flt-1 (clone FB5, Imclone Systems); CD68 (clone PG-M1, Dako North America); matrix metalloprotease-9 (MMP-9; clone 56-2A4) and HIF-1α (clone H1α67; Calbiochem); and SDF-1α (clone 79018; R&D Systems). Sections were incubated with primary antibodies (2 μg/mL, 4°C, 12 hours) followed by appropriate secondary antibody (2 μg/mL, 21°C, 30 minutes), and developed with 0.02% 3-3-diaminobenzamidine tetrahydrochloride (DAB) or immunofluorescence as previously described.24 Serial sections (cryostat; Leica) were mounted with Vectashield containing DAPI (4,6-diamidino-2-phenylindole), and visualized with an ultraviolet fluorescent microscope (Nikon Eclipse E800) with a Retiga camera (Q Imaging) through IP Lab Version 3.65a imaging software (Scanalytics). Imaging medium/solution used was Vectashield containing DAPI and Permount for paraffin-embedded sections. Images were captured by a Nikon Eclipse microscope. The numeric apertures of the objective lens are as follows: 4×/0.13, 10×/0.3, 20×/0.75, 40×/0.75, 40× oil/1.3, 100× oil/1.4. Controls were hemophilic synovium incubated with nonimmune IgG or normal synovium, as detailed in “Subjects and samples.”
Measurement of plasma cytokine expression
VEGF, SDF-1, and MMP-9 concentrations from plasma of experimental and control groups A and B were measured with specific ELISA kits (R&D Systems) per the manufacturer's instructions.
Flow cytometry
Mononuclear cells were isolated from peripheral blood samples of experimental and control groups A and B via separation with Ficoll. Fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, and allophycocyanin (APC)–labeled antibodies, namely, anti-VEGFR1 and VEGFR2 APC-conjugated mouse IgG1 (R&D Systems), PE-conjugated CD133/CD34/CD45 and FITC-conjugated CD11b (Miltenyi Biotec), and CD45APC and CD45 FITC (BD Biosciences PharMingen), were used. Three antibody triplet combinations (anti-hVEGFR1 APC /CD34 PE/CD45 FITC, anti-hVEGFR2 APC/CD133 PE/CD45 FITC, and anti-VEGFR1 APC/CD11b FITC/CD45 PE) and one negative control were used for flow cytometry analysis on each sample processed (each containing 1 × 106 cells). Flow cytometry was performed on a dual argon and diode laser FACSCalibur system (BD Biosciences). All data acquisition and analysis were performed using CELLQuest software Version 7.5.3 (BD Biosciences) and FlowJo software Version 7.2.5 (TreeStar). Live gates were set to exclude cell debris, and 100 000 cellular events were acquired. To prevent spectral overlap, compensation was performed before running samples.
Quantitative PCR
RNA isolation from peripheral blood samples of experimental and control groups A and B was accomplished using the RNAqueous-4PCR Kit (Ambion). cDNA was synthesized in a 20-μL reaction volume via reagents and protocol included with the iScript cDNA Synthesis Kit (Bio-Rad). Quantification of mRNA transcripts using iTaq SYBR Green Supermix with Rox (Bio-Rad), and 30 ng cDNA for VEGFR1, VEGFR2, and β-actin using the standard procedures with 40 cycles of denaturation for 2 minutes at 50°C, annealing for 10 minutes at 95°C, and extension for 15 seconds at 95°C was carried out. Expression of target genes was normalized to β-actin expression.RNA isolation from synovial cell cultures (after exposure to serum from HJD subjects or control sera for 6 hours) was carried out as above, with similar thermacycler conditions. Primers for VEGFR1, VEGFR2, CXCR4, and HIF-1α were designed using Primer Quest and purchased from Integrated DNA Technologies. Forward and reverse primers, respectively, were 5′-TGCGAGCTCCGGCTTTC-3′ and 5′-AAACCGTCAGAATCCTCTTC-3′ (VEGFR1); 5′-CCCAAGCCAAGCCTTAAGTG-3′ and 5′-TCCAGTACTCTCCAAAGCAAGGT-3′ (VEGFR2) and 5′-TGCCACATCATCACCA TATAGAGA-3′and 5′-TCCTTTTCCTGCTCTGTTTGG-3′ (HIF-1-α); 5′ ACT GTT GTC TGA AAC CCA TCC-3′ and 5′-CGT GCT GGG CAG AGG TTT TA-3′ (CXCR4). All polymerase chain reaction (PCR) was performed on an ABI PRISM 7700 analytical thermal cycler, according to the manufacturer's recommendations.
Statistical analyses
Descriptive statistics were used to define subjects' characteristics. Results were expressed as mean plus or minus SD or SEM of at least 3 experiments performed in duplicate. Categorical variables were compared using the 2-tailed Student t test. A P value less than .05 was considered significant. Analyses were also performed using the program GraphPad Prism Version 3.0 (Strata Corporation). Power calculations were not performed to determine patient/sample population size because this was a single-institution pilot study, and we used all available samples of subjects enrolled on the study.
Results
HJD synovium expresses proangiogenic mediators and myeloid cells
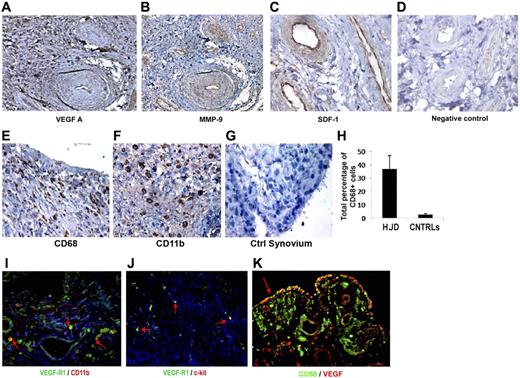
We examined hemophilic synovium for expression of VEGF-A, a principal mediator of angiogenesis. Immunohistochemical examination of 14 specimens using antihuman VEGF antibody showed localization of VEGF-A to vascular endothelial cells, perivascular tissue, and synoviocytes in the lining and sublining regions (Figure 1A). As expected, normal human synovium (n = 11) showed minimal VEGF-A staining (Figure 1D). Because mobilization of EPCs and proangiogenic HPCs from the BM requires activation of MMPs, particularly MMP-9, and because SDF-1α has been shown to potently promote the migration and activation of mononuclear cells, including HPCs,25,26 we stained HJD and control synovium for these mediators. We observed that MMP-9 (Figure 1B) and SDF-1α (Figure 1C), were highly expressed in the vascular endothelium, with moderate expression in synovial cells in the lining and sublining regions of the hemophilic synovium (n = 12). Previously, cells of monocyte/macrophage lineage have been reported to be involved in clearing breakdown products after hemarthroses in hemophilic synovium.2,3 To further characterize whether monocytes/macrophages of myeloid lineage were expressed in the synovium, we stained 12 hemophilic and 10 control synovial specimens for CD68 and CD11b, well-characterized markers of the same. We observed that hemophilic (Figure 1E), but not control (Figure 1G), synovium had increased numbers of CD68+ (Figure 1E,G) and CD11b+ (Figure 1F) cells, suggesting the involvement of myeloid cells in synovial pathology. Furthermore, CD68 staining colocalized with VEGF (Figure 1K) in hemophilic synovium (n = 12), suggesting that macrophages may be involved in synovial angiogenesis. In addition, CD11b+ cells coexpressed VEGFR1 (Figure 1I) (n = 10), and a subset also expressed the early HPC marker c-kit (Figure 1J; n = 11), demonstrating the presence of proangiogenic mediators and myeloid progenitor cells in HJD synovium.
Hemophilic synovium expresses proangiogenic mediators and myeloid cells. Immunohistochemical analyses using DAB revealed positive staining for proangiogenic mediators VEGF-A (A), MMP-9 (B), and SDF-1 (C), in hemophilic synovium but not control synovium (D) (original magnification ×200). Immunohistochemical staining for CD68 (E) and CD11b (F) provided evidence for the presence of myeloid cell infiltration not observed in control synovium (G). Quantitative assessment of CD68+ cells in hemophilic and control synovium demonstrated an increased percentage of CD68+ cells in hemophilic synovium (HJD) compared with control (CNTRLs) (H). Immunofluorescent staining (red arrow) demonstrates CD11b+ cells coexpressing VEGF-R1 (I) in hemophilic synovium. Hemophilic synovium also contained VEGFR1 (red arrows) cells coexpressing the early HPC marker c-kit (J). These CD68+ cells expressed VEGF showing coexpression within the hemophilic synovium (K). Total number of sections stained for both experimental and control groups detailed in “HJD synovium expresses proangiogenic mediators and myeloid cells” (original magnification ×400).
Hemophilic synovium expresses proangiogenic mediators and myeloid cells. Immunohistochemical analyses using DAB revealed positive staining for proangiogenic mediators VEGF-A (A), MMP-9 (B), and SDF-1 (C), in hemophilic synovium but not control synovium (D) (original magnification ×200). Immunohistochemical staining for CD68 (E) and CD11b (F) provided evidence for the presence of myeloid cell infiltration not observed in control synovium (G). Quantitative assessment of CD68+ cells in hemophilic and control synovium demonstrated an increased percentage of CD68+ cells in hemophilic synovium (HJD) compared with control (CNTRLs) (H). Immunofluorescent staining (red arrow) demonstrates CD11b+ cells coexpressing VEGF-R1 (I) in hemophilic synovium. Hemophilic synovium also contained VEGFR1 (red arrows) cells coexpressing the early HPC marker c-kit (J). These CD68+ cells expressed VEGF showing coexpression within the hemophilic synovium (K). Total number of sections stained for both experimental and control groups detailed in “HJD synovium expresses proangiogenic mediators and myeloid cells” (original magnification ×400).
Angiogenic factors are up-regulated in the plasma of HJD subjects
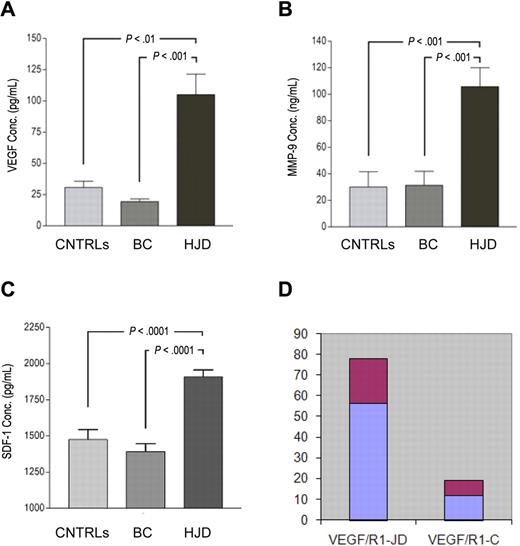
In addition to the observed up-regulation of VEGF-A, SDF-1, and MMP-9 in the synovium, we sought to determine their expression in the peripheral blood of experimental and control group A and B subjects. Experimental subjects had sustained a median of 10 (range, 6 to > 20) bleeds into a joint over a median of 7 years (range, 3-43 years) and 36% (FVIII deficiency 32% 3 times a week, FIX deficiency 4% twice a week) were being treated with prophylactic factor concentrates. The white blood cell counts, along with monocyte counts, were within normal limits at the time of these blood draws. Enzyme-linked immunosorbent assays performed on plasma from this prospective cohort of HJD subjects revealed 4-fold elevated levels of VEGF-A (n = 76), MMP-9 (n = 25), and SDF-1α (n = 46) compared with control group A (VEGF, n = 41; MMP-9, n = 28; SDF-1α, n = 17) and control group B subjects (VEGF-A, n = 26; MMP-9, n = 18; SDF-1α, n = 16) (Figure 2A-C). Notably, subjects with HJD and elevated VEGF-A levels had increased expression of VEGFR1 mRNA (Figure 2D).
Angiogenic mediators are elevated in the plasma of HJD subjects. Enzyme-linked immunosorbent assays were performed as per the manufacturer's protocol using anti VEGF-A, anti-MMP-9, anti-SDF-1 antibodies. (A) A 4-fold elevation of plasma VEGF-A was observed in HJD subjects (prospective cohort) compared with control group A (BC; P < .001) and control group B (CNTRLs; P < .01). (B) MMP-9 levels were also significantly elevated in HJD subjects (prospective cohort) compared with control group A (BC; P < .001) and control group B (CNTRLs; P < .001). (C) SDF-1 levels were also significantly elevated in HJD subjects compared with control group A (BC; P < .0001) and control group B (CNTRLs; P < .0001). (D) These subjects with elevated VEGF levels also had increased expression of VEGFR1 mRNA (VEGF/R1-JD) compared with control groups A and B grouped together (VEGF/R1-C; P < .05). Data are mean ± SEM.
Angiogenic mediators are elevated in the plasma of HJD subjects. Enzyme-linked immunosorbent assays were performed as per the manufacturer's protocol using anti VEGF-A, anti-MMP-9, anti-SDF-1 antibodies. (A) A 4-fold elevation of plasma VEGF-A was observed in HJD subjects (prospective cohort) compared with control group A (BC; P < .001) and control group B (CNTRLs; P < .01). (B) MMP-9 levels were also significantly elevated in HJD subjects (prospective cohort) compared with control group A (BC; P < .001) and control group B (CNTRLs; P < .001). (C) SDF-1 levels were also significantly elevated in HJD subjects compared with control group A (BC; P < .0001) and control group B (CNTRLs; P < .0001). (D) These subjects with elevated VEGF levels also had increased expression of VEGFR1 mRNA (VEGF/R1-JD) compared with control groups A and B grouped together (VEGF/R1-C; P < .05). Data are mean ± SEM.
In the retrospective HJD cohort (described in “Methods”), we observed that 10 subjects with early joint disease had a 10-fold increase in VEGF-A levels (mean ± SEM, 227.7 ± 33.4 pg/mL) compared with 13 subjects with advanced joint disease, who had levels of VEGF-A at 22.4 ± 1.2 pg/mL (n = 13, P < .001). These data suggested that neoangiogenesis in HJD might occur locally in the synovium with the presence of angiogenic mediators in the peripheral blood.
Circulating levels of HPCs expressing VEGFR1 and CXCR4 are increased in peripheral blood of HJD subjects
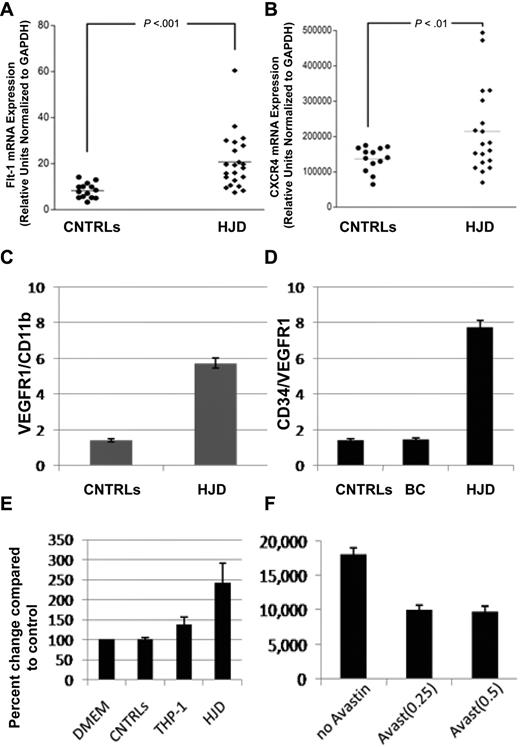
VEGF and SDF-1α, both observed in HJD synovium, exert their peripheral effects through their receptors VEGFR1, VEGFR2 (VEGF), and CXCR4 (SDF-1α), respectively.14,15,26 Using quantitative PCR, we observed that VEGFR1 (Figure 3A) and CXCR4 (Figure 3B) mRNA levels were increased in the peripheral blood of HJD subjects. Furthermore, a larger number of VEGFR1+/CD11b+ cells in the peripheral blood of 16 HJD subjects were observed on flow cytometry compared with 19 controls: control group A (n = 12) and control group B (n = 7) (Figure 3C). In addition, HPCs expressing CD34/VEGFR1, markers of early myeloid cells, were increased 4-fold in 48 HJD subjects compared with both control group A (n = 26) and control group B (n = 16; Figure 3D). Moreover, PBMCs from 17 HJD subjects stimulated a 2.5-fold increase in proliferation of normal human synovial cells (Figure 3E) that could be abrogated by bevacizumab (Avastin), a VEGF inhibitor (Figure 3F). These results provided data for association of immature HPCs as a possible source of localized proangiogenic growth factors eliciting synovial proliferation.
Circulating levels of HPCs expressing VEGFR1 and CXCR4 are increased in peripheral blood of HJD subjects. (A) mRNA from the peripheral blood of HJD subjects (prospective cohort as defined in “Subjects and samples”) show significant up-regulation of VEGFR1 mRNA (labeled Flt-1 mRNA, mouse equivalent of human VEGFR1; P < .001; A) and CXCR4 (P < .01; B) compared with controls (CNTRLs, control group A). Results represent data from 3 experiments. (C) Circulating HPCs (VEGFR1+/CD11b+) are elevated 4-fold in the peripheral blood of HJD subjects compared with controls by flow cytometry. (D) HPCs, which express CD34/VEGFR1-early myeloid cells, were also increased 4-fold in HJD subjects compared with control group A (BC) and control group B (CNTRLs) by flow cytometry. (E) PBMCs from subjects with HJD increased synovial cell proliferation by 2.5-fold compared with medium alone (DMEM) and control groups A and B (CNTRLs). THP-1 cells (immortalized cells of the monocyte/macrophage lineage used as a positive control) stimulated synovial proliferation up to 1.5-fold (THP-1). These results are expressed as a percentage change compared with the controls. (F) Synovial proliferation by PBMCs from HJD subjects (prospective cohort) could be abrogated by Avastin (Avast), an inhibitor of VEGF, by 50% using 2 different concentrations (0.25 mg/mL, 0.5 mg/mL) of the drug. These results are expressed as number of cells before and after treatment with Avastin.
Circulating levels of HPCs expressing VEGFR1 and CXCR4 are increased in peripheral blood of HJD subjects. (A) mRNA from the peripheral blood of HJD subjects (prospective cohort as defined in “Subjects and samples”) show significant up-regulation of VEGFR1 mRNA (labeled Flt-1 mRNA, mouse equivalent of human VEGFR1; P < .001; A) and CXCR4 (P < .01; B) compared with controls (CNTRLs, control group A). Results represent data from 3 experiments. (C) Circulating HPCs (VEGFR1+/CD11b+) are elevated 4-fold in the peripheral blood of HJD subjects compared with controls by flow cytometry. (D) HPCs, which express CD34/VEGFR1-early myeloid cells, were also increased 4-fold in HJD subjects compared with control group A (BC) and control group B (CNTRLs) by flow cytometry. (E) PBMCs from subjects with HJD increased synovial cell proliferation by 2.5-fold compared with medium alone (DMEM) and control groups A and B (CNTRLs). THP-1 cells (immortalized cells of the monocyte/macrophage lineage used as a positive control) stimulated synovial proliferation up to 1.5-fold (THP-1). These results are expressed as a percentage change compared with the controls. (F) Synovial proliferation by PBMCs from HJD subjects (prospective cohort) could be abrogated by Avastin (Avast), an inhibitor of VEGF, by 50% using 2 different concentrations (0.25 mg/mL, 0.5 mg/mL) of the drug. These results are expressed as number of cells before and after treatment with Avastin.
Endothelialization occurs in HJD synovium
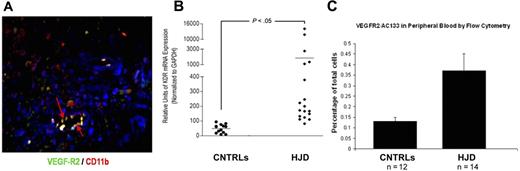
Endothelialization, as occurs in neoangiogenesis with recruitment of BM-derived HPCs and EPCs, is a vital process during tumor growth and progression.27,28 HJD synovium, known to be highly vascularized, expressed VEGFR2, an EPC marker (Figure 4A). VEGFR2 mRNA levels were increased in the peripheral blood of HJD subjects compared with controls (control groups A and B) as quantified by quantitative PCR (Figure 4B). Furthermore, we observed increased VEGFR2+/AC133+ early EPCs in the peripheral blood of 14 HJD subjects (P < .01), compared with 12 controls (7 control group A and 5 control group B) by flow cytometry (Figure 4C). Taken together, these results provided evidence for endothelialization and proangiogenic cell mobilization in HJD subjects with synovitis.
Endothelialization occurs in HJD synovium. (A) HJD synovium coexpressed VEGFR2/CD 11b (indicated by yellow staining with red arrows), late EPC markers. (B) VEGFR2 mRNA (labeled kdr, mouse equivalent of human VEGFR2) levels as quantified by quantitative PCR were increased in the peripheral blood of HJD subjects compared with control groups A and B (CNTRLs). (C) Significantly increased numbers of VEGFR2+/AC133+ early EPCs were also observed in the peripheral blood of HJD subjects compared with control groups A and B (CNTRLs). These data are expressed as a percentage of total peripheral blood mononuclear cells analyzed by flow cytometry.
Endothelialization occurs in HJD synovium. (A) HJD synovium coexpressed VEGFR2/CD 11b (indicated by yellow staining with red arrows), late EPC markers. (B) VEGFR2 mRNA (labeled kdr, mouse equivalent of human VEGFR2) levels as quantified by quantitative PCR were increased in the peripheral blood of HJD subjects compared with control groups A and B (CNTRLs). (C) Significantly increased numbers of VEGFR2+/AC133+ early EPCs were also observed in the peripheral blood of HJD subjects compared with control groups A and B (CNTRLs). These data are expressed as a percentage of total peripheral blood mononuclear cells analyzed by flow cytometry.
Hemophilic plasma elicits an angiogenic response from ECs that is VEGF-dependent
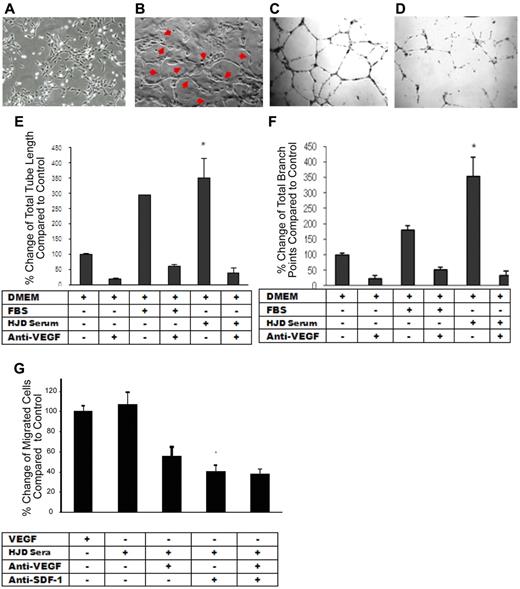
After observing that proangiogenic mediators are up-regulated in the plasma of HJD subjects, we speculated whether these mediators might aid in maintaining increased vascularity in the HJD synovium. We hypothesized that the plasma from HJD subjects may contain angiogenic stimuli to promote neoangiogenesis in the synovium. In a functional in vitro assay,29 the plasma from 18 HJD subjects, unlike 12 control group A subjects, elicited a marked angiogenic response from ECs. The morphologic changes of capillary-like tubular structure formation, cord-like processes connecting cellular nodes, increased tube length, and branchpoints of tubular nodes demonstrated the angiogenic capacity of HJD plasma (Figure 5C,E,F). Moreover, the increased total tube length and the number of branch points of tubular nodes could be almost completely abrogated by a VEGF-blocking peptide (P < .001), suggesting that this process was VEGF-dependent (Figure 5D-F).
HJD plasma elicits an angiogenic response from ECs that is VEGF-dependent. (A) Endothelial cells incubated with control group A sera for 22 hours showing normal morphology. (B) Endothelial cells incubated with HJD sera for 22 hours revealing the presence of “tube formation” representing blood vessels as indicated by the red arrows. Results replicated 4 times and represent data from 1 experiment. Human umbilical vein endothelial cells grown in DMEM culture medium (10 000/well) were plated on Matrigel-coated 48-well plates with (D) or without (C) preincubation with anti-VEGF antibody (10 μg/mL) and with or without FBS or HJD sera. Images reflected tube formation after 16-hour incubation detected by phase-contrast microscopy (original magnification ×40). Representative results from 4 experiments. Quantitative assessment of mean and total tube lengths and branch points using Metamorph software Version 40002 (Molecular Devices) revealed an 84% reduction in total tube length (SEM). *P < .001. (E) An 82% reduction in mean (SEM) tube length (data not shown). P < .001. A 93% reduction in mean (SEM) branch points. P < .001. (F) Representative results from 3 experiments. Migration across a transwell barrier was induced by VEGF and HJD serum and abrogated by anti-VEGF and anti–SDF-1 antibody. When human umbilical vein endothelial cells were plated on 24-well plates with or without VEGF, HJD serum, anti-VEGF blocking peptide, or anti-SDF-1 antibody, a 55% reduction in human umbilical vein endothelial cell migration with anti-VEGF antibody and a 60% reduction with anti-SDF-1 antibody (P < .01) was observed. There was no additive effect of anti-VEGF and anti–SDF-1 antibodies. (G) Results are expressed as a percent of the control, which is human umbilical vein endothelial cells grown in the presence of VEGF. Representative results from 3 experiments.
HJD plasma elicits an angiogenic response from ECs that is VEGF-dependent. (A) Endothelial cells incubated with control group A sera for 22 hours showing normal morphology. (B) Endothelial cells incubated with HJD sera for 22 hours revealing the presence of “tube formation” representing blood vessels as indicated by the red arrows. Results replicated 4 times and represent data from 1 experiment. Human umbilical vein endothelial cells grown in DMEM culture medium (10 000/well) were plated on Matrigel-coated 48-well plates with (D) or without (C) preincubation with anti-VEGF antibody (10 μg/mL) and with or without FBS or HJD sera. Images reflected tube formation after 16-hour incubation detected by phase-contrast microscopy (original magnification ×40). Representative results from 4 experiments. Quantitative assessment of mean and total tube lengths and branch points using Metamorph software Version 40002 (Molecular Devices) revealed an 84% reduction in total tube length (SEM). *P < .001. (E) An 82% reduction in mean (SEM) tube length (data not shown). P < .001. A 93% reduction in mean (SEM) branch points. P < .001. (F) Representative results from 3 experiments. Migration across a transwell barrier was induced by VEGF and HJD serum and abrogated by anti-VEGF and anti–SDF-1 antibody. When human umbilical vein endothelial cells were plated on 24-well plates with or without VEGF, HJD serum, anti-VEGF blocking peptide, or anti-SDF-1 antibody, a 55% reduction in human umbilical vein endothelial cell migration with anti-VEGF antibody and a 60% reduction with anti-SDF-1 antibody (P < .01) was observed. There was no additive effect of anti-VEGF and anti–SDF-1 antibodies. (G) Results are expressed as a percent of the control, which is human umbilical vein endothelial cells grown in the presence of VEGF. Representative results from 3 experiments.
Migration of ECs is VEGF-A– and SDF-1α–dependent
Previous studies have reported that neoangiogenesis requires migration of ECs.30 As we observed increased expression of the proangiogenic mediators VEGF-A and SDF-1α in the synovium and peripheral blood of HJD subjects, we reasoned that these molecules may be critical for proangiogenic cell recruitment and migration. Chemotaxis of ECs was increased in response to HJD plasma. A VEGF-blocking peptide decreased chemotaxis by 50% (± 2.6%, SEM), whereas an anti-SDF-1α antibody reduced it by 60% (± 3.4%, SEM, P < .01). Interestingly, there was no additive effect of a combined blockade of VEGF and SDF-1α (Figure 5G), suggesting that both VEGF and SDF-1α act through a common pathway to promote endothelial migration.
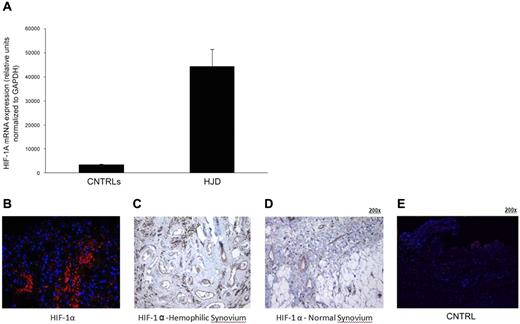
Sera from HJD subjects induce expression of HIF-1α–in synovial cells
Hypoxia has been shown to induce angiogenic mediators.31 To determine the angiogenic trigger in the HJD synovium, we decided to investigate the role of HIF-1α previously shown to induce SDF-1α during progenitor cell trafficking,31 in the angiogenic process. Human synovial cells were incubated with sera from 16 HJD or 14 (8 group A and 6 group B) control subjects for 6 hours. After the incubation, mRNA was extracted and subjected to quantitative PCR analysis. We observed that there was a significant up-regulation in the mRNA signal for HIF-1α from synovial cells incubated with serum from HJD subjects (Figure 6A). HIF-1α was overexpressed in HJD synovium (n = 8) compared with control synovium (n = 6; Figure 6B-E). These data indicated that HJD sera could potentially stimulate synovial cells to produce HIF-1α and mobilize HPCs and EPCs to the synovium.
Sera from HJD subjects induce expression of HIF-1α in synovial cells. (A) Synovial cells were incubated with HJD serum and serum from control groups A and B for 10, 30, and 60 minutes, after which mRNA was extracted and subjected to quantitative PCR. (A) Significantly increased mRNA expression for HIF-1α was observed at 60 minutes in cells incubated with HJD serum compared with control serum (CNTRLs; P < .0001). No significant difference was observed at the 10- or 30-minute time points. Results represent data from 3 independent experiments. (B) Immunofluorescence of hemophilic synovium expressing HIF-1α. Immunofluorescence using a monoclononal antibody against HIF-1α demonstrated abundant expression of HIF-1α in hemophilic synovium in the endothelial cells and lining cells (B) but not in control synovium (E). Similarly, immunohistochemical staining using DAB confirmed HIF-1α positivity in similar regions in hemophilic synovium (C, original magnification ×40) and expression of HIF-1α only in endothelial cells in normal synovium (D, original magnification ×40). Results were replicated in 8 different hemophilic samples and 6 control samples and represent data from 1 set of samples.
Sera from HJD subjects induce expression of HIF-1α in synovial cells. (A) Synovial cells were incubated with HJD serum and serum from control groups A and B for 10, 30, and 60 minutes, after which mRNA was extracted and subjected to quantitative PCR. (A) Significantly increased mRNA expression for HIF-1α was observed at 60 minutes in cells incubated with HJD serum compared with control serum (CNTRLs; P < .0001). No significant difference was observed at the 10- or 30-minute time points. Results represent data from 3 independent experiments. (B) Immunofluorescence of hemophilic synovium expressing HIF-1α. Immunofluorescence using a monoclononal antibody against HIF-1α demonstrated abundant expression of HIF-1α in hemophilic synovium in the endothelial cells and lining cells (B) but not in control synovium (E). Similarly, immunohistochemical staining using DAB confirmed HIF-1α positivity in similar regions in hemophilic synovium (C, original magnification ×40) and expression of HIF-1α only in endothelial cells in normal synovium (D, original magnification ×40). Results were replicated in 8 different hemophilic samples and 6 control samples and represent data from 1 set of samples.
Discussion
HJD secondary to recurrent hemarthroses is composed of synovial neovascularization and bony arthropathy.2-4 Previous work has suggested the role of iron and cytokines in the pathogenesis of HJD.7-9 Observing that the hemophilic synovium is not only hyperproliferative but also highly vascular,2,3 we embarked on a study to examine human blood and synovium from subjects with HJD, hypothesizing that angiogenic mediators may play a role in the pathogenesis of HJD.
In the present study, we observed that angiogenic mediators were expressed in the peripheral blood and synovium of pediatric and adult subjects with hemophilia. Our results suggest that cells of early and late myeloid lineage produced by, or acting in concert with, macrophages/monocytes of hematopoietic progenitor cell origin in HJD subjects could play a pathogenetic role in the development of hemophilic synovitis. Early pathologic features of hemophilia have been well elucidated in both hemophilic dogs32 and in an experimental murine model of hemophilic synovitis.20 Fourteen days after a single massive hemarthroses, vascular synovial hyperplasia with inflammatory cell infiltrate and irregularity of cartilage and pannus formation were observed.20 However, the signals responsible for synovial proliferation and hypertrophy are not well understood, and no large systematic studies have been carried out in patients with hemophilia. Other investigators have reported iron-induced abrogation of synovial apoptosis via MDM2 up-regulation in vitro in the pathophysiology of HJD.11 We postulated that the introduction of blood components, including endothelial cells capable of producing cytokines and angiogenic growth factors, may disrupt the homeostatic balance, thus favoring villous hypertrophy and increased vascularity.
Our data confirm the vascular nature of human hemophilic synovium and observe the up-regulation of proangiogenic mediators VEGF-A, MMP-9, and SDF-1α, as well as the transcription factor HIF-1α in hemophilic synovitis. Moreover, we observed that VEGF-A colocalized with CD68, a well-characterized macrophage marker in humans.33 It is unclear whether these are resident macrophages, type A synoviocytes,34 which were activated in response to hemarthroses, or recruited monocytes/macrophages from the BM, or a combination of all 3. Furthermore, when we incubated normal human synovial cells (which display fibroblast-like morphology, presumably type B synoviocytes) with PBMCs, we observed that PBMCs containing BM-derived HPCs and EPCs from HJD subjects, but not control subjects, stimulated synovial proliferation that could be abrogated by a VEGF inhibitor. Thus, macrophages and HPCs, mobilized from the BM, may be a potential source of VEGF in the synovium. These data further suggest that the hemophilic synovium has undergone alteration of the microenvironment with alteration of the extracellular matrix, release of chemoattractants, leading to neovascularization. The increased expression of both hematopoietic (CD34+/VEGFR1+) and endothelial progenitor (VEGFR2+/AC133+) cells in the peripheral blood of HJD subjects suggests that these BM-derived cells may be recruited to the hemophilic synovium by HIF-1α to stimulate neoangiogenesis. HIF-1α is a transcription factor induced by hypoxia, previously shown to be involved in progenitor cell trafficking.31 Hypoxic conditions are possibly created in the joint after the initial bleed followed by synovial proliferation stimulated by iron or other mediators, consequently contributing to neoangiogenesis. The subsequent local and systemic mobilization of proangiogenic mediators in response to hypoxia could promote and maintain neovascularization in the hemophilic joint.
We found evidence of both localized (synovial) and systemic (peripheral blood) mediators of neoangiogenesis, suggesting a mechanism for the development of a highly vascularized joint and leading to persistent joint damage. Subjects with inherited bleeding disorders other than hemophilia typically bleed into the skin, mucus membranes, and muscles, whereas hemophilic subjects tend to bleed into their knees, ankles, and elbows. The findings of elevated levels of VEGF-A in the peripheral blood of subjects with HJD and increased expression of VEGF-A in the synovium suggested that this elevation of VEGF-A levels in HJD subjects may be specific to the pathogenesis of joint arthropathy. In other arthritides, including osteoarthritis and rheumatoid arthritis, serum VEGF levels have been directly correlated with disease activity.18 Unfortunately, because of limitations of synovium sample procurement, we could not demonstrate mRNA expression of these angiogenic mediators in the synovium, which may have further strengthened evidence for a VEGF-induced cascade leading to synovitis.
Measurement of serum VEGF levels has proven useful in evaluating the disease severity, more rapid disease progression,35 and response to treatment in both rat collagen-induced arthritis and patients with ovarian cancer.36,37 In HJD subjects, elevated VEGF-A levels as well as levels of circulating hematopoietic and endothelial progenitor cells may signify the onset and progression of synovitis. In support of this hypothesis, we observed a 10-fold increase in plasma VEGF-A levels in a retrospective cohort of hemophilic subjects with active synovitis diagnosed by MRI, compared with subjects with advanced joint arthropathy. Interestingly, in this retrospective cohort, subjects with joint arthropathy demonstrated VEGF-A levels similar to control subjects (data not shown). This finding suggests that VEGF-A levels could rise during the phase of active synovitis playing a role in synovitis initiation and progression, and fall after progression to chronic synovitis, characterized by a predominance of fibrous tissue and decreased vascularity. Therefore, VEGF-A levels along with the presence of circulating HPCs and EPCs may serve as surrogate biologic markers for active synovitis. As our results have demonstrated the blockade of VEGF-induced synovial cell proliferation, VEGF inhibition may also be an effective treatment strategy in preventing synovial proliferation.
Apart from VEGF-A, levels of MMP-9 and SDF-1α were also up-regulated 4-fold in HJD subjects (prospective cohort). SDF-1 binding to CXCR4 triggers chemotaxis, and the up-regulation of the cell surface integrins and MMPs (eg, MMP-9) is necessary for cell migration and trafficking.38 Moreover, increased MMP-9 activity has also been reported to mediate SDF-1α–stimulated transendothelial migration of hematopoietic cell types.39 We hypothesized that SDF-1α and VEGF-A may play similar roles in progenitor cell recruitment into the hemophilic synovium. HJD sera could induce endothelial cell migration and tube formation, which was almost completely abrogated by a VEGF-blocking peptide (Figure 5). Likewise, transwell migration of endothelial cells was stimulated by HJD sera and inhibited 50% to 60% by antibodies to VEGF-A and SDF-1α (Figure 5). These data provided further functional evidence for the role of proangiogenic factors in the pathogenesis of HJD. In addition, it suggested that blockade of local chemokine signaling may abrogate the cellular infiltration causing hypervascularity characteristic of hemophilic synovitis.
The exact mechanisms by which bleeding into the joint leads to changes within the synovium have not been well elucidated. Here we have shown that hypoxia induced by recurrent hemarthroses in hemophilia through synovial proliferation potentially induces synovial angiogenesis and infiltration of BMD cells of monocyte/macrophage origin in hemophilic synovitis. Elevated levels of angiogenic mediators in hemophilic subjects could signify the presence of active synovitis, and our observations suggest that neoangiogenesis mediated by BMD-cell recruitment and infiltration could play a pathogenetic role in hemophilic synovitis. Future prospective studies that use an angiogenic profile (including VEGF-A, SDF-1, MMP-9, and progenitor cells) may help better delineate the temporal and etiologic role of angiogenesis in hemophilic synovitis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Scott Kerns for help with imaging and graphics, Dr Edward DiCarlo for synovium samples, Simon Meas for technical support with the flow cytometry, and all our study subjects for enrolling in the study.
This work was supported by Hemophilia of Georgia Foundation (S.S.A.), Doris Duke Charitable Foundation, Hope Street Kids, ASCO, Butler Foundation, Michael Cuccione Foundation (R.N.K.); National Foundation for Cancer Research, Hartwell Foundation, Malcolm Hewitt Wiener Foundation, Nancy C. and Daniel P. Paduano Foundation, American Hellenic Educational Progressive Foundation, Charles and Meryl Witmer Family Foundation, Stavros S. Niarchos Foundation, and Champalimaud Foundation (D.L.); and Susan G. Komen for the Cure (R.N.K., D.L.), Children's Cancer and Blood Foundation Laboratories, and the A. P. Alexander Family donation for hemophilia research.
Authorship
Contribution: S.S.A. and R.N.K. designed the research and wrote and edited the manuscript; D.M. performed the flow studies, immunofluorescence, and immunohistochemistry; O.T.F. performed the quantitative PCR, migration, and proliferation studies; D.L. helped plan and edit the paper; and D.D. helped in clinical data correlation and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suchitra S. Acharya, Cohen Children's Medical Center of New York, Pediatric Hematology/Oncology/BMT, 269-01 76th Ave, Suite 255, New Hyde Park, NY 11040; e-mail: sacharya@nshs.edu.
References
Author notes
S.S.A. and R.N.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal