Abstract
Sepsis is a systemic host response to invasive infection by bacteria. Despite treatment with antibiotics, current mortality rates are in the range of 20%-25%, which makes sepsis the most important cause of death in intensive care. Gram-negative bacteria are a prominent cause of sepsis. Lipopolysaccharide (LPS), one of the major constituents of the outer membrane of Gram-negative bacteria, plays a major role in activating the host's immune response by binding to monocytes and other cells. Several proteins are involved in neutralization and clearance of LPS from the bloodstream. Here, we provide evidence that β2-glycoprotein I (β2GPI) is a scavenger of LPS. In vitro, β2GPI inhibited LPS-induced expression of tissue factor and IL-6 from monocytes and endothelial cells. Binding of β2GPI to LPS caused a conformational change in β2GPI that led to binding of the β2GPI-LPS complex to monocytes and ultimately clearance of this complex. Furthermore, plasma levels of β2GPI were inversely correlated with temperature rise and the response of inflammatory markers after a bolus injection of LPS in healthy individuals. Together, these observations provide evidence that β2GPI is involved in the neutralization and clearance of LPS and identify β2GPI as a component of innate immunity.
Introduction
Lipopolysaccharide (LPS), a major constituent of the outer membrane of Gram-negative bacteria, plays a role in activating the host's immune response by binding to white blood cells.1 One of the important components in neutralization and clearance of LPS from the bloodstream is high-density lipoprotein (HDL). Ulevitch2 was the first to show that preincubation of LPS with HDL resulted in a decreased pyrogenic response when injected into rabbits. Furthermore, infusion of reconstituted HDL attenuated cytokine release on intravenous injection of low-dose LPS in healthy volunteers.3 Other major candidates for LPS clearance have been identified and tested, such as bactericidal/permeability-increasing protein,4 LPS binding protein,5 and soluble CD14.6
β2GPI is a highly abundant (∼ 4-5μM) 43-kDa plasma protein. It is composed of 5 homologous domains (domains I-V) that are complement control protein repeats.7,8 These complement control protein repeats are mostly found in proteins from the complement system, and they mediate binding of complement factors to viruses and bacteria.9,10 Recently, we described that β2GPI can adopt 2 different conformations: it circulates in a closed circular conformation, but it is converted into an open “activated” conformation on interaction with anionic surfaces or antibodies.11 β2GPI is known from its role in antiphospholipid syndrome (APS), in which it serves as the antigen for antiphospholipid antibodies.12,13 APS is one of the most common causes of acquired thrombophilia,14 especially at younger age.
Although the role of β2GPI in APS has been firmly established, its physiologic function remains unclear. Because of the high affinity of β2GPI for anionic phospholipids, it was thought that β2GPI, by inhibition of the contact phase activation of coagulation, could play a role in maintaining the hemostatic balance.15-17 Furthermore, it has been suggested that β2GPI is involved in platelet prothrombinase activity and ADP-mediated platelet aggregation.18,19 β2GPI binds liposomes and microparticles via an interaction with phosphatidylserine, and it also is involved in the clearance of these negatively charged cellular fragments in mice.20-22 Recently, β2GPI also has been identified as a regulator of von Willebrand function.23,24 However, individuals deficient in β2GPI do not express hemostatic abnormalities.25 Here, we show that β2GPI can bind LPS and that the binding of LPS to β2GPI inactivates LPS both in vitro and in vivo.
Methods
Human plasma β2GPI
Plasma β2GPI was isolated from fresh citrated human plasma as described previously.11 Purity of β2GPI was determined with SDS-PAGE (GE Healthcare). Purified plasma β2GPI showed a single band, with a molecular mass of approximately 43 kDa, under nonreducing conditions. The concentration of the protein was determined with the bicinchoninic acid protein assay (Thermo Fisher Scientific). Matrix-assisted laser desorption ionization/time of flight analysis of the purified protein showed that it was > 99.9% pure. The limulus amoebocyte lysate chromogenic end point assay (Hycult Biotechnology) showed that plasma-purified β2GPI contained < 0.6pM LPS. Conversion from the closed circular conformation of β2GPI to the open conformation11 was performed in a Slide-A-Lyzer 3.5K molecular weight cutoff dialysis cassette (Thermo Fisher Scientific) by dialysis against 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) containing 1.15M NaCl, pH 11.5, for 48 hours at 4°C followed by dialysis with 20mM HEPES and 150mM NaCl, pH 7.4. Conversion from the open conformation of β2GPI to the closed conformation was achieved by dialysis against 20mM HEPES and 150mM NaCl, pH 3.4, for 48 hours at 4°C followed by dialysis against 20mM HEPES and 150mM NaCl, pH 7.4. Samples were concentrated with an Ultrafree-0.5 centrifugal filter unit (Millipore). Samples were snap-frozen in liquid nitrogen and stored at 80°C for analysis.
Labeling of β2GPI and LPS
One milligram of open β2GPI at a concentration of 40μM was fluorescently labeled with Alexa Fluor 488 (Invitrogen) according to the manufacturer's instructions. Labeling of closed β2GPI was performed on a heparin column. First, 0.5 mL (40μM) of native closed β2GPI was applied to the column, after which the reactive dye was applied at a rate of 10 μL/min. Subsequently, the column was washed with 0.01M potassium phosphate, 0.15M NaCl, pH 7.2, and 0.2mM sodium azide. Fluorescently labeled closed β2GPI was eluted from the column with the same buffer containing 0.5M NaCl. The labeling of closed β2GPI did not result in a preparation that could inhibit the activated partial thromboplastin time, suggesting that the β2GPI was still in its closed conformation. Escherichia coli J5 or Salmonella minnesota R595 LPS (Sigma-Aldrich) also was Alexa Fluor 488 (Invitrogen) fluorescently labeled according to the manufacturer's instruction. The moles of dye per mole of protein was calculated via the formula (A494 × dilution factor):(71.000 × protein concentration [M]), where 71.000 cm−1 M−1 is the approximate molar extinction coefficient of the Alexa Fluor 488 dye at 494 nm. Four moles of dye per mole of β2GPI (both closed and open) and 3 moles of dye per mole of LPS were calculated.
Depletion of β2GPI from human pooled plasma
Construction, expression, and purification of individual domains of β2GPI
Human β2GPI cDNA was used for the construction of domains I, I to IV, and V of β2GPI. cDNA was subcloned into a PCR-Blunt II-TOPO vector (Invitrogen), and the separate domains were constructed with a set of primers with BamHI and NotI restriction sites. For domain I, the primers GGATCCGGACGGACCTGTCCCAAGCC and GCGGCCGCTTATACTCTGGGTGTACATTTCAGAGTG were used. For domains I to IV, the primers GGATCCGGACGGACCTGTCCCAAGCC and GCGGCCGCA-GATGCTTTACAACTTGGCATGG were used. For domain V, GATCCGCAT-CTTGTAAAGTACCTGTGAAAAAAGC and GCGGCCGCTTAGCATGGCTTTACA-TCGG were used. The PCR product was cloned into a PCR-Blunt II-TOPO vector, and sequence analysis was performed to confirm the sequence of domains I to IV and V. From this vector, the PCR product was subcloned into the expression vector HisN-Tev (Promega) and expressed in human embryonic kidney 293E cells. Recombinant domains were purified via their His-tag with nickel-Sepharose beads and eluted by 25mM tris(hydroxymethyl)aminomethane, 0.5M NaCl, and 0.5M imidazole, pH 8.2. Recombinant domain concentrations were determined using the bicinchoninic acid protein assay.
Surface plasmon resonance measurements
Surface plasmon resonance analysis was performed with a BIAcore 2000 surface plasmon resonance system (GE Healthcare). Binding of LPS or lipid A to β2GPI or LPS to domains I to IV or V of β2GPI was investigated with surface plasmon resonance. β2GPI or domains I to IV or V was immobilized on a CM5 sensor chip, and increasing concentrations of LPS (0-1μM; E coli J5 or S minnesota R595), or lipid A (0.1-1μM; S minnesota R595) were injected at time points 0, 350, and 700 seconds. Every injection was stopped after 100 seconds. Specific binding of LPS to β2GPI or domain V was corrected for nonspecific binding to the deactivated control channel. The nonspecific binding was < 1% of total binding.
Immunosorbent assay of β2GPI
NUNC MaxiSorp High Protein-Binding Capacity ELISA plates (Nalge Nunc International) were coated with 3.1μM goat anti-β2GPI (Cedarlane Labs) by incubation in 50mM carbonate buffer, pH 9.6, 100 μL in each well for 1 hour at room temperature (RT). After washing with 20mM Tris, 150mM NaCl, and 0.1% Tween 20, pH 7.4 (wash buffer), the plates were blocked by the addition of 200μL/well of 3% bovine serum albumin (Sigma-Aldrich) in 20mM Tris and 150mM NaCl, pH 7.4 (blocking buffer) for 2 hours at RT. After washing the wells 3 times with wash buffer, 100μL of plasma purified β2GPI (0-25pM in blocking buffer; as standards) or diluted pooled normal plasma in blocking buffer (1:2000) was added to the wells and incubated for 1 hour at RT. Subsequently, after washing 3 times with wash buffer, 100μL of peroxidase-conjugated goat anti-β2GPI antibodies (0.6μM; Cedarlane Labs) was added to the wells and incubated for 1 hour at RT. After the removal of unbound antibodies by washing with wash buffer, peroxidase activity of the bound antibody was measured by addition of 3,3′,5,5′-tetramethylbenzidine substrate (50μL/well; Tebu-bio Laboratories). After 20 minutes, color development was stopped by adding an equal volume of 2.0M sulfuric acid. The optical density was measured at 450 nm with a spectrophotometer (Molecular Devices). The immunosorbent assay used recognizes both the native closed form as well as the open activated form. The antibodies used are directed against domains I and IV (not the patient antibody binding site) that are accessible in both conformations. The detection signal for β2GPI in the absence or presence of LPS was the same.
Cell culture
Suspensions of human monocyte-like THP-1 cells (ATCC) were cultured in RPMI 1640 medium + GlutaMAX II (Invitrogen) and supplemented with 10% FCS, 0.1% penicillin-G, and 0.1% streptomycin sulfate (Invitrogen). Cells (1 × 106 cells/mL) in FCS-free RPMI 1640 medium were incubated (for 4 hours at 37°C) with 1nM LPS (E coli J5 or S minnesota R595) and purified plasma β2GPI (0-1μM) or 1nM LPS with 2.0μM recombinant domains I to V or V. LPS with β2GPI or domains of β2GPI were preincubated for 15 minutes at 37°C before addition to cells. Human umbilical vein endothelial cells were isolated from anonymous leftover human umbilical cords from the Department of Obstetrics, the University Medical Center Utrecht, Utrecht, the Netherlands. Incubations of endothelial cells with LPS, β2GPI, and domains of β2GPI were as described for THP-1 cells.
TF assay
Release of cell surface tissue factor (TF) was measured by determining the thrombin generation time as described previously.26 Thrombin generation time was measured spectrophotometrically by the fibrin polymerization method. All experiments were conducted in β2GPI-depleteded normal pool plasma. Cells in FCS-free RPMI 1640 medium were incubated for 4 hours at 37°C with 1nM LPS or preincubated LPS with β2GPI (0-1μM) for 15 minutes at 37°C or with recombinant domain I or V (2μM). In experiments with receptor-associated protein (RAP), cells (1 × 106 cells/mL) were first preincubated with RAP (0-10μM) for 30 minutes at 37°C before addition of LPS or β2GPI. RAP in the absence or presence of LPS in monocytes did not alter the TF expression. After incubation, cells were washed and resuspended in phosphate-buffered saline and kept at 4°C. Seventy-five microliters of cell suspension samples or TF standard was added to 100μL of NPP. Thrombin generation was initiated by the addition of 75μL of calcium chloride (38mM). The clotting time was measured spectrophotometrically and expressed as time to reach the midpoint of clear to maximum turbid density. TF release was quantified as picomolar per 1 × 106 cells measured by reference to the TF standard curve (200 to 128 000-fold dilutions of Innovin (Siemens Healthcare Diagnostics). Results are expressed as percentage as mean ± SEM relative to LPS alone (n ≥ 3).
Whole blood stimulation assay
Blood was drawn from healthy volunteers in sterile and pyrogen-free 5-mL tubes containing citrate. LPS (1nM), β2GPI (0-1μM), and recombinant domains I, I to IV, and V of β2GPI (all 2μM) in the presence or absence of LPS were added to blood and left for 6 hours at 37°C. Subsequently, samples were centrifuged for 15 minutes at 2000g, and plasma was measured for interleukin-6 (IL-6) expression with a human IL-6 enzyme-linked immunosorbent assay (Sanquin) according to the manufacturer's instructions. Results were expressed as percentage as mean ± SEM relative to LPS alone (n ≥ 3).
Intravenous LPS bolus injection
The study was approved by the institutional scientific and ethics committees (MEC 03/124). Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Twenty-three healthy male volunteers (mean ± SEM age, 23.9 ± 0.7 years) were admitted to the Clinical Research Unit of the Academic Medical Center (University of Amsterdam, Amsterdam, the Netherlands).27 Medical history, physical examination, hematologic and biochemical screening, and electrocardiograms were all normal. All participants were challenged at t = 0 hours with LPS (E coli LPS, lot G; US Pharmacopeia) as a bolus intravenous injection at a dose of 4 ng/kg body weight. Oral temperature, blood pressure, and heart rate were measured at 0.5-hour intervals. Blood was collected from a cannulated forearm vein at 2 hours and at 1 hour before LPS injection, directly before LPS administration (t = 0 hours) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, and 24 hours thereafter.
AT assay
Antithrombin (AT) levels of healthy volunteers were measured using the Berichrom antithrombin III kit (Siemens Healthcare Diagnostics) according to the manufacturer's instructions. Results were expressed as percentage relative to normal pooled plasma calibrated to the 3rd International WHO standard (06/166).
Cytokine assays
For cytokine and chemokine measurements, blood was drawn into sterile 4.5-mL tubes containing ethylenediaminetetraacetic acid (K3). Plasma was obtained by immediate centrifugation (at 1200g for 10 minutes at 4°C) and stored at −20°C until assayed. Cytokine concentrations (tumor necrosis factor [TNF]-α, IL-6, and IL-8) were measured using a cytometric bead array immunoassay (BD Biosciences/BD Biosciences Pharmingen) according to the manufacturer's instructions. The data are expressed as area under the curve (AUC) by using all time points and were determined with the InStat 5.01 software package (GraphPad Software).
Clinical sepsis study
Thirty-six patients without sepsis and 35 consecutive patients diagnosed with Gram-negative severe sepsis and admitted at the Intensive Care Unit at the Academic Medical Center in Amsterdam and University Medical Center in Utrecht were enrolled. Written informed consent was obtained from all subjects or relatives in accordance with the Declaration of Helsinki. Criteria for the diagnosis of severe sepsis, as defined previously,28 had to be met within 24 hours before enrollment. Patients were not eligible if they were < 18 years of age, if they had undergone organ transplantation, if there was an uncontrolled hemorrhage, if there was a cardiogenic shock, or if the primary acute underlying condition was a burn injury. Patients were recruited in a consecutive manner, and neither premorbid conditions, such as hypothyroidism and renal insufficiency, nor medications were excluded. Patients in the 2 groups were selected by sex and age and were confirmed for Gram-negative–induced sepsis. We did not check Gram-positive sepsis patients, because we did not find an interaction between β2GPI and Lipoteichoic acid (composite of Gram-positive bacterial outer layer) in binding assays. All patients met the criteria for severe sepsis.
Negative staining transmission electron microscopy
Plasma-purified β2GPI with or without preincubation with gold-labeled LPS (E coli J5 or S minnesota R595) or albumin, in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 150mM NaCl, pH 7.4, was analyzed by negative staining electron microscopy as described previously.11 S minnesota R595 LPS was conjugated with 5-nm colloidal gold particles by means of titration and stored in Tris-buffered saline at 4°C. Gold labeling of LPS did not change LPS activity in the TF expression assay. Solutions of β2GPI (10-20nM) were mixed with equal concentrations of gold-labeled LPS samples, allowed to react for 30 minutes at RT, and negatively stained with 0.75% uranyl formate before electron microscopy. Specimens were examined in a JEM 1230 transmission electron microscope (JEOL) at 60-kV accelerating voltage. Images were recorded with a Multiscan 791 charge-coupled device camera (Gatan) by using the software provided by the manufacturer. Figures were prepared in Photoshop CS5 (Adobe Systems).
Flow cytometric analysis
For dose-dependent binding, different concentrations of fluorescently labeled open β2GPI (0-1μM) were added to 100 μL of monocytes (1 × 106 cells/mL). For time-dependent binding 0.2μM fluorescently labeled open β2GPI was added at time points 0, 10, 20, 25, and 30 minutes to 100 μL of 1 × 106 cells/mL monocytes. For binding of LPS and fluorescently labeled plasma β2GPI, monocytes (100 μL; 1 × 106 cells/mL) were incubated for 30 minutes at 4°C with 1μM LPS (E coli J5 or S minnesota R595) or preincubated LPS (1μM) with 0.2μM fluorescently labeled plasma β2GPI. Subsequently, cells were centrifuged at 130g for 5 minutes at 4°C, supernatant was removed, and cells were taken up in 100 μL of 2% bovine serum albumin in 10mM phosphate-buffered saline, pH 7.4 (fluorescence-activated cell sorting buffer). Inhibition of time-dependent binding of β2GPI by RAP was achieved by preincubation of different concentrations of RAP (0-4μM) with 100 μL of monocytes (1 × 106 cells/mL) for 30 minutes at 4°C. Subsequently, 0.2μM fluorescently labeled open β2GPI was added and incubated for 10 minutes at 4°C. Cells were centrifuged and taken up in 100 μL of fluorescently activated cell sorting buffer. Flow cytometric analysis was performed on an FACSCalibur system (BD Biosciences). Ten thousand cells were counted, and forward scatter, side scatter, and fluorescence signals were determined for each sample and stored in list mode data files in FCS 2.0 format. The data files were acquired and analyzed by CellQuest Pro 4.0.2 software (BD Biosciences).
Statistical analysis
Differences between intensive care groups were analyzed using the Student t test. All statistical analyses were performed using InStat software (GraphPad Software), and P values < .05 were considered statistically significant. Values presented are given as mean ± SEM.
Results
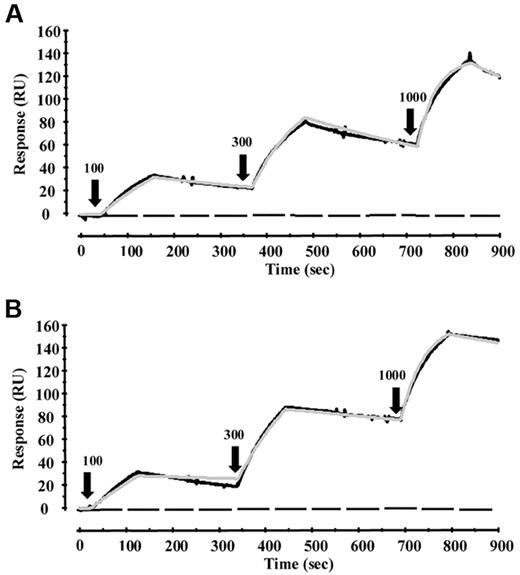
Surface plasmon resonance experiments revealed direct binding of plasma-purified β2GPI to LPS from E coli J5 or S minnesota R595 but not to lipid A (S minnesota R595). The binding was mediated via domain V but not domains I to IV of β2GPI (Figure 1). Fitting of the data to a 1-to-1 model revealed a KD value of 62nM for LPS E coli J5 and 23nM for LPS S minnesota R595.
Binding analysis of LPS and β2GPI. Binding of LPS or lipid A to β2GPI or domains I to IV and V of β2GPI was investigated with surface plasmon resonance. β2GPI or domains I to IV or V was immobilized on a CM5 sensor chip, and increasing concentrations of LPS E coli J5 (A) or LPS S minnesota R595 (B) were injected at time points 0, 350, and 700 seconds (100, 300, and 1000nM, respectively, as indicated by black arrows), and every injection was stopped after 100 seconds. Binding of both LPSs to β2GPI or domain V could be observed (black line), whereas no binding could be detected to domains I to IV (black dotted line). Also, no binding could be observed between lipid A and β2GPI or domain V (black dotted line). Fitting of the data to a 1-to-1 model revealed a KD value of 62nM for LPS E coli J5 and 23nM for LPS S minnesota R595 (visualized by the gray line).
Binding analysis of LPS and β2GPI. Binding of LPS or lipid A to β2GPI or domains I to IV and V of β2GPI was investigated with surface plasmon resonance. β2GPI or domains I to IV or V was immobilized on a CM5 sensor chip, and increasing concentrations of LPS E coli J5 (A) or LPS S minnesota R595 (B) were injected at time points 0, 350, and 700 seconds (100, 300, and 1000nM, respectively, as indicated by black arrows), and every injection was stopped after 100 seconds. Binding of both LPSs to β2GPI or domain V could be observed (black line), whereas no binding could be detected to domains I to IV (black dotted line). Also, no binding could be observed between lipid A and β2GPI or domain V (black dotted line). Fitting of the data to a 1-to-1 model revealed a KD value of 62nM for LPS E coli J5 and 23nM for LPS S minnesota R595 (visualized by the gray line).
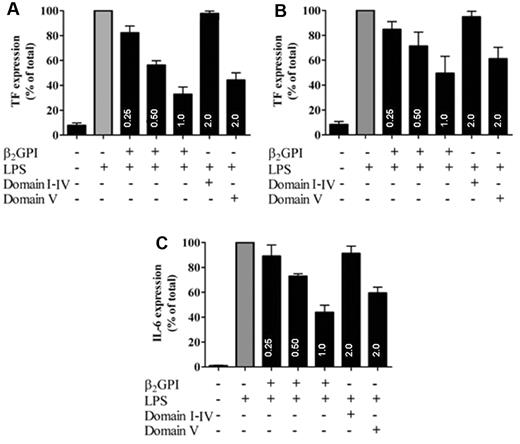
A possible functional consequence of binding of β2GPI was investigated in a cellular model of LPS-induced TF expression on monocytic cells. LPS incubation with monocytic cells resulted in TF expression and addition of plasma-purified β2GPI dose-dependently inhibited this TF expression (Figure 2A). Recombinant domain V but not domains I to IV of β2GPI inhibited LPS-induced TF expression on monocytic cells (Figure 2A). Similarly, β2GPI and recombinant domain V also inhibited LPS-induced expression of TF in human umbilical vein endothelial cells in a dose-dependent manner (Figure 2B). To extend these observations, the effects of β2GPI were investigated in a whole blood model of LPS-induced IL-6 release. Also, in this model a concentration-dependent inhibition by both β2GPI and domain V was observed on LPS-induced IL-6 expression (Figure 2C). These results show that domain V of β2GPI is able to bind and neutralize LPS in vitro.
β2GPI inhibits LPS-induced TF and IL-6 expression. LPS-induced TF expression in monocytes and LPS-induced IL-6 expression in whole blood was measured in the absence or presence of plasma-purified β2GPI or recombinant domains I to IV and V of β2GPI. A concentration-dependent inhibition of LPS-induced tissue factor expression (▩) by plasma-purified β2GPI (0.25-1μM) or recombinant domain V of β2GPI (2μM) but not with recombinant domains I to IV of β2GPI (2μM) could be observed in monocytes (A) and endothelial cells (B) after a 15-minute preincubation of β2GPI or domain I-IV or V with LPS. Similar results were obtained with LPS-induced IL-6 expression (C), where preincubation of LPS with β2GPI or recombinant domain V of β2GPI, but not with domains I to IV of β2GPI led to inhibition of the LPS induced IL-6 expression in whole blood. Data are represented as mean ± SEM relative to LPS alone (n = 3).
β2GPI inhibits LPS-induced TF and IL-6 expression. LPS-induced TF expression in monocytes and LPS-induced IL-6 expression in whole blood was measured in the absence or presence of plasma-purified β2GPI or recombinant domains I to IV and V of β2GPI. A concentration-dependent inhibition of LPS-induced tissue factor expression (▩) by plasma-purified β2GPI (0.25-1μM) or recombinant domain V of β2GPI (2μM) but not with recombinant domains I to IV of β2GPI (2μM) could be observed in monocytes (A) and endothelial cells (B) after a 15-minute preincubation of β2GPI or domain I-IV or V with LPS. Similar results were obtained with LPS-induced IL-6 expression (C), where preincubation of LPS with β2GPI or recombinant domain V of β2GPI, but not with domains I to IV of β2GPI led to inhibition of the LPS induced IL-6 expression in whole blood. Data are represented as mean ± SEM relative to LPS alone (n = 3).
To obtain insight into the in vivo relevance of the interaction between β2GPI and LPS, 23 healthy volunteers were intravenously challenged with LPS.24 The febrile response to LPS challenge was associated with tachycardia and transient flu-like symptoms, including headache, chills, nausea, and myalgia. Immediately after LPS injection, a decrease in β2GPI levels was observed in all healthy volunteers, with a mean reduction of 25% of baseline values (Figure 3A), suggesting an in vivo interaction between β2GPI and LPS. A similar reduction could not be observed for AT, a protein with similar molecular mass and plasma levels (Figure 3A). Considering that binding of LPS to β2GPI inhibited cellular LPS responsiveness in vitro, we sought to correlate constitutive plasma β2GPI concentrations with LPS-induced cytokine release in vivo. Plasma levels of β2GPI in the volunteers before infusion of LPS were highly significantly, negatively associated with plasma levels of TNF-α, IL-6, and-IL-8 after the challenge (Figure 3B-D). In agreement with this, the observed temperature rise on LPS challenge was found to be highly significantly inversely related to the baseline β2GPI level (Figure 3E).
β2GPI in experimental endotoxemia. (A) Twenty-three healthy volunteers were challenged with a bolus injection of LPS. Immediately after LPS injection (black dotted arrow at time point 0), a reduction of 25% in β2GPI levels (●) occurred in all volunteers. As a control, AT levels were measured (▴). Data are represented as mean ± SEM. β2GPI levels before LPS infusion were negatively associated with the AUC for TNF-α (B), IL-6 (C), and IL-8 (D; all P < .01). AUC for temperature rise on LPS challenge was highly significantly inversely related to baseline β2GPI levels (P = .0003; E).
β2GPI in experimental endotoxemia. (A) Twenty-three healthy volunteers were challenged with a bolus injection of LPS. Immediately after LPS injection (black dotted arrow at time point 0), a reduction of 25% in β2GPI levels (●) occurred in all volunteers. As a control, AT levels were measured (▴). Data are represented as mean ± SEM. β2GPI levels before LPS infusion were negatively associated with the AUC for TNF-α (B), IL-6 (C), and IL-8 (D; all P < .01). AUC for temperature rise on LPS challenge was highly significantly inversely related to baseline β2GPI levels (P = .0003; E).
A potential in vivo interaction between β2GPI and LPS was assessed in intensive care patients. We measured plasma β2GPI levels in 36 nonsepsis patients and 35 sepsis patients, the latter of whom had sepsis because of a Gram-negative pathogen. A significant difference in β2GPI levels was observed between nonsepsis and sepsis patients in the intensive care unit: 2.96 ± 0.20 [mean ± SEM] versus 2.14 ± 0.13μM; these levels returned to normal levels after recovery (> 7 days): 3.84 ± 0.17 versus 3.52 ± 0.20μM (Figure 4).
β2GPI in clinical sepsis. Plasma β2GPI levels in 36 nonsepsis (○) patients and 35 sepsis patients (▵, ▴), who had sepsis because a Gram-negative pathogen was measured. A significant difference in β2GPI levels could be observed between nonsepsis and sepsis patients in the intensive care unit (*P = .003), which almost returned to baseline levels after discharge (> 7 days) for both nonsepsis and sepsis patients (P = .30). Eleven sepsis patients died (▴).
β2GPI in clinical sepsis. Plasma β2GPI levels in 36 nonsepsis (○) patients and 35 sepsis patients (▵, ▴), who had sepsis because a Gram-negative pathogen was measured. A significant difference in β2GPI levels could be observed between nonsepsis and sepsis patients in the intensive care unit (*P = .003), which almost returned to baseline levels after discharge (> 7 days) for both nonsepsis and sepsis patients (P = .30). Eleven sepsis patients died (▴).
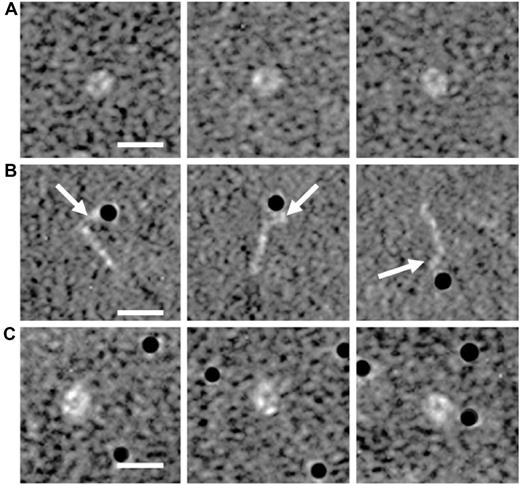
To observe a possible change in the conformation of β2GPI11 after interaction with LPS, we performed electron microscopy analysis. Incubation of plasma-purified β2GPI (Figure 5A) with gold-labeled LPS induced a conformational change in β2GPI: a conversion of the closed plasma conformation to the open form of β2GPI (Figure 5B). This conformational change was not observed when gold-labeled albumin was added to plasma-purified β2GPI (Figure 5C). Moreover, close inspection of the electron microscopy graphs indicated that LPS was bound to the bent end of the β2GPI molecule, which has been identified as domain V.11
Electron microscopy analysis of β2GPI. Purified human plasma β2GPI was visualized with electron microscopy in the absence or presence of gold-labeled (black dots) LPS or albumin. Magnifications of purified plasma β2GPI show a circular conformation (A). Purified plasma β2GPI in the presence of gold-labeled LPS shows on magnification an open fishhook-like shape of β2GPI (B), where LPS is bound to domain V of β2GPI, as indicated with the white arrows. Incubation of plasma β2GPI with gold-labeled albumin, as a control, does not induce a conformational change of β2GPI (C). White bar represents 10 nm.
Electron microscopy analysis of β2GPI. Purified human plasma β2GPI was visualized with electron microscopy in the absence or presence of gold-labeled (black dots) LPS or albumin. Magnifications of purified plasma β2GPI show a circular conformation (A). Purified plasma β2GPI in the presence of gold-labeled LPS shows on magnification an open fishhook-like shape of β2GPI (B), where LPS is bound to domain V of β2GPI, as indicated with the white arrows. Incubation of plasma β2GPI with gold-labeled albumin, as a control, does not induce a conformational change of β2GPI (C). White bar represents 10 nm.
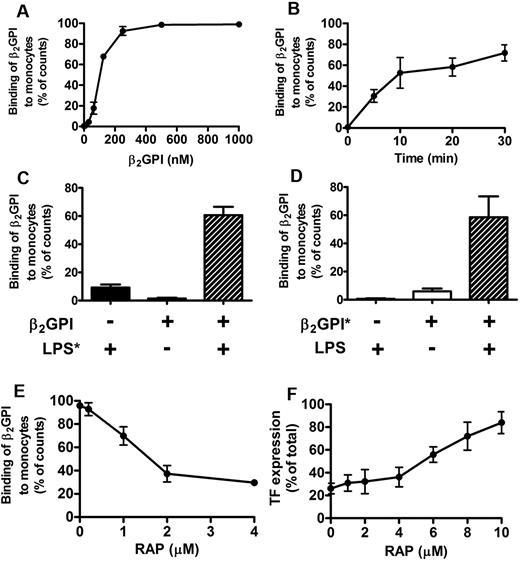
To investigate whether the reduction in β2GPI levels after LPS challenge coincided with an uptake of β2GPI by monocytes, we studied binding of β2GPI to monocytes. Fluorescently labeled β2GPI (β2GPI*) in the closed conformation did not bind to monocytes, whereas open β2GPI* bound in a concentration-dependent manner (Figure 6A). Next to a concentration-dependent binding, we also observed a time-dependent binding of open β2GPI* to monocytes (Figure 6B). To check whether the conformational change within β2GPI after binding to LPS caused binding of closed β2GPI* to monocytes, we first preincubated closed β2GPI* with LPS. We observed that incubation of LPS with closed β2GPI* led to binding of the β2GPI*-LPS complex to monocytes (Figure 6C-D), which also could be seen with open β2GPI*. Interestingly, the binding of the complex could be dose-dependently inhibited by a 4- to 10-fold excess of RAP (Figure 6E), indicating that binding of β2GPI was mediated via a receptor of the lipoprotein-related protein-receptor (LRP) family.29-31 A similar result was obtained by addition of a 4- to 10-fold excess of RAP to monocytes, in which the dose-dependent inhibition of LPS-induced TF expression by plasma-purified β2GPI was reduced (Figure 6F). In line with this, confocal microscopy revealed that preincubated closed plasma-purified β2GPI* with LPS led to internalization of the β2GPI*-LPS complex, whereas plasma-purified closed β2GPI* did not bind and therefore could not be internalized by monocytes (data not shown).
Flow cytometry with β2GPI. Binding of open or closed β2GPI to monocytes was investigated. Cells (1 × 106 cells/mL) were incubated for 30 minutes with fluorescently labeled β2GPI ± LPS or β2GPI ± fluorescently labeled LPS. Open β2GPI (●), but not plasma-purified closed β2GPI (▴), showed a concentration- (A) and time-dependent (B) binding to monocytes (both n = 5). Incubation of plasma-purified closed β2GPI (0.2μM) with fluorescently labeled LPS showed binding of β2GPI to monocytes ( ; n = 3; C). Similar binding could be observed with preincubated fluorescently labeled plasma-purified β2GPI (0.2μM) and LPS (
; n = 3; C). Similar binding could be observed with preincubated fluorescently labeled plasma-purified β2GPI (0.2μM) and LPS ( ; n = 3) (D). (E) Binding of open β2GPI (0.3μM) to monocytes could be partially inhibited (approximately 70%) by addition of RAP. (F) Addition of increasing concentrations of RAP (0-10μM) also reduced the inhibitory effect of β2GPI (1μM) on LPS-induced TF expression on monocytes (n = 3). Results are expressed as mean ± SEM.
; n = 3) (D). (E) Binding of open β2GPI (0.3μM) to monocytes could be partially inhibited (approximately 70%) by addition of RAP. (F) Addition of increasing concentrations of RAP (0-10μM) also reduced the inhibitory effect of β2GPI (1μM) on LPS-induced TF expression on monocytes (n = 3). Results are expressed as mean ± SEM.
Flow cytometry with β2GPI. Binding of open or closed β2GPI to monocytes was investigated. Cells (1 × 106 cells/mL) were incubated for 30 minutes with fluorescently labeled β2GPI ± LPS or β2GPI ± fluorescently labeled LPS. Open β2GPI (●), but not plasma-purified closed β2GPI (▴), showed a concentration- (A) and time-dependent (B) binding to monocytes (both n = 5). Incubation of plasma-purified closed β2GPI (0.2μM) with fluorescently labeled LPS showed binding of β2GPI to monocytes ( ; n = 3; C). Similar binding could be observed with preincubated fluorescently labeled plasma-purified β2GPI (0.2μM) and LPS (
; n = 3; C). Similar binding could be observed with preincubated fluorescently labeled plasma-purified β2GPI (0.2μM) and LPS ( ; n = 3) (D). (E) Binding of open β2GPI (0.3μM) to monocytes could be partially inhibited (approximately 70%) by addition of RAP. (F) Addition of increasing concentrations of RAP (0-10μM) also reduced the inhibitory effect of β2GPI (1μM) on LPS-induced TF expression on monocytes (n = 3). Results are expressed as mean ± SEM.
; n = 3) (D). (E) Binding of open β2GPI (0.3μM) to monocytes could be partially inhibited (approximately 70%) by addition of RAP. (F) Addition of increasing concentrations of RAP (0-10μM) also reduced the inhibitory effect of β2GPI (1μM) on LPS-induced TF expression on monocytes (n = 3). Results are expressed as mean ± SEM.
Discussion
The neutralization of LPS is fundamental in our protection against the toxic sequelae of severe Gram-negative infections. Different candidates have been identified as involved in the neutralization of LPS, and it is not surprising that redundant mechanisms exist for such an important process.2-6 Here, we show that β2GPI belongs to the family of LPS-neutralizing proteins, and we propose that β2GPI is an important member of this family. The levels of β2GPI in healthy volunteers immediately drop within 30 minutes after being challenged with LPS, whereas the levels of known scavengers do not decrease but increase after hours,4-6 indicating that β2GPI takes part in the immediate neutralization of LPS. The disappearance of β2GPI has very different kinetics from parameters of coagulation and fibrinolysis. Global markers such as prothrombin fragment 1 + 2, thrombin-antithrombin complexes, and plasmin-α2-antiplasmin complexes, and individual coagulation factors all show a delayed response in comparison with β2GPI.32,33 It is therefore unlikely that β2GPI was consumed by coagulation or fibrinolytic proteases. Furthermore, the levels of β2GPI are inversely correlated with the expression of inflammatory markers and with the temperature rise after a bolus injection of LPS in healthy volunteers. Apparently, β2GPI is involved in the neutralization of LPS. We also show that binding of plasma-derived β2GPI to LPS causes a conformational change within β2GPI after which the β2GPI-LPS complexes bind to and are internalized by monocytes. This could explain the significant reduction of β2GPI levels in healthy volunteers after an LPS challenge. The physiologic function of β2GPI, an abundant plasma protein, has long been a mystery. Here, we show that β2GPI acts as a direct scavenger of LPS.
Recently, we have shown that β2GPI exists in 2 completely different conformations. In plasma, it circulates in a closed conformation, but after interaction with anionic phospholipid surfaces it is converted into an open “activated” conformation.11 The interaction between domain I and domain V in the closed conformation of β2GPI is destabilized by interaction of negatively charged phospholipids with the positive charged cluster of amino acids in domain V. This results in the exposure of a “hidden” epitope for autoantibodies that characterize APS. Here, we show that interaction of LPS with domain V of β2GPI also results in a conformational change from the closed to the open conformation, suggesting that binding of LPS leads to interference of the interaction between domain I and V of β2GPI. LPS is composed of lipid A, a negatively charged inner and outer core and repeating sugar units. We established that the negatively charged domain of LPS and not the lipid A part is able to bind to the positive patch in domain V of β2GPI, thereby interfering with the intramolecular interaction between domain I and V of β2GPI. These observations could hold an important lead to our insight into the pathophysiology of APS. Direct binding of LPS to domain V of β2GPI results in a conformational change in β2GPI, which subsequently will lead to exposure of a cryptic epitope in domain I that is recognized by antiphospholipid antibodies.11,34 LPS infections could be one of the inducers of an autoimmune response to β2GPI. The incidence of thrombosis in APS is variable and is often related to coinfections.35
We could inhibit the binding of the open conformation of β2GPI to monocytes with RAP, a universal inhibitor of the members of the low-density lipoprotein (LDL)-receptor family. In addition, RAP also reduced the inhibitory effect of β2GPI on LPS-induced TF expression on monocytes. This would suggest that binding of β2GPI to LPS alone is not sufficient for the neutralization of LPS: a second step is needed in which the β2GPI-LPS complex has to bind to one of the members of the LDL-receptor family after which the complex is internalized.
β2GPI can bind to many members of the LRP-receptor family,27 but the best-studied member of this family is LRP-8.30,31 This receptor is induced during the differentiation of monocytes to macrophages.36 Similar induction of LRP expression also was demonstrated in a human monocytic cell line, THP-1.34 Interestingly, there are several publications claiming that β2GPI also can bind to known LPS receptors, Toll-like receptors (TLRs) 2 and 4.37-39 The evidence for this binding is indirect and no direct binding between β2GPI and the receptors has been shown: the addition of β2GPI to cultured endothelial cells or fibroblasts causes a cellular reaction comparable with the response induced after addition of LPS.
Here, we clearly show that in vivo the interaction of β2GPI with LDL-receptor family members is dominant over a possible interaction of LPS-β2GPI complexes with TLRs. Although Toll-like receptors are abundantly available on the monocytes of the volunteers injected with LPS, the inverse relation between β2GPI plasma levels and the clinical responses in the volunteers clearly shows that LPS-β2GPI complexes are efficiently scavenged by the LDL-receptor family members. Although we cannot exclude that part of the LPS-β2GPI complexes also bind to the TLRs on monocytes, we have shown here that binding of the complexes to LDL-receptor family members efficiently neutralizes the responses to LPS in humans.
The ability of native β2GPI to inactivate LPS in vivo offers opportunities to use β2GPI for the treatment of sepsis. We have shown that β2GPI binds to LPS via domain V of β2GPI. It seems logical to use domain V of β2GPI and not the whole molecule for sepsis treatment. When native β2GPI binds to LPS, β2GPI changes from the closed to the open conformation, in which the cryptic epitope in domain I for autoantibodies present in APS is exposed.11 The use of the whole protein could induce the formation of autoantibodies against this cryptic epitope, which could lead to the development of APS.13 The use of only domain V could potentially avoid the development of autoantibodies against β2GPI.
Figure 7 represents a schematic overview of our current findings of β2GPI. Native β2GPI in its closed conformation does not bind to the LRP receptor. On interaction of LPS with domain V of β2GPI, a conformational change occurs in β2GPI. The “active” fishhook-like conformation of β2GPI in complex with LPS is then able to bind to the receptor after which the complex is internalized. The scavenging of LPS by β2GPI leads to a decreased binding of LPS to the TLR4 receptor, resulting in a decreased expression of the inflammatory markers TNF-α, IL-6, and IL-8. The evidence provided here introduces β2GPI as a novel component of innate immunity.
Schematic overview. Plasma purified β2GPI in its closed conformation does not bind to the LRP receptor. (1) On interaction of LPS with domain V of β2GPI, (2) A conformational change occurs in β2GPI. The “active” fishhook-like conformation of β2GPI in complex with LPS is then able to bind to the receptor (3) after which the β2GPI-LPS complex is cleared. (4) The scavenging of LPS by β2GPI leads to a decreased binding of LPS to the TLR4 receptor (5), resulting in a decreased expression of the inflammatory markers TNF-α, IL-6, and IL-8. (6)
Schematic overview. Plasma purified β2GPI in its closed conformation does not bind to the LRP receptor. (1) On interaction of LPS with domain V of β2GPI, (2) A conformational change occurs in β2GPI. The “active” fishhook-like conformation of β2GPI in complex with LPS is then able to bind to the receptor (3) after which the β2GPI-LPS complex is cleared. (4) The scavenging of LPS by β2GPI leads to a decreased binding of LPS to the TLR4 receptor (5), resulting in a decreased expression of the inflammatory markers TNF-α, IL-6, and IL-8. (6)
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marieke A. van Zoelen and Martijn D. de Kruif for providing experimental endotoxemia samples, Maria Baumgarten and J. Arnoud Marquart for technical support, and Rita Wallén and Eric Hallberg for help with electron microscopy.
This study was supported in part by grants from the Swedish Medical Research Council (project 2008-7480); the Foundations of Crafoord, Greta och Johan Kock's stiftelser, Alfred Österlund's stiftelse, Konung Gustav V's 80-Årsfond; the Swedish Society for Medicine, the Medical Faculty, Lund University (M.M.); a grant from the Netherlands Organization for Scientific Research (ZonMW; J.C.M.M. and P.G.d.G.); and an Academic Medical Center stimulation grant (J.C.M.M). B.d.L. is a postdoctoral fellow of the Netherlands Heart Foundation (grant 2006T053).
National Institutes of Health
Authorship
Contribution: Ç.A., M.M., S.D.D.C.M., and B.d.L. performed experiments; Ç.A., P.G.d.G., M.M., S.D.D.C.M., G.M.A.v.O., J.H.M.L., B.d.L., R.T.U., H.H., T.v.d.P., and J.C.M.M. analyzed the results; Ç.A. and M.M, created the figures; P.G.d.G., J.H.M.L., B.d.L., R.T.U., H.H., T.v.d.P., and J.C.M.M. participated in the analysis, discussion, and interpretation of the data; J.C.M.M, and P.G.d.G. designed the research; Ç.A., P.G.d.G., and J.C.M.M. drafted the manuscript; and all authors were involved in finalizing the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joost C. M. Meijers, Department of Experimental Vascular Medicine, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands; e-mail: j.c.meijers@amc.uva.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal