Abstract
Nilotinib is a potent selective inhibitor of the BCR-ABL tyrosine kinase approved for use in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP), and in CML-CP and CML-accelerated phase after imatinib failure. Nilotinib (400 mg twice daily) was approved on the basis of the initial results of this phase 2 open-label study. The primary study endpoint was the proportion of patients achieving major cytogenetic response (CyR). All patients were followed for ≥ 24 months or discontinued early. Of 321 patients, 124 (39%) continue on nilotinib treatment. Overall, 59% of patients achieved major CyR; this was complete CyR (CCyR) in 44%. Of patients achieving CCyR, 56% achieved major molecular response. CyRs were durable, with 84% of patients who achieved CCyR maintaining response at 24 months. The overall survival at 24 months was 87%. Adverse events were mostly mild to moderate, generally transient, and easily managed. This study indicates that nilotinib is effective, with a manageable safety profile, and can provide favorable long-term benefits for patients with CML-CP after imatinib failure. This trial was registered at www.clinicaltrials.gov as #NCT00109707.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm resulting in expansion of hematopoietic cells carrying the oncogenic BCR-ABL fusion gene, which encodes the constitutively active BCR-ABL protein tyrosine kinase. The fusion event that generates the Philadelphia chromosome (Ph) is the result of a reciprocal translocation between the long arms of chromosomes 9 and 22, t(9;22)(q34;q11), and can be detected by cytogenetic analysis.1,2 Imatinib mesylate (Gleevec; Novartis Pharmaceuticals Corporation; imatinib [Glivec]; Novartis Pharmaceuticals Corporation) is a BCR-ABL tyrosine kinase inhibitor that has dramatically improved the outcome of patients with CML in chronic-phase (CML-CP). Imatinib inhibits BCR-ABL by binding to an inactive conformation of the kinase, thereby preventing signal transduction that leads to cellular proliferation.3,4 Despite the success of imatinib in the treatment of patients with CML-CP, 34% of patients randomly assigned to the imatinib arm in the landmark International Randomized Study of Interferon and STI571 trial were no longer on study drug at 6 years, for reasons that included lack of efficacy (12%) and the occurrence of adverse events (4%).5 Resistance can develop through several mechanisms, including point mutations in the BCR-ABL kinase domain.6 For patients resistant or intolerant to imatinib, second-generation tyrosine kinase inhibitors are available with improved potency over imatinib.
Nilotinib (Tasigna; Novartis Pharmaceuticals Corporation) is a rationally designed highly potent and selective inhibitor of BCR-ABL.7-9 In a phase 1 dose-escalation study of patients with imatinib-resistant CML, nilotinib treatment resulted in a significant proportion of patients achieving hematologic and cytogenetic responses (CyRs) in all phases of CML.10 Results from a single-arm, open-label phase 2 study in patients with CML-CP treated with nilotinib (minimum follow-up of 6 months; 280 evaluable patients) have been previously reported.11 The results presented herein include additional patients and longer follow-up (N = 321; 70% imatinib-resistant, 30% imatinib-intolerant). All patients had a minimum follow-up of 24 months or discontinued early.
Methods
Patient population and study design
Eligibility criteria for this trial have been previously described.11 Briefly, patients with CML-CP positive for the Philadelphia chromosome who were ≥ 18 years of age were eligible if they had imatinib resistance or intolerance, adequate performance status (World Health Organization Performance Score ≤ 2), and normal hepatic, renal, and cardiac functions. Patients in accelerated phase (AP) or blastic phase (BP)12,13 or patients who received treatment with imatinib for < 5 days before nilotinib treatment were excluded, as were patients with a corrected QT interval (QTc) > 450 milliseconds. Imatinib resistance was also previously defined11 as either treatment with imatinib ≥ 600 mg/d (for ≥ 3 months) with disease progression (≥ 50% increase in white blood cells), or no hematologic response after 4 weeks, or patients receiving < 600 mg/d with mutations at any of the following ABL amino acids: L248, G250, Q252, Y253, E255, T315, F317, H396.
Resistance was defined as no complete hematologic response (CHR) at or after 3 months, no minimal CyR by 6 months, no major CyR (MCyR) by 12 months, loss of CHR, loss of minor CyR, loss of MCyR or complete cytogenetic response (CCyR), or development of clonal evolution. Imatinib intolerance was defined as a lack of MCyR and discontinuation because of a grade 3/4 imatinib-related adverse event (AE), despite optimal supportive care, or because of a persistent grade 2 imatinib-related AE, despite optimal supportive care, which persists ≥ 1 month or recurs > 3 times with imatinib dose reduction. Thus, patients with imatinib intolerance in this study are also selected with an element of resistance because they were required not to have achieved MCyR at any time on imatinib therapy.
Trial design and objectives
This study was conducted in accordance with the Declaration of Helsinki, and all patients gave written informed consent according to institutional guidelines. The protocol was reviewed and approved by an appropriate institutional review board or ethics committee at each participating center. The primary objective of this phase 2, single-treatment arm, open-label study was to determine the incidence of MCyRs in patients resistant or intolerant to imatinib. This is the best cumulative response, with cytogenetic assessments performed at months 1 and 3 and every 3 months thereafter in the first year and every 3-6 months in subsequent years. Secondary objectives were to determine the incidence of CCyR, CHR, and molecular response; duration of MCyR and CCyR; and progression-free survival (PFS), event-free survival (EFS), and overall survival (OS), as well as to determine the safety profile of nilotinib. Definitions of hematologic, cytogenetic, and molecular response have been previously described.11 Duration of response was defined as the time from the start of response to the date of discontinuation due to progression as assessed by the investigator (hematologic or cytogenetic relapse, transformation to AP or BP) or death. PFS was defined as the time from the start of nilotinib to the earliest date of progression to AP or BP, discontinuation due to progression (as assessed by the investigator), or death from any cause on nilotinib therapy. EFS was defined as the time from the start of nilotinib to the earliest date of any of the following events: loss of CHR, loss of MCyR, progression to AP or BP, discontinuation due to CML progression as assessed by the investigator, or death from any cause on nilotinib therapy. Patients taken off nilotinib therapy for reasons other than progression or death were censored at nilotinib discontinuation (because progression or events related to CML may be influenced by subsequent disease-modifying therapies). Survival was calculated from the start of nilotinib to death due to any cause. All time-to-event analyses were performed with the use of Kaplan-Meier methods and presented by Kaplan-Meier curves. Patients taken off nilotinib therapy were followed for survival (information on date and cause of death were collected). Nilotinib was administered at a dose of 400 mg twice daily (800 mg/d) on the basis of the safety and tolerability established in the phase 1 study.10
Results
Patient demographics
Of the 321 patients included in this analysis, 70% were imatinib resistant and 30% were imatinib intolerant. The median age was 58 years (Table 1). The median duration of CML was 58 months, and the median duration of prior imatinib therapy was 32 months. Patients were heavily pretreated with imatinib (72% receiving ≥ 600 mg/d), hydroxyurea (83%), and interferon (58%); some were also pretreated with cytarabine (24%). Overall, 24% of patients had additional chromosomal abnormalities, and 42% had BCR-ABL kinase domain mutations at baseline.
Patient demographics at baseline (N = 321)
| . | Values . |
|---|---|
| Median age, y (range) | 58 (21-85) |
| Median duration of CML, mo (range) | 58 (5-275) |
| Median duration of prior imatinib therapy, mo (range) | 32 (< 1 to 94) |
| Imatinib resistant | 39 (2-94) |
| Imatinib intolerant | 14 (< 1 to 64) |
| Active disease before therapy, n (%) | 207 (64) |
| Imatinib resistant/intolerant (%) | 70/30 |
| Prior imatinib ≥ 12 mo, n (%) | 266 (83) |
| Imatinib resistant, n/N (%) | 215/226 (95) |
| Imatinib intolerant, n/N (%) | 51/94* (54) |
| Prior highest imatinib dose ≥ 600 mg/d, n (%) | 232 (72) |
| Imatinib resistant, n/N (%) | 201/226 (89) |
| Imatinib intolerant, n/N (%) | 31/94 (33) |
| Other prior therapy | |
| Hydroxyurea, n (%) | 266 (83) |
| Interferon, n (%) | 187 (58) |
| Cytarabine, n (%) | 78 (24) |
| Response at baseline, n (%) | |
| Complete hematologic response | 114 (36) |
| Major cytogenetic response | 35 (11) |
| Complete cytogenetic response | 10 (3) |
| . | Values . |
|---|---|
| Median age, y (range) | 58 (21-85) |
| Median duration of CML, mo (range) | 58 (5-275) |
| Median duration of prior imatinib therapy, mo (range) | 32 (< 1 to 94) |
| Imatinib resistant | 39 (2-94) |
| Imatinib intolerant | 14 (< 1 to 64) |
| Active disease before therapy, n (%) | 207 (64) |
| Imatinib resistant/intolerant (%) | 70/30 |
| Prior imatinib ≥ 12 mo, n (%) | 266 (83) |
| Imatinib resistant, n/N (%) | 215/226 (95) |
| Imatinib intolerant, n/N (%) | 51/94* (54) |
| Prior highest imatinib dose ≥ 600 mg/d, n (%) | 232 (72) |
| Imatinib resistant, n/N (%) | 201/226 (89) |
| Imatinib intolerant, n/N (%) | 31/94 (33) |
| Other prior therapy | |
| Hydroxyurea, n (%) | 266 (83) |
| Interferon, n (%) | 187 (58) |
| Cytarabine, n (%) | 78 (24) |
| Response at baseline, n (%) | |
| Complete hematologic response | 114 (36) |
| Major cytogenetic response | 35 (11) |
| Complete cytogenetic response | 10 (3) |
CML indicates chronic myeloid leukemia; active disease refers to patients not in complete hematologic response at the time of study entry.
Imatinib dosing information was missing for 1 patient.
Efficacy
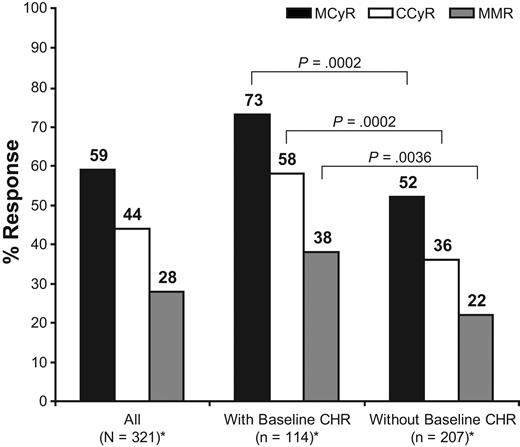
Overall, 59% of patients achieved MCyR (45% if measured only by metaphase analysis and excluding fluorescence in situ hybridization analysis and also excluding MCyR responders who had an MCyR at baseline or who had a missing cytogenetic analysis at baseline); 56% for imatinib-resistant patients and 66% for imatinib-intolerant patients (Figure 1). Most patients who achieved MCyR also attained CCyR. Overall, 44% of patients achieved CCyR (37% if measured only by metaphase analysis, excluding fluorescence in situ hybridization analysis, and also excluding CCyR responders who had a CCyR at baseline or who had a missing cytogenetic analysis at baseline). Complete cytogenetic response was achieved in 41% of imatinib-resistant patients and in 51% of imatinib-intolerant patients (Figure 1). The proportion of patients who achieved MCyR (73% vs 52%; P = .0002) and CCyR (58% vs 36%; P = .0002) was higher among patients who entered the study with a baseline CHR compared with patients who did not (Figure 2).
Major(MCyR) andcomplete cytogenetic response(CCyR)in patients with a minimum follow up of 24 months(N = 321).
Major(MCyR) andcomplete cytogenetic response(CCyR)in patients with a minimum follow up of 24 months(N = 321).
Cytogenetic andmolecular responses in patients with and withoutCHR atbaseline (N = 321). CCyR indicates complete cytogenetic response; CHR, complete hematologic response; MCyR, major cytogenetic response; MMR, major molecular response (BCR-ABL ≤ 0.1% on the international scale). *n for MMR as follows: 294 patients for all, 105 with baseline CHR, and 189 without baseline CHR.
Cytogenetic andmolecular responses in patients with and withoutCHR atbaseline (N = 321). CCyR indicates complete cytogenetic response; CHR, complete hematologic response; MCyR, major cytogenetic response; MMR, major molecular response (BCR-ABL ≤ 0.1% on the international scale). *n for MMR as follows: 294 patients for all, 105 with baseline CHR, and 189 without baseline CHR.
Overall, CyRs were achieved rapidly, with a median time to MCyR of 1.4 months for MCyR responders with a baseline CHR and 2.8 months for MCyR responders without baseline CHR (Kaplan-Meier analysis of time to MCyR among all patients showed a significant difference between patients with and without CHR at baseline, P < .0001). The median time to CCyR was 3.2 months for CCyR responders with a baseline CHR and 3.3 months for CCyR responders without a baseline CHR (Kaplan-Meier analysis of time to CCyR among all patients showed a significant difference between patients with and without CHR at baseline, P = .0003). Cytogenetic responses were durable, with 77% of MCyR responders and 84% of CCyR responders maintaining their response at 24 months.
Molecular response was assessed for 294 of 321 patients who had BCR-ABL transcript levels available after baseline. Overall, 28% (82 of 294) of patients achieved major molecular response (MMR) up to the cutoff. Among patients who entered the study with baseline CHR, 38% achieved MMR versus 22% of patients without baseline CHR (P = .0036; Figure 2). An MMR occurred in 44% of patients who achieved an MCyR and in 56% of patients who achieved CCyR. MMR was achieved by 32% (43 of 134) and 39% (41 of 105) of evaluable patients who had BCR-ABL transcript levels available at 12 and 18 months, respectively.
Response to nilotinib among imatinib-resistant patients in this study by BCR-ABL mutation status was analyzed. Fifty-five percent (109 of 200) of the imatinib-resistant patients had BCR-ABL mutation(s) at baseline and 14% (27 of 200) had mutations at baseline that, with the results of the present study, are known to be insensitive to nilotinib (E255K/V, Y253H, F359C/V).14 Four imatinib-resistant patients had the T315I mutation at baseline. MCyR and CCyR were achieved by 68% and 50% of imatinib-resistant patients without mutations at baseline, respectively, versus 49% (P = .006) and 35% (P = .037) of patients with mutations at baseline. Response to nilotinib in this study by BCR-ABL mutation status has been reported in detail elsewhere.14 Among imatinib-intolerant patients, 13% (12 of 90) had a BCR-ABL mutation(s) at baseline and 2% (2 of 90) had a mutation at baseline known to be insensitive to nilotinib. Two imatinib-intolerant patients had the T315I mutation at baseline.
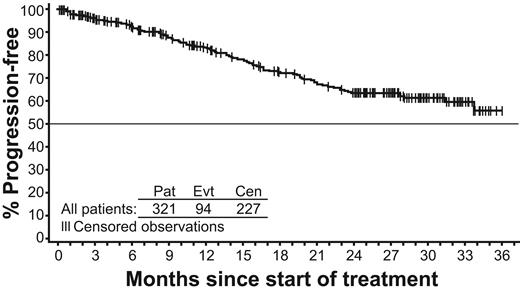
Overall, the estimated PFS (progression to AP or BP or discontinuation due to progression or death) at 24 months was 64% (Figure 3). The estimated PFS at 24 months was higher for patients with baseline CHR (77%) compared with patients without CHR at study entry (56%). The EFS is shown in Figure 4. A total of 117 events have occurred: loss of CHR, 10; loss of MCyR, 38; progression to AP or BP 10 (2 of these 10 patients were reported to have progressed to AP or BP because of data errors that were identified after database lock); “disease progression” as assessed by investigator, 57; and death, 2.
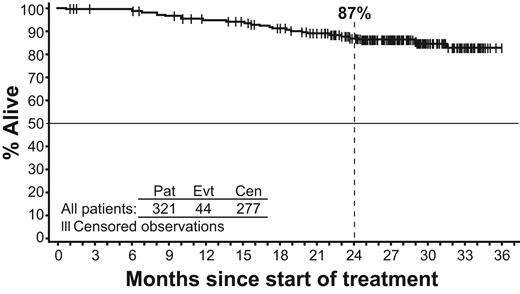
The estimated 24-month OS was 87% (Figure 5). Of the 321 patients, 44 have died, 8 on study or within 28 days of treatment discontinuation and 36 after 28 days of treatment discontinuation. The coded causes of deaths in the 8 patients on treatment or within 28 days of treatment discontinuation were as follows: myocardial infarction/coronary heart disease, 3; pulmonary embolism, 1; sepsis, 1; subdural hematoma, 1; multiorgan failure, 1; CML progression, 1. The coded causes of death in the 36 patients beyond 28 days off therapy were as follows: CML progression, 18; myocardial infarction/coronary heart disease, 1; sepsis, 3; bleeding events, 2; graft-versus-host or other complications after transplantation, 4; other cancers, 2; organ infarcts, 1; suicide, 1; pneumonia, 1; unknown, 3.
Patient status
At the time of data cutoff (April 20, 2008), 124 of 321 patients (39%) remained on nilotinib therapy (Table 2). Treatment discontinuation occurred in 197 patients (61%), with 88 patients (27%) discontinuing because of disease progression and 61 patients (19%) because of AEs, of which 50 (16%) were drug-related (6% were because of myelosuppression). The median nilotinib dose intensity was 789 mg/d, close to the planned dose of 800 mg/d. The median duration of exposure to nilotinib was 18.4 months (< 1.0 to 36.0 months), with 62% of patients on therapy for ≥ 12 months and 42% on therapy for ≥ 24 months. Fifty-eight percent of patients required a dose interruption, with a median cumulative duration of interruption of 20 days (median of 4% of days of exposure).
Patient disposition (by investigator assessment) (N = 321)
| . | No. (%) . |
|---|---|
| Ongoing treatment | 124 (39) |
| Discontinued treatment | 197 (61) |
| Disease progression | 88 (27) |
| Adverse events | 61 (19) |
| Drug related | 50 (16)* |
| Non-drug related | 11 (3) |
| Abnormal test results | 4 (1) |
| Abnormal laboratory values | 2 (< 1) |
| Other† | 39 (12) |
| Death | 3 (< 1) |
| . | No. (%) . |
|---|---|
| Ongoing treatment | 124 (39) |
| Discontinued treatment | 197 (61) |
| Disease progression | 88 (27) |
| Adverse events | 61 (19) |
| Drug related | 50 (16)* |
| Non-drug related | 11 (3) |
| Abnormal test results | 4 (1) |
| Abnormal laboratory values | 2 (< 1) |
| Other† | 39 (12) |
| Death | 3 (< 1) |
Drug-related adverse events causing discontinuation included myelosuppression (n = 20; 6%), elevations of lipase or pancreatitis (n = 2; < 1%), elevations of liver enzymes or bilirubin (n = 3; < 1%), skin disorders (n = 5; 2%), diarrhea (n = 1; < 1%).
Other includes protocol violation, withdrawn consent, lost to follow-up, not stated, and administrative problems.
Safety and tolerability
Nilotinib therapy remained well tolerated after 24 months, and no changes were observed in the overall safety profile with longer follow-up. Severe nonhematologic AEs were infrequent, with grade 3/4 rash, headache, and diarrhea in 2% of patients, and all other grade 3/4 AEs in < 1% of patients (Table 3). Grade 3/4 fluid retention (< 1%) or bleeding (< 1%) events were uncommon. Biochemical laboratory abnormalities were generally mild, transient, and easily managed with grade 3/4 lipase elevation (18%), hypophosphatemia (17%), and hyperglycemia (12%) being most common. Grade 3/4 elevations of alanine aminotransferase and aspartate aminotransferase occurred in 4% and 3% of patients, respectively. Only 2 patients discontinued nilotinib therapy for elevated liver transaminases. Nilotinib-associated myelosuppressive events were generally easily managed with dose interruptions or reductions or both. The change from baseline in mean time-averaged QTc interval calculated from the use of the Fridericia correction (QTcF) on day 8 at steady-state was 5.1 milliseconds. By the data cut-off, 8 patients (2.5%) had a > 60-millisecond change from baseline in QTcF interval, and 4 patients (1.2%) had a QTcF interval after baseline of > 500 milliseconds. No episodes of torsades de pointes or death due to arrhythmias were noted.
Drug-related hematologic laboratory abnormalities, nonhematologic adverse events (frequency > 10%), events associated with fluid retention (any frequency), and biochemical abnormalities (frequency > 10%) (N = 321)
| . | All grades . | Grades 3/4 . |
|---|---|---|
| Hematologic laboratory abnormalities,* n/N† (%) | ||
| Anemia | 169/318 (53) | 34/318 (11) |
| Neutropenia | 168/316 (53) | 98/316 (31) |
| Thrombocytopenia | 184/319 (58) | 95/319 (30) |
| Nonhematologic adverse events, n (%) (N = 321) | ||
| Rash | 99 (31) | 6 (2) |
| Pruritus | 84 (26) | 3 (< 1) |
| Nausea | 79 (25) | 3 (< 1) |
| Fatigue | 65 (20) | 4 (1) |
| Headache | 57 (18) | 5 (2) |
| Diarrhea | 39 (12) | 6 (2) |
| Vomiting | 41 (13) | 2 (< 1) |
| Constipation | 43 (13) | 1 (< 1) |
| Drug-related adverse events associated with fluid retention and bleeding, n (%) (N = 321) | ||
| Peripheral edema | 20 (6) | 0 |
| Pericardial effusion | 2 (< 1) | 1 (< 1) |
| Pleural effusion | 4 (1) | 1 (< 1) |
| Pulmonary edema | 1 (< 1) | 1 (< 1) |
| CNS bleeding | 1 (< 1) | 1 (< 1) |
| GI bleeding | 3 (< 1) | 1 (< 1) |
| Biochemical laboratory abnormalities,* n/N† (%) | ||
| Lipase elevation | 140/301 (47) | 54/301 (18) |
| Hypophosphatemia | 167/301 (56) | 51/301 (17) |
| Hyperglycemia | 218/311 (70) | 38/311 (12) |
| Bilirubin elevation (total) | 226/316 (72) | 23/316 (7) |
| ALT elevation | 218/318 (69) | 13/318 (4) |
| AST elevation | 173/316 (55) | 8/316 (3) |
| Hypocalcemia | 160/315 (51) | 5/315 (2) |
| Creatinine | 77/317 (24) | 4/317 (1) |
| Hypomagnesemia | 48/289 (17) | 1/289 (< 1) |
| . | All grades . | Grades 3/4 . |
|---|---|---|
| Hematologic laboratory abnormalities,* n/N† (%) | ||
| Anemia | 169/318 (53) | 34/318 (11) |
| Neutropenia | 168/316 (53) | 98/316 (31) |
| Thrombocytopenia | 184/319 (58) | 95/319 (30) |
| Nonhematologic adverse events, n (%) (N = 321) | ||
| Rash | 99 (31) | 6 (2) |
| Pruritus | 84 (26) | 3 (< 1) |
| Nausea | 79 (25) | 3 (< 1) |
| Fatigue | 65 (20) | 4 (1) |
| Headache | 57 (18) | 5 (2) |
| Diarrhea | 39 (12) | 6 (2) |
| Vomiting | 41 (13) | 2 (< 1) |
| Constipation | 43 (13) | 1 (< 1) |
| Drug-related adverse events associated with fluid retention and bleeding, n (%) (N = 321) | ||
| Peripheral edema | 20 (6) | 0 |
| Pericardial effusion | 2 (< 1) | 1 (< 1) |
| Pleural effusion | 4 (1) | 1 (< 1) |
| Pulmonary edema | 1 (< 1) | 1 (< 1) |
| CNS bleeding | 1 (< 1) | 1 (< 1) |
| GI bleeding | 3 (< 1) | 1 (< 1) |
| Biochemical laboratory abnormalities,* n/N† (%) | ||
| Lipase elevation | 140/301 (47) | 54/301 (18) |
| Hypophosphatemia | 167/301 (56) | 51/301 (17) |
| Hyperglycemia | 218/311 (70) | 38/311 (12) |
| Bilirubin elevation (total) | 226/316 (72) | 23/316 (7) |
| ALT elevation | 218/318 (69) | 13/318 (4) |
| AST elevation | 173/316 (55) | 8/316 (3) |
| Hypocalcemia | 160/315 (51) | 5/315 (2) |
| Creatinine | 77/317 (24) | 4/317 (1) |
| Hypomagnesemia | 48/289 (17) | 1/289 (< 1) |
CNS indicates central nervous system; GI, gastrointestinal tract; ALT, alanine aminotransferase; and AST, aspartate aminotransferase.
Most frequent newly occurring or worsening regardless of causality.
Total number of patients with available data at baseline and after baseline who had less than grade 4 at baseline.
Discussion
The 24-month follow-up results show that nilotinib therapy continued to be effective in patients with CML after imatinib failure, inducing MCyR and CCyR in 59% and 44% of patients, respectively, and was associated with an estimated 24-month OS of 87% and PFS of 64%. Patients with MCyR at baseline were not permitted on study, and responses were more likely in patients with CHR at baseline, suggesting that patients with imatinib resistance and intolerance with loss of CyR but not hematologic response have a more favorable outcome than do patients with loss of hematologic response. Nilotinib treatment was well tolerated, with a median dose intensity near the intended dose. The safety profile of nilotinib remained unchanged at 24 months, without significant increases in AEs from the 6-month follow-up data. No cumulative risk of hepatic, pancreatic, or cardiac events was observed with longer follow-up. QT prolongation was uncommon.
Patients in the nilotinib trial were quite resistant to imatinib therapy; most patients required high doses of imatinib for ≥ 3 months, and enrollment of imatinib-intolerant patients in MCyR at study entry was not permitted. The rigorous definition of imatinib intolerance resulted in the imatinib-intolerant population having disease characteristics more closely resembling imatinib resistance. Because patients with a baseline CHR were more likely to achieve CyR on nilotinib therapy, inclusion of more patients with baseline CHR and MCyR may influence overall response rates.
Nilotinib is also now approved as frontline therapy in patients with newly diagnosed CML-CP. At 12 months, results from the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENEST-nd) showed superior molecular and CyRs, as well as a decreased rate of progression to advanced disease for nilotinib (300 mg and 400 mg twice daily doses) compared with imatinib.15
In summary, this study update confirms that nilotinib is an effective and well-tolerated therapeutic option for patients with CML-CP resistant or intolerant to imatinib therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michelle Boehm, PhD, Daniel Hutta, PhD, Erinn Goldman, PhD, and Michael Mandola, PhD, for medical editorial assistance with this manuscript.
This work was supported by Novartis Pharmaceuticals for medical editorial assistance.
Authorship
Contribution: H.M.K., F.J.G., A.H., and M.B. designed the study, provided study and patient data, and contributed to data analysis and interpretation; J.P.R. designed the study and collected, analyzed, and interpreted data; T.P.H. designed the study and analyzed and interpreted data; D.-W.K., J.C., and P.D.l.C. provided study and patient data and collected, analyzed, and interpreted data; K.N.B., N.G., O.G.O., and G.S. provided and collected study and patient data; J.P.-I., R.A.L., and G.M. provided study and patient data; Y.S. and N.J.G collected, analyzed and interpreted data; R.B. analyzed and interpreted data; and all authors drafted and approved the manuscript.
Conflict-of-interest disclosure: H.M.K. received research funding from Novartis, Bristol-Myers Squibb, Wyeth, and ARIAD; A.H. and T.P.H. received research funding and honoraria and provided consultancy for Novartis and Bristol-Myers Squibb; M.B. received honoraria and provided consultancy for Novartis and Bristol-Myers Squibb; J.P.R. received research funding from Novartis and received honoraria and provided consultancy to Novartis and Bristol-Myers Squibb; D.-W.K. received research funding and provided consultancy to Novartis, Bristol-Myers Squibb, and Wyeth and received honoraria from Novartis and Bristol-Myers Squibb; J.C. received research funding from Novartis, Bristol-Myers Squibb, and Wyeth; P.D.l.C. received research funding from Novartis and honoraria from Novartis and Bristol-Myers Squibb; F.J.G., K.N.B., O.G.O., J.P.-I., and R.A.L. have received research funding and honoraria and provided consultancy for Novartis; G.S. has received research funding from Novartis and received honoraria and provided consultancy for Novartis and Bristol-Myers Squibb; N.G. has received honoraria and research funding from Novartis; G.M. has received honoraria from Novartis, Bristol-Myers Squibb, and Merck Sharpe & Dohme; Y.S. and N.J.G. are employees and stockholders of Novartis; and R.B. is an employee of Novartis.
Correspondence: Hagop M. Kantarjian, The University of Texas, M.D. Anderson Cancer Center, Leukemia Department, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal