Abstract
Retention of poorly deformable red blood cells (RBCs) by the human spleen has been recognized as a critical determinant of pathogenesis in hereditary spherocytosis, malaria, and other RBC disorders. Using an ex vivo perfusion system, we had previously shown that retention of Plasmodium falciparum–infected RBCs (Pf-RBCs) occur in the splenic red pulp, upstream from the sinus wall. To experimentally replicate the mechanical sensing of RBCs by the splenic microcirculation, we designed a sorting device where a mixture of 5- to 25-μm-diameter microbeads mimics the geometry of narrow and short interendothelial splenic slits. Heated RBCs, Pf-RBCs, and RBCs from patients with hereditary spherocytosis were retained in the microbead layer, without hemolysis. The retention rates of Pf-RBCs were similar in microbeads and in isolated perfused human spleens. These in vitro results directly confirm the importance of the mechanical sensing of RBCs by the human spleen. In addition, rigid and deformable RBC subpopulations could be separated and characterized at the molecular level, and the device was used to deplete a stored RBC population from its subpopulation of rigid RBCs. This experimental approach may contribute to a better understanding of the role of the spleen in the pathogenesis of inherited and acquired RBC disorders.
Introduction
Retention of poorly deformable red blood cells (RBCs) by the human spleen has been recognized as a critical determinant of pathogenesis of several RBC disorders, including hereditary spherocytosis and malaria. The severity of hereditary spherocytosis (HS), a syndrome resulting from mutations of RBC membrane proteins,1 is related to the extent of decreased membrane surface area. Splenic retention is the dominant mechanism responsible for reduced life span of HS-RBCs, such that splenectomy is beneficial in reducing anemia in symptomatic HS patients.1 Changes of RBC mechanical properties have also been observed in blood stored for 3 weeks or longer.2 Prolonged storage influences the survival of RBCs after transfusion3 and probably contributes to transfusion-related side effects, including respiratory distress and systemic sepsis.3-5 Not least, the deformability of Plasmodium falciparum–infected red blood cells (Pf-RBCs) is also central in malaria pathogenesis. It progressively decreases as P falciparum matures in its host RBCs, from rings (ring-RBCs)6 to trophozoites and then to schizonts.7,8 When mature stages are sequestered in small vessels, they escape retention in the spleen. Ring-RBCs only rarely adhere to endothelial cells9 and are found in the peripheral blood circulation.10,11 We recently showed that a fraction of ring-RBCs is retained in isolated-perfused human spleens.12 Ring-RBCs accumulated upstream from interendothelial slits, a spleen-specific microanatomic structure that retains RBCs with reduced deformability (rigid-RBCs).13-15
In brief, in malaria as in several other RBC disorders, extensive RBC retention in the spleen is associated with anemia,1,16,17 whereas impaired retention potentially favors adverse events, such as acute microvascular obstruction in malaria patients,18,19 late vascular disorders in splenectomized patients with HS or thalassemia,20,21 and acute organ dysfunction in critically ill patients transfused with “older” blood.4 Exploration of how the human spleen senses poorly deformable RBCs would be facilitated by a physiologically relevant in vitro approach simpler than the ex vivo spleen perfusion system. To experimentally replicate the mechanical sensing of RBC by the splenic microcirculation, we first analyzed the fine structure of interendothelial slits in the human spleen. We realized that the geometry of narrow and short interendothelial slits would be accurately mimicked by spaces between microbeads. We then allowed RBCs to flow through a mixture of 5- to 25-μm-diameter microbeads. Heated RBCs, Pf-RBCs, and RBCs from patients with HS were retained in the microbead layer without hemolysis. The retention rates of Pf-RBCs were similar in microbeads and in isolated-perfused human spleens. These in vitro results directly confirmed the importance of the mechanical sensing of RBCs by the human spleen. In addition, rigid and deformable RBC subpopulations could be separated and characterized at the molecular level, and the device could be used to deplete a stored RBC population from its rigid-RBC subpopulation.
Methods
Microbeads and sorting process
Calibrated metal microbeads (96.50% tin, 3.00% silver, and 0.50% copper; Industrie des poudres Sphériques) with 2 different size distributions (5- to 15-μm-diameter and 15- to 25-μm-diameter) each from a single batch were used throughout. Particle shape and size are controlled by the manufacturer through visual observation with a microscope at a set magnification and by image analyzer to define the percentage of spherical or elliptical particles, and to guarantee that more than 90% of beads are in the specified size range.
A total of 2 g of dry microbeads of each sort was mixed and then suspended in 8 mL of phosphate-buffered saline (PBS)/1% AlbuMAX II (Invitrogen). A total of 600 μL of this bead suspension was poured into an inverted 1000-μL antiaerosol pipette tip (Neptune, BarrierTips) and allowed to settle, leading to the formation of a 5-mm-thick bead layer above the antiaerosol filter. A total of 600 μL of a 2% hematocrit RBC suspension containing less than 10% of potentially “retainable” RBCs (to avoid bead saturation) was introduced upstream from the microbead layer. RBCs were perfused through the bead layer at a flow rate of 60 mL/h using an electric pump (Syramed μsp6000, Arcomed'Ag). The bead layer was then washed with 8 mL of PBS/1% AlbuMAX II. The downstream sample was retrieved. RBCs retained in the bead layer at the end of the entire procedure (filtration and washing steps) were separated from the microbeads by 3 successive settling steps. The concentration of heated, HS, or parasitized RBCs in upstream, downstream, and retained RBC samples was determined by examination of Giemsa-stained blood films or counting the percentage of (PK Horan)–labeled RBCs in cell suspensions. In a control experiment, Pf-iRBCs were allowed to flow through 24-μm-thick polycarbonate membranes perforated with 2- or 3-μm-wide channels (Sterlitech).
Measurement of RBC elongation index
RBC and iRBC elongation index was measured over a range of shear stresses (0.3-30 Pa) by ektacytometry using a laser-assisted optical rotational cell analyzer (LORCA; Mechatronics) as previously described.12 The extent of RBC deformability, namely, the elongation index, was defined as the ratio between the difference between the 2 axes of the ellipsoid diffraction pattern and the sum of these 2 axes.
Human spleen retrieval and ex vivo spleen perfusion
Human spleens were retrieved from patients undergoing left spleno-pancreatectomy for pancreatic diseases and processed as reported previously22 and briefly described in the next sentences. Medical and surgical care was not modified, and written consent was obtained from the patient. The project was approved by the Ile-de-France II Investigational Review Board. On removal of the organ, the main splenic artery was cannulated; the spleen was flushed with cold Krebs-albumin solution for transport to our laboratory. Once in the laboratory, the spleen was connected to the perfusion device, and the patient's RBCs were flushed from the spleen. RBCs with modified deformability were introduced into the circulation system. During perfusion, key physiologic markers were maintained at normal levels. The spleen effluents were sampled at different time points during perfusion.
Parasite culture
The FCR3 strain (FUP/CB line) of P falciparum was used throughout the experiments. Parasites were cultured using the method described by Trager and Jensen23 with the modifications described by Ralph et al.24 Cultures were tightly synchronized by lysing maturing forms through treatment with 0.3M alanine at 2 successive cycles, starting 3 hours after ring-RBCs appeared in the culture.24 Parasites were harvested at different stages of development (ring-RBCs, 0-8 hours and 8-16 hours; trophozoites, 16-30 hours; and schizonts, 30-48 hours).
Clinical isolates
Clinical isolates were derived from patients' blood samples obtained for diagnosis purposes. Use of clinical samples was approved by the Ile-de-France VI Institutional Review Board. One part of each sample was perfused through the microbeads and another was cultured for one parasite cycle, allowing reinvasion to occur. Cultured clinical isolates were then perfused through microbeads.
RBC suspensions and PKH67 labeling
A total of 200 μL of packed RBCs was washed twice in RPMI 1640. Approximately 2 × 109 washed cells were stained with PKH67 (Sigma-Aldrich) according to the manufacturer's instructions. In brief, after the removal of the RPMI medium, the cells were then suspended in 1.8 mL of diluent C (PKH67 Fluorescent Cell Linker Kit; Sigma-Aldrich), immediately mixed with 2 mL of a 1/500 dilution of PKH67 stock solution in diluent C, and gently rocked at room temperature for 1.5 minutes. The cell suspension was then gently rocked for 1 minute, after which 4 mL of RPMI 2% AlbuMAX II (Invitrogen) was added. RBCs were pelleted, transferred to a fresh centrifuge tube, and washed twice in PBS/1% Albumax II. Labeled RBCs were diluted 1:10 with unlabeled RBCs. A 2% hematocrit RBC suspension in PBS/1% AlbuMAX II was prepared with this cell mixture.
Heated RBCs
RBCs were heated at 50°C during 20 minutes after which they were PKH67-labeled. Labeled RBCs were mixed 1:10 with control unlabeled RBCs. A 2% hematocrit RBC suspension in PBS/1% AlbuMAX II was prepared with this cell mixture.
Hereditary spherocytosis RBCs
RBCs from hereditary spherocytosis patients were obtained in the context of patient follow-up as approved by the Ile-de-France VI Institutional Review Board. RBCs were either studied by LORCA or labeled with PKH67 and then mixed 1:10 with normal unlabeled RBCs. A 2% hematocrit RBC suspension in PBS/1% AlbuMAX II was prepared with this cell mixture and then perfused through the microbeads as described.
Lactate dehydrogenase measurement
Lactate dehydrogenase was quantified using Dimension (Dade Behring, clinical chemistry system) according to the manufacturer's instructions.
Western blot analysis
A total of 120 μL of RBCs from a culture of VarO-expressing P falciparum parasites (VarO) or parasites expressing another member of the PfEMP1 family (FUP/CB) were perfused through microbeads (this required 20 sorting devices). RBCs were harvested, washed 3 times with PBS, pH 7.4, and then pelleted by centrifugation (1000g for 10 minutes at room temperature). Pellets were lysed in 20 volumes of PBS/1% Triton X-100 with protease inhibitors (Roche Diagnostics) for 30 minutes at 4°C followed by a centrifugation step (1000g for 30 minutes at 4°C). Pellets were resuspended in 4 times sample buffer (Bio-Rad). The electrophoresis of samples using reducing 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel precast Bio-Rad was followed by transfer onto nitrocellulose. The membrane was blocked with 5% skimmed milk/PBS, pH 7.4, and probed at 37°C for 1 hour with anti-Var0 mouse antiserum at a 1/100 dilution in 5% skimmed milk/0.05% Tween 20, followed by 1 hour with alkaline phosphatase-conjugated antimouse IgG secondary antibodies (Promega) at a 1:5000 dilution. Pf-EMP1 VarO band was visualized using nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt as chromogenic substrates.
Depletion of rigid RBCs from stored blood
Leukocyte-depleted blood was stored at 4°C during 42 days under the same conditions as done in blood banks, in storage medium (0.87% sodium chloride, 0.017% adenine, 0.9% dextrose monohydrate, 0.52% mannitol) in blood bank bags as provided by the Institut National de la Transfusion Sanguine.3 The elongation index (Lorca) of the RBC population and its retention rate in the microbeads were determined at weekly intervals during storage as described (“Heated RBCs”). Fresh blood (collected on the day of each experiment) was used as a control. To facilitate comparison with clinical data, data from weekly measurement during the 3 first and the 3 last weeks of the 6-week storage period were pooled.
Light microscopy
Pictures were acquired on a Nikon E800 microscope, using Nikon digital still DXM1200 camera controlled by ACT-1 Version 2 Nikon software. Images were visualized with a Plan Apo 40×/0.95 DiCM or a Plan Apo 100×/1.40 oil-immersion DiCM objective lens (Nikon).
Electronic microscopy
Samples were processed as described,12 then examined and photographed with a JEOL JSM 6700F field emission scanning electron microscope operating at 5kv or 7kv (images were acquired with the upper SE dector on the lower secondary dector), or with a JEOL 1200 EX electron microscope operating at 80 kv.
Results
The geometry of interendothelial slits is mimicked by interbead spaces
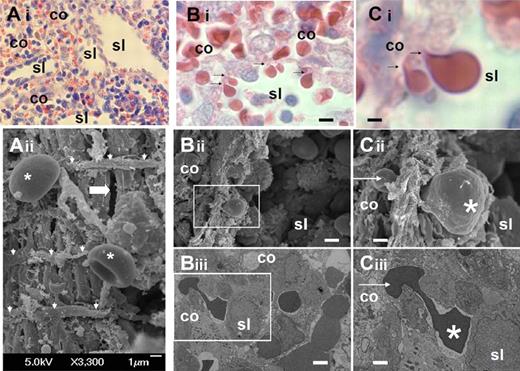
We analyzed the structure of the sinus wall in the human spleen red pulp (Figure 1A). Samples were collected from ex vivo perfusion experiments12,22 and processed at the end of the perfusion for histologic analysis and transmission or scanning electron microscopy. This confirmed the marked squeezing of RBCs (Figure 1C) when they cross the sinus wall from the cords to the sinus lumen (Figure 1B).
Fine structure of interendothelial slits in the sinus wall of the human splenic red pulp. (Ai) Reticular meshwork in the human spleen red pulp made of cords (co) and sinus lumens (sl) on a Giemsa-stained histologic section (original magnification ×200). (Aii) Typical scanning electron micrograph of the abluminal side of a sinus wall in the red pulp, with periodic helical basal fibers (white arrowheads), normal RBC (stars), and a narrow interendothelial slit (horizontal thick arrow). (B) Squeezing of RBCs crossing an interendothelial slit, from the cord (co) to the sinus lumen (sl) on a Giemsa-stained histologic section (original magnification ×1000): (i black arrows), scanning (ii, white square), or transmission (iii, white square) electron micrograph of a human spleen. (C) Same pictures at original magnification ×4000. On electron micrographs (ii-iii), one part of the RBC (star) has emerged in the sinus lumen, although the other is still in the process of leaving the cord (white arrow). This illustrates that the interendothelial slits are not long enough (typically < 3.5 μm) to accommodate the whole elongated RBCs as they squeeze through. Bi-iii: bar represents 4 μm; Ci-iii: bar represents 1 μm.
Fine structure of interendothelial slits in the sinus wall of the human splenic red pulp. (Ai) Reticular meshwork in the human spleen red pulp made of cords (co) and sinus lumens (sl) on a Giemsa-stained histologic section (original magnification ×200). (Aii) Typical scanning electron micrograph of the abluminal side of a sinus wall in the red pulp, with periodic helical basal fibers (white arrowheads), normal RBC (stars), and a narrow interendothelial slit (horizontal thick arrow). (B) Squeezing of RBCs crossing an interendothelial slit, from the cord (co) to the sinus lumen (sl) on a Giemsa-stained histologic section (original magnification ×1000): (i black arrows), scanning (ii, white square), or transmission (iii, white square) electron micrograph of a human spleen. (C) Same pictures at original magnification ×4000. On electron micrographs (ii-iii), one part of the RBC (star) has emerged in the sinus lumen, although the other is still in the process of leaving the cord (white arrow). This illustrates that the interendothelial slits are not long enough (typically < 3.5 μm) to accommodate the whole elongated RBCs as they squeeze through. Bi-iii: bar represents 4 μm; Ci-iii: bar represents 1 μm.
The dimensions of interendothelial slits were then determined (as shown in Figure 2A) from 11 transmission electron microscopy pictures. Mean length (SD, range) and width measured were 1.89 μm (0.9, 0.9-3.2 μm) and 0.65 μm (0.5, 0.25-1.2 μm), respectively. These specific geometric characteristics could be recapitulated by 3 adjacent 5-μm beads, which generate a less than 1.85-μm-wide aperture, altogether shorter and narrower than the narrowest polycarbonate or microfluidic channels through which RBCs can flow without hemolysis (ie, 3-μm-wide; Figure 2Bi-iii). We purchased mixtures of 5- to 25-μm beads to generate narrow apertures (when 3 small microbeads are adjacent) interspersed with wider apertures (Figure 2Biv). Fixation followed by microscopic examination of RBCs in the process of crossing the microbead layer showed shape deformations closely reminiscent to that observed in human spleens (Figure 2Bi,iii).
Size and geometry of the interendothelial slit and resulting RBC deformation. (Ai) Transmission electron micrograph (original magnification ×2000) of RBCs squeezing through an interendothelial slit (ie, between 2 adjacent endothelial cells [EC], from the cord [Co] to the sinus lumen [SL], white star showing the anterior pole of the RBCs). (Aii) Width (red double-headed arrow) and length (black double-headed arrow) of the interendothelial slit at high original magnification (×5000) as measured on 11 pictures. (B) Dumbbell-shaped RBCs as observed on a transmission electron micrograph (Bi, white star showing the anterior pole of the RBCs), or as expected to occur as a RBCs squeeze between microbeads (Bii), or as observed under the contrast-phase microscope after formaldehyde fixation of RBCs engaged within the microbead layer (Biii). (Biv) Schematic flow routes and shape deformation of RBCs squeezing through a narrow and short interbead space interspersed in wider spaces (left route), or only through wide spaces (right route). (Bv) Schematic longitudinal elongation of RBCs flowing though a narrow and long channel in a polycarbonate sieve. (C) Morphologic aspect of RBCs on Giemsa-stained smears showing either a normal discoid aspect downstream from the microbead layer (Ci), or dacryocytes (vertical arrows), schizocytes (horizontal arrows), poikilocytes (stars), and anisocytosis downstream from a narrow, 2-μm-wide, 24-μm-long channels (Cii).
Size and geometry of the interendothelial slit and resulting RBC deformation. (Ai) Transmission electron micrograph (original magnification ×2000) of RBCs squeezing through an interendothelial slit (ie, between 2 adjacent endothelial cells [EC], from the cord [Co] to the sinus lumen [SL], white star showing the anterior pole of the RBCs). (Aii) Width (red double-headed arrow) and length (black double-headed arrow) of the interendothelial slit at high original magnification (×5000) as measured on 11 pictures. (B) Dumbbell-shaped RBCs as observed on a transmission electron micrograph (Bi, white star showing the anterior pole of the RBCs), or as expected to occur as a RBCs squeeze between microbeads (Bii), or as observed under the contrast-phase microscope after formaldehyde fixation of RBCs engaged within the microbead layer (Biii). (Biv) Schematic flow routes and shape deformation of RBCs squeezing through a narrow and short interbead space interspersed in wider spaces (left route), or only through wide spaces (right route). (Bv) Schematic longitudinal elongation of RBCs flowing though a narrow and long channel in a polycarbonate sieve. (C) Morphologic aspect of RBCs on Giemsa-stained smears showing either a normal discoid aspect downstream from the microbead layer (Ci), or dacryocytes (vertical arrows), schizocytes (horizontal arrows), poikilocytes (stars), and anisocytosis downstream from a narrow, 2-μm-wide, 24-μm-long channels (Cii).
The bead-sorting device retains poorly deformable RBCs from different sources but does not induce morphologic alterations of RBCs
The designed bead-sorting device consisted of a 5-mm-thick microbead layer deposited above the antiaerosol filter of a tip (Figure 3A). In principle, bead diameter heterogeneity generates a wide range of aperture sizes probably preventing clogging (Figure 2Biv). The system was first evaluated with RBCs incubated for 20 minutes at 50°C, a treatment known to impair their deformability and induce their spleen clearance when injected in vivo.25 Retention rates for heated RBCs were consistently more than 95% (Figure 3B-C). We then studied the retention of Pf-RBCs obtained from in vitro culture at different maturation stages.12,22 Retention rates correlated with parasite maturation, with a mean (SD) retention rate of 46.0% (1.4%), 64.2% (7.0%), 81% (5.6%), and 97.8% (3.8%) for young ring-RBCs (0-8 hours), late ring-RBCs (8-16 hours), trophozoites, and schizonts, respectively (Figures 2D-E, 3B).
Poorly deformable RBC flow-through microbeads: setup and results. Metal beads from a single batch were purchased as calibrated mixtures of 5- to 15-μm and 15- to 25-μm-diameter (IPS Industrie des Poudres Sphériques). Optimal results were obtained with a 50/50 mixture of 5- to 15-μm and 15- to 25-μm-diameter beads deposited onto the antiaerosol filter of a commercial 1000-μL tip. (A) Decantation of microbeads after their introduction into an inverted 1000-μL tip, leading to the formation of a 5-mm-thick bead layer (black arrow) above the antiaerosol filter (Ai1). RBCs from a volunteer or a patient blood or from a P falciparum culture were resuspended at 2% hematocrit in medium (PBS/1% albumin) and then introduced in the tubing upstream from the microbead layer (①). An electric pump containing medium was then immediately switched on (②), gently flowing RBCs through the microbead layer (③). On rinsing of the microbead layer with 7 mL of medium, the downstream sample was retrieved (④). The RBC subset retained in the microbead layer at the end of the rinsing procedure was separated from the microbeads by a 3-step decantation procedure. The proportion of rigid RBCs in “upstream,” “downstream,” and “retained” RBC samples was determined by PKH or Giemsa staining. (B) Retention rate of heated, HS, and Pf-RBCs: Heated normal RBCs (50°C for 20 minutes) or HS-RBCs were PKH-labeled and retention rate expressed as the Δ = {[(% of PKH-positive RBC in downstream sample) − (% of PKH-positive RBC in upstream sample)]/(% of PKH-positive in the upstream sample)} ×100. The same procedure was applied to determine parasitized RBC retention (parasitemia determined on Giemsa-stained blood films). Retention in the microbeads was partial at the ring stage (R), subtotal at the trophozoite stage (T), and complete at the schizont stage (S). When ring-RBCs from patients' blood were immediately processed (ex vivo ring), retention rate was low; but on culture to the next generation of ring-RBCs (cultured ring), the mean retention rate was similar to that observed with reference laboratory isolates maintained in long-term culture. (C-G) Typical upstream to downstream decrease of heated (C1-C2), or parasitized (D-E) RBC proportion as observed by PKH fluorescence or on Giemsa-stained smears. (F) Correlation rate between elongation index (LORCA) and retention rate for HS-RBCs.
Poorly deformable RBC flow-through microbeads: setup and results. Metal beads from a single batch were purchased as calibrated mixtures of 5- to 15-μm and 15- to 25-μm-diameter (IPS Industrie des Poudres Sphériques). Optimal results were obtained with a 50/50 mixture of 5- to 15-μm and 15- to 25-μm-diameter beads deposited onto the antiaerosol filter of a commercial 1000-μL tip. (A) Decantation of microbeads after their introduction into an inverted 1000-μL tip, leading to the formation of a 5-mm-thick bead layer (black arrow) above the antiaerosol filter (Ai1). RBCs from a volunteer or a patient blood or from a P falciparum culture were resuspended at 2% hematocrit in medium (PBS/1% albumin) and then introduced in the tubing upstream from the microbead layer (①). An electric pump containing medium was then immediately switched on (②), gently flowing RBCs through the microbead layer (③). On rinsing of the microbead layer with 7 mL of medium, the downstream sample was retrieved (④). The RBC subset retained in the microbead layer at the end of the rinsing procedure was separated from the microbeads by a 3-step decantation procedure. The proportion of rigid RBCs in “upstream,” “downstream,” and “retained” RBC samples was determined by PKH or Giemsa staining. (B) Retention rate of heated, HS, and Pf-RBCs: Heated normal RBCs (50°C for 20 minutes) or HS-RBCs were PKH-labeled and retention rate expressed as the Δ = {[(% of PKH-positive RBC in downstream sample) − (% of PKH-positive RBC in upstream sample)]/(% of PKH-positive in the upstream sample)} ×100. The same procedure was applied to determine parasitized RBC retention (parasitemia determined on Giemsa-stained blood films). Retention in the microbeads was partial at the ring stage (R), subtotal at the trophozoite stage (T), and complete at the schizont stage (S). When ring-RBCs from patients' blood were immediately processed (ex vivo ring), retention rate was low; but on culture to the next generation of ring-RBCs (cultured ring), the mean retention rate was similar to that observed with reference laboratory isolates maintained in long-term culture. (C-G) Typical upstream to downstream decrease of heated (C1-C2), or parasitized (D-E) RBC proportion as observed by PKH fluorescence or on Giemsa-stained smears. (F) Correlation rate between elongation index (LORCA) and retention rate for HS-RBCs.
Two experiments were performed to assess the reproducibility of retention rates, one with with young (0-8 hours, experiment 1), the other with old (8-16 hours, experiment 2) ring-RBCs. In both cases, 3 technical replicates showed good reproducibility (experiment 1: mean retention rate, 64.1%; range, 63.4-64.5; experiment 2: mean retention rate, 43.9%; range, 43.5-44.4). Importantly, ring-RBCs collected from the peripheral blood of P falciparum–infected patients (ie, having successfully crossed inter-endothelial slits in the spleen of patients) were retained at a low rate in microbeads (Figure 3B). However, on cultivation and in vitro reinvasion into freshly collected RBCs, the mean retention rate of the cultured clinical isolates was much higher and similar to the observed retention of laboratory isolates (Figure 2B).
The pressure generated by the electric pump, which corresponds to the pressure across the device, was assessed every 15 to 60 seconds during 7 experiments. The peak pressure (mean, 63.2 cm H2O; range, 48.5-75.5; mean variability, 8.5%) was reached after 60 to 90 seconds and then decreased to a plateau (mean, 51.3 cm H2O; range, 39.7-58.4; mean variability, 10%), which was reached 150 to 180 seconds after initiation of the process. The initial phase of higher pressure observed 30 to 150 seconds after switching on the pump corresponded to the passage of the bulk of the RBC sample (as assessed visually), whereas the plateau phase corresponded to the rinsing phase of the process. When Pf-RBCs where flown through microbeads with larger diameter (15-25 μm), no retention occurred (data not shown).
We then tested retention of RBC collected from HS patients. Retention was partial, ranging from 44% to 88% (Figure 3B). Interestingly, the retention rate of HS-RBC in microbeads correlated with the elongation index determined by LORCA (Spearman correlation coefficient R2 = 0.96; Figure 3F).
Hemolysis was estimated by measuring lactate dehydrogenase released in the supernatant of the flow-through sample and was consistently less than 3% for both heat-treated and HS-RBC. Importantly, there were no schizocytes, dacryocytes, or poikilocytes downstream from the microbeads (Figure 2Ci), contrasting with filtration through narrow polycarbonate sieves (2-μm-wide, 24-μm-long channels, Figure 2Cii). This indicated that, despite its ability to retain rigid RBCs, the bead-sorting process did not induce cell fragmentation and preserved RBC morphology.
Retention of ring-RBCs occurs to a similar extent in isolated human spleens and in microbeads
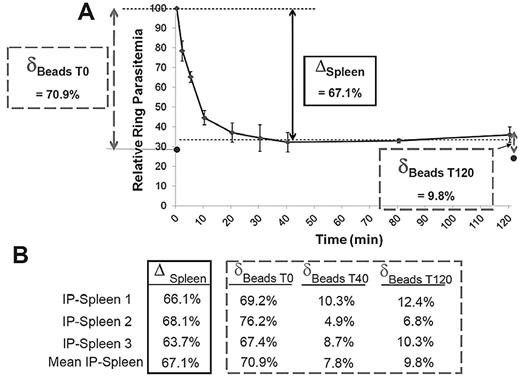
To assess how closely filtration through microbeads reflected the retention in the spleen, ring-RBC retention was explored in parallel in the microbeads and in the isolated-perfused human spleens.22 Ring-RBCs collected from in vitro cultures of the FCR3 laboratory line were either allowed to flow through microbeads or perfused in a human spleen. An aliquot of the circulating, unretained Pf-RBCs was collected from the spleen perfusate at different time points as described12 and the retention of Pf-RBCs in microbeads was quantified (Figure 4A). In 3 individual experiments, the microbead retention rate of ring-RBCs was similar to the cumulative retention rate in the isolated-perfused spleen (mean, 72.7% for the initial sample vs an overall mean 67.1% splenic retention). After a 40-minute spleen perfusion, ring-RBCs collected from the perfusate (ie, having passed through the slow open microcirculation of the spleen12 ) were no longer retained by the microbeads (Figure 4B).
A. Retention of ring-RBCs occurs to a similar extent in isolated human spleens and in microbeads. Mean relative ring-RBC parasitemia (100% of initial parasitemia at time 0) in the perfusate as a function of time of perfusion from 3 independent experiments. As previously observed,12 a fraction of ring-RBCs is retained by isolated-perfused human spleens, the initial 20-minute retention phase being followed by a plateau-phase during which the concentration of ring-RBCs in the perfusate is stable at 20% to 50% of the initial concentration (50%-80% retention). The retention rate in the isolated-perfused spleen (ΔSpleen) was expressed as the difference between the relative ring-RBC parasitemia at T0 and at T40 (onset of plateau phase). The initial retention rate in microbeads was determined as defined in Figure 3, with a sample collected at T0 (δT0 Beads). ● represents the mean relative parasitemia downstream from microbeads. RBCs were then collected from the perfusate at different time points, allowed to flow through the microbeads, and the retention rates determined (δTx Beads). This explored the ability of RBCs that had already crossed the spleen several times to cross the microbead layer. (B) Relative retention rate in isolated-perfused spleens and in microbeads at different time points during perfusion as observed in 3 independent experiments.
A. Retention of ring-RBCs occurs to a similar extent in isolated human spleens and in microbeads. Mean relative ring-RBC parasitemia (100% of initial parasitemia at time 0) in the perfusate as a function of time of perfusion from 3 independent experiments. As previously observed,12 a fraction of ring-RBCs is retained by isolated-perfused human spleens, the initial 20-minute retention phase being followed by a plateau-phase during which the concentration of ring-RBCs in the perfusate is stable at 20% to 50% of the initial concentration (50%-80% retention). The retention rate in the isolated-perfused spleen (ΔSpleen) was expressed as the difference between the relative ring-RBC parasitemia at T0 and at T40 (onset of plateau phase). The initial retention rate in microbeads was determined as defined in Figure 3, with a sample collected at T0 (δT0 Beads). ● represents the mean relative parasitemia downstream from microbeads. RBCs were then collected from the perfusate at different time points, allowed to flow through the microbeads, and the retention rates determined (δTx Beads). This explored the ability of RBCs that had already crossed the spleen several times to cross the microbead layer. (B) Relative retention rate in isolated-perfused spleens and in microbeads at different time points during perfusion as observed in 3 independent experiments.
Large amounts of rigid RBCs sorted out by microbeads can be retrieved and analyzed
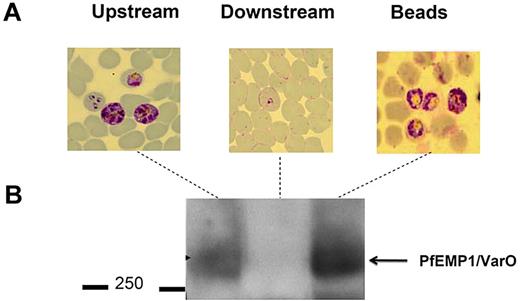
Microbeads were then used to separate RBCs with distinct deformability properties for further analysis. RBCs retained within the microbeads could readily be retrieved by differential decantation at 1g. At the end of the filtration process, microbeads containing retained RBCs were collected from the tip. Because they are denser than RBCs, microbeads sedimented within seconds, whereas most RBCs remained in the supernatant from which they were retrieved. To confirm that the retained and flow-through RBCs corresponded to distinct subpopulations with differing phenotypes, we fractionated a Pf-RBC culture containing mature stages displaying the parasite-specific VarO adhesin onto the Pf-RBC surface. Western blot analysis using a VarO-specific antibody26 (Figure 5) showed enrichment of the VarO adhesion in the microbead-retained fraction (a more intense band in the “retained” sample) and its depletion from the flow-through sample (no observable band; Figure 5). When the FUP/CB line that does not express VarO was used, as expected, no band corresponding to the VarO protein was observed (data not shown). As VarO is essentially expressed in schizonts, this reflects the total retention of schizonts by the microbead device. This was consistent with Giemsa staining of the various fractions, which showed that mature Pf-RBCs concentrated in the microbeads (Figure 5). This result showed the efficiency of microbeads to concentrate and retrieve less deformable RBCs for further molecular analyses.
Sorted RBC subsets are amenable to further immunochemical characterization. (A) Giemsa-stained blood films of upstream, retained/retrieved from microbeads, and downstream samples showing the total retention of schizonts expressing VarO protein. (B) Pooled extracts of upstream, retained/retrieved, and downstream samples analyzed by a Western blot probed with mouse anti-VarO immune serum.
Sorted RBC subsets are amenable to further immunochemical characterization. (A) Giemsa-stained blood films of upstream, retained/retrieved from microbeads, and downstream samples showing the total retention of schizonts expressing VarO protein. (B) Pooled extracts of upstream, retained/retrieved, and downstream samples analyzed by a Western blot probed with mouse anti-VarO immune serum.
The bead-sorting device can deplete a stored RBC population from its subpopulation of poorly deformable RBCs
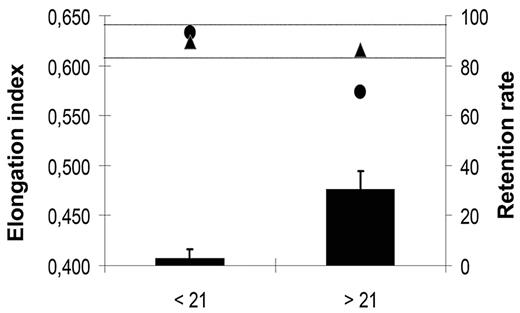
Prolonged storage of RBC concentrates is associated with a decrease in the mean deformability of RBC concentrates and with an increase in the incidence of transfusion-related side effects.3,4 To evaluate the potential of microbeads to sort out poorly deformable RBCs from stored RBC concentrates, and thereby improve the quality of the transfused blood, we studied deformability of RBCs kept under blood bank storage conditions for 6 weeks using the LORCA methodology and in parallel monitored their retention in microbeads. In 2 independent experiments, the elongation index of RBC concentrates decreased significantly after 3 weeks of storage (Figure 6). A parallel increase of the retention rate of stored RBCs in microbeads was observed. No retention was detected with RBCs stored for 3 weeks or less (data not shown), although the 30.6% mean retention of RBCs stored for 3 to 6 weeks matched the 26.5% 24-hour clearance rate of RBCs transfused to patients after a 25- to 35-day storage period.3 Importantly, the elongation index of the stored RBC population was restored to normal after rigid RBCs had been removed by filtration through microbeads (Figure 6).
Elongation index and retention rate of “fresh” versus “old” RBCs. Mean (SD) retention rates (black bars) in microbeads of an RBC population measured weekly during a 6-week storage period in blood bank storage conditions. Data from the 3 first (< 21) and 3 last (> 21) weeks of storage period were pooled. Mean (SD) elongation index of a RBC population upstream (●) and downstream (▲) from the microbead layer. Data from the 3 first (< 21) and 3 last (> 21) weeks of the 6-week storage period were pooled. In our experimental conditions, the fresh RBC population elongation index values range from 0.610 to 0.640 (horizontal dotted lines).
Elongation index and retention rate of “fresh” versus “old” RBCs. Mean (SD) retention rates (black bars) in microbeads of an RBC population measured weekly during a 6-week storage period in blood bank storage conditions. Data from the 3 first (< 21) and 3 last (> 21) weeks of storage period were pooled. Mean (SD) elongation index of a RBC population upstream (●) and downstream (▲) from the microbead layer. Data from the 3 first (< 21) and 3 last (> 21) weeks of the 6-week storage period were pooled. In our experimental conditions, the fresh RBC population elongation index values range from 0.610 to 0.640 (horizontal dotted lines).
Discussion
We showed here that heated RBCs, HS-RBCs, and Pf-RBCs are selectively retained by inert, metal microbeads, with retention rates similar to that observed in parallel in the isolated-perfused human spleen model. These in vitro results directly confirm the importance of the mechanical sensing of RBCs by the human spleen. They show that splenic retention of HS-RBCs and ring-RBCs is based predominantly on their mechanical properties and can occur in the absence of a ligand-receptor interaction. The perfused-isolated human spleen system has provided new insight in the field of malaria and splenic physiology.12,17,22,27 However, access to human spleens is limited, and it was important to develop an in vitro system that could reflect the mechanical sensing of RBCs in the human spleen as they cross interendothelial slits. We have confirmed in this study that interendothelial slits in the human spleen are altogether narrower and shorter than capillaries, thereby imposing an original dumbbell-shape deformation on RBCs (Figures 1–2) different from their bullet-shape deformation in narrow capillaries28 or their cylindrical elongation in microfluidic channels.29,30 The hypothesis that different microanatomic structures or devices create different mechanical constraints is further supported by frequent RBC alterations observed downstream from 2-μm-wide channels in polycarbonate sieves, although these were not observed downstream from microbead layers (Figure 2). We reasoned that squeezing across the intersphere spaces would force RBCs to deform in a way resembling the crossing of interendothelial slits, as interbead apertures offer the appropriate association of narrowness and shortness. The retention rate of heated-RBCs in microbeads, and HS-RBCs correlated with RBC rigidity, a feature thought to be the basis for retention of such RBCs by the spleen.1 The retention rate of Pf-RBCs in microbeads correlated with the parasite stage and with the progressive decrease of RBC deformability as the parasite develops in its host cell.6 The results support the conclusion that interendothelial slits and microbeads force RBCs to deform in similar ways, this similarity being observed with various alterations of RBC mechanical properties. In particular, there was an excellent correlation of Pf-RBC retention rates in microbeads and isolated-perfused human spleens (Figure 3).12,17 In the context of malaria, this original innate retention process can probably operate very early during parasite intraerythrocytic development. Because ring-RBC retention corresponds to the clearance of circulating parasites, it probably reduces the speed of parasite load increase and, therefore, the kinetics of infection. This hypothesis is perfectly consistent with the observation that the spleen is more protective against severe manifestations of malaria in naive than in immune subjects.18,31
When RBC samples from patients were perfused through microbeads immediately after collection, HS-RBCs and ring-RBCs were retained at different rates (moderate and very mild, respectively, Figure 2). We were initially surprised by this difference as, when assessed by Lorca, the deformability of circulating HS-RBCs (Figure 2) and that of cultured ring-RBCs12 was decreased to similar extents, both compatible with retention in the spleen (Figure 2), suggesting that both RBC populations should be retained equally. However, published data suggest that splenic RBC filtering function is modified in different ways in patients with either HS or P falciparum malaria. Although the clearance of mechanically altered, surface altered, and normal RBCs is generally accelerated in P falciparum–infected adults,32-34 splenic RBC filtration function is partially impaired in HS patients, as illustrated by the abnormally high proportion of circulating “pocked” RBCs35 and the occasional observation of Howell-Jolly bodies on the blood smears of HS patients.35 Therefore, the different retention rates of freshly collected HS-RBCs and ring-RBCs in microbeads may result from the fact that the spleen of HS patients leaves a proportion of poorly deformable HS-RBCs in circulation, although the spleen of malaria patients rapidly retains most poorly deformable ring-RBCs. The modification of spleen-filtering function in HS patients is mild compared with that of patients with severe functional or anatomic asplenia,35,36 probably explaining why putative impaired RBC retention is not associated with an increased risk of overwhelming pneumococcal infection in HS (as opposed to what occurs in splenectomized subjects). Taken together, these observations suggest that hyposplenism is not an all-or-nothing phenomenon and that new markers, like the retention rate of circulating RBC in microbeads, would help delineate its heterogeneity in diverse clinical situations. Along the same line, there is still much to be learned on how RBC deformability assessed by different methods predicts the ability of RBCs to cross splenic interendothelial slits. Three major parameters (ie, the RBC surface-to-volume ratio, the cytoplasmic viscosity, and the intrinsic membrane deformability) regulate the RBC capacity to deform and probably to cross interendothelial slits.37 The mechanism that determines the reduced deformability of HS-RBCs is predominantly decreased surface-to-volume ratio.1 In HS, reduced deformability correlates with RBC retention in the spleen and in microbeads1 (Figure 2). In the context of P falciparum malaria, the mechanisms of decreased RBC deformability and increased splenic retention are less precisely explored and probably multifactorial. Insertion of parasite proteins in the RBC membrane38 and interactions with the RBC cytoskeleton contribute to altering Pf-RBC mechanical properties, but the impact of parasitization on cell surface-to-volume ratio39 and internal viscosity is not well known. Yet, we observed a very close correspondence between retention rates of ring-RBCs in both isolated-perfused human spleens and in microbeads (Figure 3), suggesting that microbeads may be a powerful tool to more precisely address these issues in the future.
In addition to its relevance, filtration through microbeads displays several practical strengths and advantages. It is an easy method for separating RBC subpopulations based on their mechanical properties. Importantly, subpopulations can be concentrated or removed by the device, thereby generating samples enriched in or depleted from less deformable RBCs. Sample quantity retrieved from 2 mL of microbeads was sufficient for biochemical studies (Figure 5), a key advantage over available methods. Not least, filtration through microbeads is simple and inexpensive, and the volume of the bead layer can be adjusted to the size of the sample to be processed. Small volumes are suitable for field or bedside diagnosis or prognosis studies, for screening compounds modifying the deformability of normal or abnormal RBCs, or for molecular analyses of subpopulations with defined mechanical phenotypes. It is anticipated that the method could be accommodated for preparative purposes, such as the removal of poorly deformable RBCs from large samples (sclererythrocytapheresis). As a proof-of-concept for this application, we used microbeads to remove the most rigid RBCs from a RBC population kept for several weeks under blood bank storage conditions. The deformability of RBCs downstream from the sorting device was restored to normal levels (Figure 6). This opens interesting prospects for optimized RBC concentrates and improved transfusion yields3 and for decreased risk of post-transfusion severe adverse events.4 Scaling-up of the microbead technology may help spare rare blood resources and improve prognosis when transfusing critically ill patients. In the longer term, the depletion of rigid RBCs from the blood of patients with RBC disorders or impaired spleen-filtering function may be envisioned as a therapeutic approach to reduce acute or chronic complications. Not least, the impact of number of experimental parameters (eg, temperature, RBC suspension medium) can now be tested rapidly in vitro, potentially leading to further optimization of the experimental approach.
The ability of microbead sorting to reflect the mechanical sensing of RBCs in the human spleen red pulp has been verified on an array of inherited and acquired RBC abnormalities and proved robust when tested with ex vivo blood samples. Although initially developed on the basis of the geometry of interendothelial slits, this method that preserves RBC morphology and integrity portends wide research and medical applications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis-Ministère de l'Enseignement Supérieur et de la Recherche-Institut Pasteur, the Centre National de la Recherche Scientifique, and the Institut Pasteur. G.D. was supported by the Délégation Générale à l'Armement (fellowship 05 60 00 032). F.L. was supported by Fondation Inckerman-Fondation de France and the Région Ile de France. I.S. was funded by a fellowship from the Région Ile de France. V.B. was supported by Fondation pour la Recherche Médicale. Research of the IMP Unit at Institut Pasteur is partly supported by the BioMalPar European Network of Excellence from the Priority 1 “Life Sciences, Genomics and Biotechnology for Health” in the 6th Framework Programme and by the Project: Mechanisms of erythrocytic Infection & Anemia in Malaria PI Kasturi Haldar, and the National Institutes of Health (Award Number 5P01HL078826-06). N.M. acknowledges the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis-Ministère de l'Enseignement Supérieur et de la Recherche-Institut Pasteur, which made possible his sabbatical stay at Institut Pasteur.
National Institutes of Health
Authorship
Contribution: G.D., I.S., F.J., and P.A.B. designed and performed research, contributed vital analytical tools, analyzed data, and wrote the paper; V.B. performed research, analyzed data, and wrote the paper; S.P., S.B., and M.G. performed research and analyzed data; F.L., C.G., S.D., B.A., A.S., D.C.H., F.P., M.T., and D.M. contributed vital analytical tools; N.M. and O.M.-P. designed research, analyzed data, and wrote the paper; and G.M. and P.H.D. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre A Buffet, Service de Parasitologie Mycologie, Groupe Hospitalier Pitié-Salpêtrière, 47 Boulevard de l'hôpital, 75651 Paris Cedex 13, France; e-mail: pierre.buffet@psl.aphp.fr.
References
Author notes
G.D. and I.S. contributed equally to this study.

![Figure 2. Size and geometry of the interendothelial slit and resulting RBC deformation. (Ai) Transmission electron micrograph (original magnification ×2000) of RBCs squeezing through an interendothelial slit (ie, between 2 adjacent endothelial cells [EC], from the cord [Co] to the sinus lumen [SL], white star showing the anterior pole of the RBCs). (Aii) Width (red double-headed arrow) and length (black double-headed arrow) of the interendothelial slit at high original magnification (×5000) as measured on 11 pictures. (B) Dumbbell-shaped RBCs as observed on a transmission electron micrograph (Bi, white star showing the anterior pole of the RBCs), or as expected to occur as a RBCs squeeze between microbeads (Bii), or as observed under the contrast-phase microscope after formaldehyde fixation of RBCs engaged within the microbead layer (Biii). (Biv) Schematic flow routes and shape deformation of RBCs squeezing through a narrow and short interbead space interspersed in wider spaces (left route), or only through wide spaces (right route). (Bv) Schematic longitudinal elongation of RBCs flowing though a narrow and long channel in a polycarbonate sieve. (C) Morphologic aspect of RBCs on Giemsa-stained smears showing either a normal discoid aspect downstream from the microbead layer (Ci), or dacryocytes (vertical arrows), schizocytes (horizontal arrows), poikilocytes (stars), and anisocytosis downstream from a narrow, 2-μm-wide, 24-μm-long channels (Cii).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/8/10.1182_blood-2010-10-312801/5/m_zh89991166590002.jpeg?Expires=1769898763&Signature=OIMwg~aCTNi~IjN1SMk8JeFFE3C3zci3jOWiCfQks5hbqqYt1Ij-fVxw2B-Zgoqte8COI6IrF-3w6sAwFNR24v6cMAdJbgiZcPQCLyxGhAtTrJ~~fXS9VbyD8yQ6oRls2NvsrxXdR2rgwzoaI-53ps9QYdIorJWZgdO53LR8dRPDtTFtY0z~YxIFelkyNeMQcGNRH5HySucCacQMYPZeEyOXF3tx9QLVssICYARBmK5R8wg-i6SMT3n4Y1myQAPedNTOhM8-JWFmXYC6VudqkX3KR-Tc~QlB7I1fBWfnH80m1L4SXsqewCN2iI2qraDhHRYBTW8dskUWAbZyZebXiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Poorly deformable RBC flow-through microbeads: setup and results. Metal beads from a single batch were purchased as calibrated mixtures of 5- to 15-μm and 15- to 25-μm-diameter (IPS Industrie des Poudres Sphériques). Optimal results were obtained with a 50/50 mixture of 5- to 15-μm and 15- to 25-μm-diameter beads deposited onto the antiaerosol filter of a commercial 1000-μL tip. (A) Decantation of microbeads after their introduction into an inverted 1000-μL tip, leading to the formation of a 5-mm-thick bead layer (black arrow) above the antiaerosol filter (Ai1). RBCs from a volunteer or a patient blood or from a P falciparum culture were resuspended at 2% hematocrit in medium (PBS/1% albumin) and then introduced in the tubing upstream from the microbead layer (①). An electric pump containing medium was then immediately switched on (②), gently flowing RBCs through the microbead layer (③). On rinsing of the microbead layer with 7 mL of medium, the downstream sample was retrieved (④). The RBC subset retained in the microbead layer at the end of the rinsing procedure was separated from the microbeads by a 3-step decantation procedure. The proportion of rigid RBCs in “upstream,” “downstream,” and “retained” RBC samples was determined by PKH or Giemsa staining. (B) Retention rate of heated, HS, and Pf-RBCs: Heated normal RBCs (50°C for 20 minutes) or HS-RBCs were PKH-labeled and retention rate expressed as the Δ = {[(% of PKH-positive RBC in downstream sample) − (% of PKH-positive RBC in upstream sample)]/(% of PKH-positive in the upstream sample)} ×100. The same procedure was applied to determine parasitized RBC retention (parasitemia determined on Giemsa-stained blood films). Retention in the microbeads was partial at the ring stage (R), subtotal at the trophozoite stage (T), and complete at the schizont stage (S). When ring-RBCs from patients' blood were immediately processed (ex vivo ring), retention rate was low; but on culture to the next generation of ring-RBCs (cultured ring), the mean retention rate was similar to that observed with reference laboratory isolates maintained in long-term culture. (C-G) Typical upstream to downstream decrease of heated (C1-C2), or parasitized (D-E) RBC proportion as observed by PKH fluorescence or on Giemsa-stained smears. (F) Correlation rate between elongation index (LORCA) and retention rate for HS-RBCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/8/10.1182_blood-2010-10-312801/5/m_zh89991166590003.jpeg?Expires=1769898763&Signature=RQbhk8~42iVBQS~lY1dd3WW4UkOsmsck5cslKwvmAvS-PhfmqeXBOkiP7TpL47R5NU0znE1Q~58-8L-e0WdAf7d1ae3cOlkWlr3DNUvFZ6BcG~jtXGK8F~4rkL8J0B5quVYFJxNh3kF65RWe3wpfn0HwHo0d-rlg9hc8pYyD1u5KWmUSDJA1jsPLWK2VDHNh56U3b7ZgVTX1stIXbKAJCm~iAuihDPv0CerWXYPQWpQpzvoRZ~kItk0g6xOBT8auIq8vzdRgpZaCC8y4MQW4fjZaQGROSsxmiZrdIMq54-OX8HMtaH7vVUBiVlJNL5QJls9UBqazVEK~nax1cW4umw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal