Abstract
Blood vessel remodeling is crucial to the formation of the definitive vasculature, but little is known about the mechanisms controlling this process. We show that Delta-like ligand 4 (Dll4)/Notch pathway regulates vessel regression in normal pathologic conditions. Genetic and pharmacologic inhibition of Dll4/Notch prevented retinal capillary regression in the oxygen-induced retinopathy (OIR) model and during normal development. Deletion of the Notch-regulated ankyrin repeat protein, a negative regulator of the Notch pathway, produced an opposite phenotype. Inhibition of Dll4/Notch reduced vessel occlusion, maintaining blood flow that is essential for survival of microvessels. Dll4/Notch inhibition up-regulated the expression of vasodilators adrenomedullin and suppressed the expression of vasoconstrictor angiotensinogen. Angiotensin II induced rapid nonperfusion and regression of developing retinal capillaries, whereas Ace1 and AT1 inhibitors and adrenomedullin attenuated vasoobliteration in OIR, indicating that both pathways are involved in modulating vessel remodeling. In contrast, inhibition of vascular endothelial growth factor-A (VEGF-A) did not result in a pervasive loss of retinal capillaries, demonstrating that reduced expression of VEGF-A is not the proximate cause of capillary regression in OIR. Modulation of VEGF-A and DII4/Notch signaling produced distinct changes in blood vessel morphology and gene expression, indicating that these pathways can have largely independent functions in vascular remodeling.
Introduction
Notch signaling pathways are evolutionary conserved and control cell-fate determination and differentiation in multiple tissues and cell types,1,2 including the vascular system.3 Signaling through Notch1 and Notch4 receptors has been implicated in regulating angiogenesis and vascular differentiation during development and in diverse pathologic conditions.4-6 Delta-like ligand 4 (Dll4) is the only Notch ligand expressed predominantly by the vascular endothelium,7-9 and mice lacking even a single Dll4 allele exhibit multiple severe vascular abnormalities, including defective arteriogenesis, resulting in embryonic lethality in most mouse strains. For other genes associated with angiogenesis, lethal haploinsufficiency has been previously observed only in vascular endothelial growth factor-A (VEGF-A) mutants,10,11 further emphasizing the critical nature of Dll4 in vascular development. The VEGF and Dll4/Notch signaling pathways interplay at several levels in vascular development and pathology.12 Notably, in all species, and under all normal and pathologic angiogenic conditions evaluated to date, Dll4/Notch inhibition stimulates vascular sprouting and endothelial cell proliferation.13,14 Under these conditions, VEGF induces Dll4 expression in the “tip” and stalk cells of angiogenic vessels, where it acts as a negative regulator of VEGF action by suppressing further tip cell formation and proliferation of stalk cells.15,16 For example, during normal retinal development, inhibition of the Dll4/Notch pathway increases angiogenic sprouting, resulting in the formation of an abnormally dense primary capillary plexus.15,17,18 In the oxygen-induced model of ischemic retinopathy (OIR),19 increased angiogenic sprouting produced by Dll4/Notch inhibition results in a more rapid revascularization of the ischemic portions of the retina.15
However, Dll4 is expressed not only in “tip” and stalk cells during active angiogenesis, it is also expressed at particularly high levels in the vascular endothelium of mature arteries and arterioles and, to a lesser extent, in capillaries but not veins.7,15 Moreover, in contrast to Dll4 expression in angiogenic vessels, the expression of Dll4 in postangiogenic vessels does not appear to be regulated by VEGF.15 Although the role of Dll4 in sprouting angiogenesis has been extensively studied, currently very little is known about the function of the Dll4/Notch pathway in postangiogenic and mature blood vessels.
In this paper, we report that genetic or pharmacologic modulation of Dll4/Notch signaling affects postangiogenic blood vessel remodeling and regression, a process that plays a critical role in determining the patterning and density of blood vessels in mature tissues. Specifically, constitutive or conditional genetic deletion of Dll4 or pharmacologic inhibition of Dll4/Notch signaling inhibits the normal developmental pruning of capillaries in the developing retinal vasculature, as well as blood vessel regression after exposure to hyperoxia. Moreover, genetic deletion of a negative feedback regulator of the Notch pathway (Notch-regulated ankyrin repeat protein [Nrarp]) results in an opposite Notch gain-of-function phenotype leading to exaggerated vaso-obliteration. In both circumstances, blood vessel regression was preceded by a loss of blood flow, which was maintained by Dll4/Notch inhibition. This effect was associated with induction of the genes that encode vasodilatory peptides (eg, adrenomedullin) while suppressing the expression of genes involved in vasoconstriction (eg, angiotensinogen). Effects of Dll4/Notch signaling modulation were largely distinct from changes produced by modulation of VEGF-A activity, at both morphologic and gene expression levels, indicating that these 2 pathways independently regulate postangiogenic blood vessel remodeling and homeostasis. Importantly, attenuation of Dll4/Notch signaling maintained blood flow and prevented capillary regression without inducing the gross morphologic changes in the postangiogenic vasculature that were evident after administration of VEGF-A. The ability of Dll4/Notch signaling to modulate blood flow and subsequent vascular regression via angiotensin and adrenomedullin pathways represents a new mechanism by which this pathway modulates vascular development and remodeling.
Methods
Animals
Generation of Dll4+/lacZ mice was previously described.7,15 Conditional Dll4 COIN mice were generated as shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), to be described in greater detail elsewhere (A.E., D. Frendewey, P. Yang, et al, Conditionals-by-inversion: a novel universal method for the generation of conditional alleles, manuscript in preparation). VelociGene technology was used to replace the entire Nrarp coding region with the β-galactosidase reporter gene in C57BL/6:129 hybrid mouse embryonic stem cells.20 C57/Bl6 mice (Taconic Farms) were used to study the effect of Dll4-constant Fc region of human IgG (Fc) on normal vascular development and vaso-obliteration in OIR. All animal manipulations were approved by the Regeneron Pharmaceuticals Institutional Animal Care and Use Committee and conformed to Association for Research in Vision and Ophthalmology guidelines for the use of animals.
Reagents and antibodies
Dll4-Fc composes the extracellular domain of human Dll4 expressed in sequence with the constant (Fc) part of human IgG1. Dll4-Fc was expressed in CHO cells and affinity purified by protein-A chromatography. Dll4-Fc binds to Notch receptors, preventing their interaction with endogenous Notch ligands and has previously been shown to inhibit Notch signaling in vitro and in vivo.15,16 DAPT (Assay Design) was dissolved in dimethyl sulfoxide and injected subcutaneously. Captopril (Sigma-Aldrich) and Losartan (Cayman Chemical) were dissolved in water and injected periocularly. HuAngiotensin II was from MP Chemical, and huAdrenomedullin was from American Peptide. Anti–collagen IV antibody was from Abcam; anti-platelet-endothelial cell adhesion molecule (PECAM) antibody was from BD Biosciences PharMingen. Fluorescein isothiocyanate-labeled Griffonia simplicifolia lectin I, Texas Red-labeled Lycopersicon esculentum (tomato) lectin, and rhodamine red-labeled conconavalin A (ConA) lectin were from Vector Laboratories. Secondary antibodies labeled with Alexa fluorochrome were from Invitrogen.
OIR and ITV microinjections
OIR was produced after the method developed by Smith et al.19 Briefly, litters of 7-day-old (postnatal day 7, P7) mouse pups and their dams were placed in hyperoxia (75% oxygen) to induce pervasive capillary obliteration in the central retina. For shorter-term pharmacologic studies designed to evaluate the mechanisms leading to capillary regression, pups were exposed to hyperoxia from P9 to P10. Intravitreal (ITV) microinjections (50-500 nL) were made between the equator and the corneal limbus using a Drummond nanoinjector equipped with a glass needle as described.15 Annexin V (Invitrogen) was injected ITV before animal exposure to high oxygen to evaluate the temporal relationship of endothelial cell apoptosis to vaso-occlusion.
Immunostaining and quantification
Immunostaining and quantification were performed as previously described.15 Images were taken using a Nikon Eclipse microscope. For each experimental condition, 3 to 5 retinas were used for quantification. Quantifications of avascular areas and blood vessel density were performed using Scion Image, Version 1.63 and Fovea, Version 4.0 (Reindeer Graphics) software. Total blood vessel length was extracted from the skeletonized (single pixel wide) images using Fovea, Version 4.0. To assess blood vessel regression, collagen IV-positive/PECAM-negative capillary segments were counted in 8 images (original magnification × 400) taken close to the optic nerve in each retina. To assess blood vessel nonperfusion, Griffonia simplicifolia lectin I (GS lectin-positive)/ConA lectin-negative capillary segments were counted in 4 to 6 images (original magnification × 100) taken close to the optic nerve in each retina. Student t test or one-way analysis of variance for multiple comparisons was used to assess statistical significance. Images were assembled into figures using Adobe Photoshop CS3 and Adobe Illustrator CS3 software.
Microarray and TaqMan gene expression analysis
For retinal gene expression studies, 1 μg of VEGF-A165, 4 μg of Dll4-Fc, or 4 μg of hFc was injected ITV at P8. Retinas were harvested 24 hours after the injection, and retinal RNA was purified using the RNeasy Mini Kit (QIAGEN). Cyanine 3/5-labeled cRNA was produced from 2 μg of total RNA using the Low RNA Input Linear Amplification Kit (Agilent). Each sample labeled in Cyanine 3 and common reference labeled in Cyanine 5 were hybridized to a custom Agilent mouse whole genome gene expression array. The arrays were scanned by Agilent Microarray scanner, and the data were extracted by Agilent Feature Extraction Software. All microarray data are available on the Gene Expression Omnibus (GEO) website under accession number GSE28516.
Results
Genetic and pharmacologic manipulation of the Dll4/Notch pathway modulates oxygen-induced vaso-obliteration in the retina
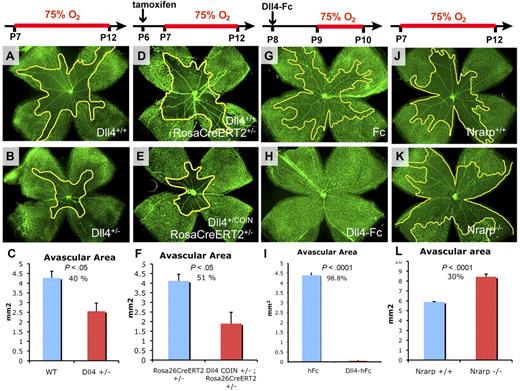
Normal blood vessel differentiation, remodeling, and pruning are thought to be driven in large part by tissue oxygenation.21 In the murine model of OIR, exposure of developing animals to hyperoxia induces profound capillary regression in the central retina.19 To determine whether acute attenuation of Dll4/Notch signaling is involved in blood vessel regression, we further evaluated the effect of genetic deletion of Dll4 on vaso-obliteration in the OIR model. We had previously observed that oxygen-induced capillary obliteration is attenuated in the retinas of mice in which one Dll4 allele had been deleted.15 Seven-day-old (P7) Dll4+/− mice together with littermate wild-type (WT) controls were subjected to hyperoxia for 5 days (until P12) followed by assessment of retinal vasculature using GS lectin staining. Retinal capillaries in Dll4+/− mice were significantly more resistant to oxygen-induced vaso-obliteration (Figure 1A-B). Quantitative analysis revealed an approximately 40% decrease of the avascular areas in retinas of Dll4+/− mice compared with WT littermates (Figure 1C). These data confirmed that reduced expression of Dll4 makes capillaries more resistant to hyperoxia-induced vaso-obliteration.
Effects of Dll4/Notch genetic and pharmacologic modulation on oxygen-induced vaso-obliteration. (A-B,D-E,G-H,J-K) GS lectin staining of the retinal vasculature in 12-day-old (A,D,J) or 10-day-old (G) control mice and in Dll4+/− (B), Dll4+/COIN;Rosa26-CreERT2+/− treated with tamoxifen, and Nrarp−/− (K) mice that were exposed to high oxygen for 5 days and WT mice treated with Dll4-Fc and exposed to high oxygen for 1 day (H). (C,F,I,L) Quantification of the avascular area. Decreased vaso-obliteration in Dll4+/− (C) and Dll4+/COIN;Rosa26-CreERT2+/− (F) mice compared with littermate controls as well as in mice treated with Dll4-Fc (I) compared with control mice treated with Fc. (L) Increased vaso-obliteration in Nrarp−/− mice compared with WT littermates. Percentages indicate changes in avascular areas compared with corresponding control animals. Original magnification, ×20.
Effects of Dll4/Notch genetic and pharmacologic modulation on oxygen-induced vaso-obliteration. (A-B,D-E,G-H,J-K) GS lectin staining of the retinal vasculature in 12-day-old (A,D,J) or 10-day-old (G) control mice and in Dll4+/− (B), Dll4+/COIN;Rosa26-CreERT2+/− treated with tamoxifen, and Nrarp−/− (K) mice that were exposed to high oxygen for 5 days and WT mice treated with Dll4-Fc and exposed to high oxygen for 1 day (H). (C,F,I,L) Quantification of the avascular area. Decreased vaso-obliteration in Dll4+/− (C) and Dll4+/COIN;Rosa26-CreERT2+/− (F) mice compared with littermate controls as well as in mice treated with Dll4-Fc (I) compared with control mice treated with Fc. (L) Increased vaso-obliteration in Nrarp−/− mice compared with WT littermates. Percentages indicate changes in avascular areas compared with corresponding control animals. Original magnification, ×20.
However, this phenomenon could be a secondary consequence of long-term, constitutive reduction in Dll4/Notch signaling and resultant alterations in retinal vascular development in Dll4+/− mice. Therefore, we evaluated the effect of conditional deletion of Dll4 coincident with exposure to hyperoxia. Mice bearing a single conditional Dll4 COIN allele (supplemental Figure 1; A.E., D. Frendewey, P. Yang, et al, Conditionals-by-inversion: a novel universal method for the generation of conditional alleles, manuscript in preparation); and a single Rosa26CreERT2 allele (Dll4+/COINRosa26-CreERT2+/−) and littermate control mice with a single Rosa26CreERT2 allele (Dll4+/+;Rosa26-CreERT2+/−) were injected with tamoxifen (25 mg/kg, intraperitoneally) at P6 followed by exposure to high oxygen from P7 to P12. Acute conditional genetic deletion of a single Dll4 allele had the same effect on the retinal vasculature as the constitutive Dll4 deletion, substantially reducing capillary regression (Figure 1D-F).
To confirm these findings, we next evaluated the effect of acute pharmacologic Dll4/Notch inhibition in the OIR model using ITV injections of 4 μg of Dll4-Fc, a soluble decoy Notch ligand, or control human Fc protein at P8, before exposure to hyperoxia on P9. Local ITV delivery was chosen to eliminate potential secondary systemic effects of Dll4/Notch inhibition. Retinal vasculature was assessed 24 hours later at P10 using GS lectin staining. Within 24 hours of exposure to high oxygen, capillaries in the central retina of the control animals treated with Fc had regressed (Figure 1G), whereas the retinal capillaries in Dll4-Fc-treated mice were well preserved (Figure 1H-I). Systemic administration of Dll4-Fc (25 mg/kg, intraperitoneally) also produced significant reduction in capillary loss (supplemental Figure 2), indicating that Dll4-Fc acts directly on the vascular endothelium rather than on other organs or other retinal cell types. Inhibition of Notch signaling with DAPT, a γ-secretase inhibitor, also reduced vaso-obliteration in OIR (supplemental Figure 3).
To determine whether increased Notch signaling would have an opposite effect on oxygen-induced blood vessel regression, we also evaluated the effect of genetic deletion of Nrarp. Nrarp is a target gene of Notch that acts as a negative feedback regulator of Notch signaling, by promoting degradation of Notch intracellular domain.20,22 Thus Nrarp deficiency results in Notch gain-of-function. As expected, homozygous deletion of Nrarp produced a phenotype that was opposite that of Dll4 inhibition or haploinsufficiency. The area of oxygen-induced vaso-obliteration at P12 was increased by 30% in Nrarp−/− mice (Figure 1J-L). These results indicate that suppression and activation of the canonical Notch pathway have opposite effects on oxygen-induced vaso-obliteration.
Inhibition of Dll4/Notch pathway reduces blood vessel constriction and capillary nonperfusion
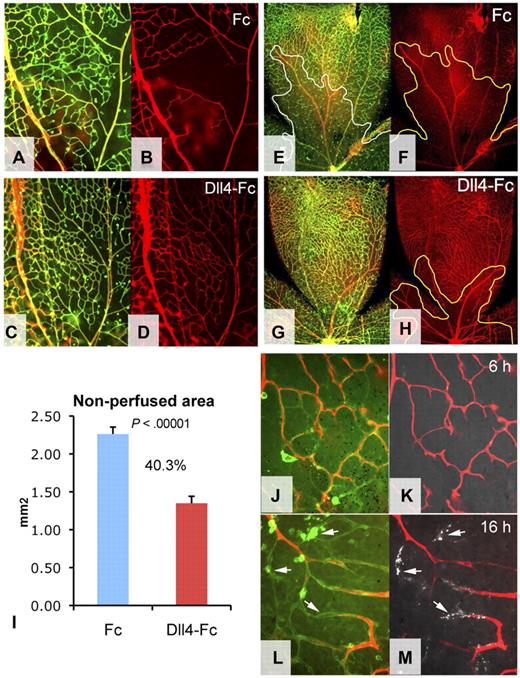
The molecular pathways that mediate oxygen-induced vaso-obliteration have not been fully elucidated. Hyperoxia might affect the retinal vasculature by reducing VEGF expression,23 leading to regression of immature capillaries still dependent on VEGF for their survival. However, this hypothesis is not entirely consistent with pervasive degeneration of more mature capillaries in the central retina. Alternatively, given the critical role of blood flow in promoting the survival of small blood vessels,24 the proximate cause of vessel regression in OIR might be an excessive vasoconstriction,25 resulting in prolonged capillary nonperfusion. To determine whether capillaries preserved by Dll4-Fc treatment in mice exposed to hyperoxia remain patent, animals were treated on P8 with Dll4-Fc or Fc (25 mg/kg intraperitoneally) followed by a brief 6-hour exposure to high oxygen on P9. Fluorescently labeled ConA lectin was then injected into left cardiac ventricle and allowed to circulate for 5 minutes, at which time retinas were removed and stained with GS lectin to visualize the entire retinal vasculature (Figure 2A,C). A pervasive lack of blood flow was evident in the capillaries of the central retina of Fc-treated controls (Figure 2B), whereas most retinal capillaries in Dll4-Fc-treated mice remained patent (Figure 2D), indicating that Dll4/Notch inhibition can prevent acute blood vessel nonperfusion caused by exposure to elevated levels of oxygen.
Blood vessel patency in mice exposed to hyperoxia. (A-H) Combined GS lectin staining (green) and ConA perfusion staining (red) (A,C,E,G) and ConA perfusion staining (B,D,F,H) of the retinal vasculature treated with Fc (A-B,E-F) or Dll4-Fc (C-D,G-H) for 24 hours followed by high oxygen exposure for 6 hours (A-D) or 24 hours (E-H). (I) Quantification of nonperfused areas per retinal quadrant; percentages indicate decrease of nonperfused area. (J-M) Combined GS lectin staining (green), ConA perfusion staining (red), and annexin V labeling (white, arrows). Six hours (J-K) and 16 hours (L-M) of hypoxia. Original magnifications: A-D, ×100; E-H, × 40; and J-L, ×400.
Blood vessel patency in mice exposed to hyperoxia. (A-H) Combined GS lectin staining (green) and ConA perfusion staining (red) (A,C,E,G) and ConA perfusion staining (B,D,F,H) of the retinal vasculature treated with Fc (A-B,E-F) or Dll4-Fc (C-D,G-H) for 24 hours followed by high oxygen exposure for 6 hours (A-D) or 24 hours (E-H). (I) Quantification of nonperfused areas per retinal quadrant; percentages indicate decrease of nonperfused area. (J-M) Combined GS lectin staining (green), ConA perfusion staining (red), and annexin V labeling (white, arrows). Six hours (J-K) and 16 hours (L-M) of hypoxia. Original magnifications: A-D, ×100; E-H, × 40; and J-L, ×400.
To test patency of the blood vessels remaining after more prolonged exposure to hyperoxia, animals were again treated at P8 with either Fc or Dll4-Fc, followed by 24-hour exposure to high oxygen starting at P9. At P10 animals were injected with fluorescent ConA, lectin and the retinal vasculature was stained with GS lectin (Figure 2E-H). Although there was some reduction in the perfusion of capillaries closest to the nerve head, measurement of nonperfused areas showed that the majority of preserved capillaries in Dll4-Fc-treated retinas remained well perfused (Figure 2I). In contrast, most capillaries in the control retinas of Fc-treated mice had already degenerated by this time. Moreover, capillary diameter in Dll4-Fc-treated retinas was similar to that seen in animals raised under normoxic conditions, whereas the few remaining blood vessels in retinas of control mice exposed to hyperoxia appeared constricted.
To evaluate the effects of Dll4/Notch inhibition during prolonged exposure to hyperoxia, Dll4+/COIN;Rosa26-CreERT2+/− and littermate control Dll4+/+;Rosa26-CreERT2+/− animals were given a single injection of tamoxifen at P6 followed by exposure to high oxygen from P7 to P12. Patency of blood vessels was tested by intracardiac infusion of red fluorescent lectin. Capillaries preserved by conditional deletion of Dll4 also remained patent (supplemental Figure 4).
It has previously been shown that short-term retinal vessel occlusion resulting from hyperoxia is reversible on return to room air and does not cause blood vessel regression.19 To test whether capillary occlusion and vessel regression are indeed sequential events, animals were exposed to high oxygen for 6 or 16 hours. The presence of the apoptotic cells in the capillaries was evaluated using ITV injections of fluoresceinated annexin V. Remarkably, although no apoptotic annexin V-positive cells were observed in nonperfused capillaries after 6 hours of hyperoxia, a more prolonged 16-hour exposure to high oxygen induced apoptosis in the nonperfused capillaries (Figure 2J-M), confirming that vessel occlusion precedes endothelial cell death and vessel regression. Taken together, these results suggest that capillary nonperfusion is the proximate cause of vascular regression in OIR.
Interestingly, deletion of even a single Dll4 allele or partial inhibition of Dll4/Notch signaling with Dll4-Fc had very significant effects on blood vessel patency and regression, indicating a critical role of Dll4/Notch in this process.
Genetic and pharmacologic inhibition of the Dll4/Notch pathway also reduces capillary regression during normal developmental capillary remodeling
Genetic or pharmacologic inhibition of Dll4/Notch signaling during retinal angiogenesis leads to formation of a denser primary vascular plexus associated with increased formation of new capillary sprouts and endothelial cell proliferation.15,17,18 As Dll4 continues to be expressed at high to moderate levels in the endothelial cells of mature arteries and capillaries, we also investigated whether inhibition of Dll4/Notch signaling might also modulate normal developmental remodeling in postangiogenic vessels. To this end, we also assessed the effects of acute conditional deletion of one Dll4 allele, or pharmacologic inhibition of Dll4/Notch signaling on the developing retinal vasculature after the superficial vascular plexus had formed and sprouting angiogenesis had ceased.
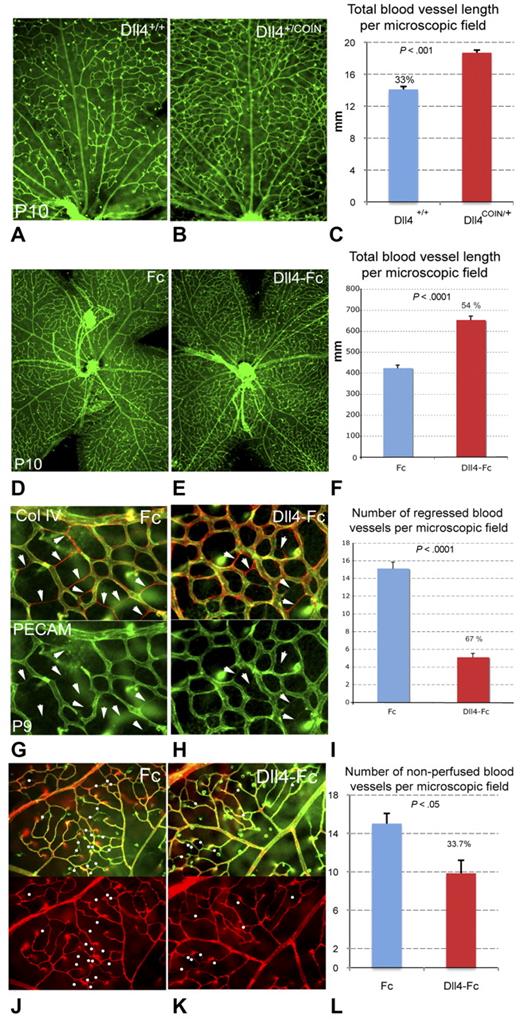
Dll4+/COIN;Rosa26-CreERT2+/− and littermate control Dll4+/+;Rosa26-CreERT2+/− animals were given a single injection of tamoxifen on P7. Retinal vasculature was assessed at P10 using GS lectin staining (Figure 3A-B). Retinal vessel density was increased by 33% (Figure 3C) in the central retina of Dll4+/COIN mice relative to controls. Similar effects were seen after acute pharmacologic inhibition of Dll4/Notch using Dll4-Fc. Mouse pups were injected intraperitoneally with Dll4-Fc or a control Fc protein at a dose of 25 mg/kg at P8, and retinal vasculature was assessed at P10 (Figure 3D-E). Retinal vessel density was increased in the Dll4-Fc-treated animals by 54% compared with control animals treated with Fc (Figure 3F).
Effects of genetic and pharmacologic inhibition of Dll4/Notch on capillary remodeling during retinal vascular development. (A-C) Reduced blood vessel pruning in conditional Dll4+/− mice. Retinal vasculature at P10 in Dll4+/+;Rosa26-CreERT2+/− (A) and Dll4+/COIN;Rosa26-CreERT2+/− (B) mice treated for 72 hours with tamoxifen; GS lectin staining. (C) Quantification of total blood vessel length in the central retina (per 100 × microscopic field); percentages indicate changes in total vessel length compared with WT (Dll4+/+;Rosa26-CreERT2+/−) control mice. (D-F) Reduced blood vessel pruning in Dll4-Fc-treated mice. Retinal vasculature in P10 mouse pups treated systemically with Fc control protein (D) or Dll4-Fc (E) for 48 hours; GS lectin staining. (F) Quantification of total blood vessel length in the central retina (per 20× microscopic field); percentages indicate increase in total vessel length. (G-I) Reduced blood vessel regression in Dll4-Fc-treated mice. Retinal vasculature in P9 mouse pups treated systemically with Fc control protein (G) or Dll4-Fc (H) for 24 hours; double labeling with PECAM (green) and collagen IV (Col IV) antibody (red) staining. (I) Quantification of the number of regressed capillary segments (empty Col IV-positive tubes; per 400× microscopic field); percentages indicate decrease in the number of regressed capillary segments. (J-L) Reduced blood vessel occlusion in Dll4-Fc-treated mice. Double labeling with GS lectin staining (green) and ConA lectin perfusion staining (red). (L) Quantification of the number of occluded capillary segments (nonperfused capillaries; per 400× microscopic field); percentages indicate decrease in the number of occluded capillary segments. Original magnifications: A-B,D-F, ×40; J-K, ×200; G-H, ×400.
Effects of genetic and pharmacologic inhibition of Dll4/Notch on capillary remodeling during retinal vascular development. (A-C) Reduced blood vessel pruning in conditional Dll4+/− mice. Retinal vasculature at P10 in Dll4+/+;Rosa26-CreERT2+/− (A) and Dll4+/COIN;Rosa26-CreERT2+/− (B) mice treated for 72 hours with tamoxifen; GS lectin staining. (C) Quantification of total blood vessel length in the central retina (per 100 × microscopic field); percentages indicate changes in total vessel length compared with WT (Dll4+/+;Rosa26-CreERT2+/−) control mice. (D-F) Reduced blood vessel pruning in Dll4-Fc-treated mice. Retinal vasculature in P10 mouse pups treated systemically with Fc control protein (D) or Dll4-Fc (E) for 48 hours; GS lectin staining. (F) Quantification of total blood vessel length in the central retina (per 20× microscopic field); percentages indicate increase in total vessel length. (G-I) Reduced blood vessel regression in Dll4-Fc-treated mice. Retinal vasculature in P9 mouse pups treated systemically with Fc control protein (G) or Dll4-Fc (H) for 24 hours; double labeling with PECAM (green) and collagen IV (Col IV) antibody (red) staining. (I) Quantification of the number of regressed capillary segments (empty Col IV-positive tubes; per 400× microscopic field); percentages indicate decrease in the number of regressed capillary segments. (J-L) Reduced blood vessel occlusion in Dll4-Fc-treated mice. Double labeling with GS lectin staining (green) and ConA lectin perfusion staining (red). (L) Quantification of the number of occluded capillary segments (nonperfused capillaries; per 400× microscopic field); percentages indicate decrease in the number of occluded capillary segments. Original magnifications: A-B,D-F, ×40; J-K, ×200; G-H, ×400.
Given that Dll4/Notch inhibition is known to increase vascular sprouting and endothelial cell proliferation earlier during the angiogenic phase of retinal vascular development, the observed increase in capillary density might have resulted from reinitiation of angiogenesis. However, examination of the retinas after conditional deletion or pharmacologic inhibition of Dll4 in the postangiogenic period did not reveal the presence of sprouts or other morphologic evidence of angiogenic vessel formation in the central retina. To confirm the alternate hypothesis that retinal capillary regression was reduced, retinal flatmounts from Dll4-Fc and control mice were double stained for PECAM and collagen IV (Figure 3G-I). Collagen IV is a component of the basal lamina surrounding blood vessels, and collagen IV-positive tubes lacking PECAM immunoreactivity represent the empty basement membrane sleeves of previously extant vessels that have regressed. Eight-day-old pups were injected intraperitoneally with Dll4-Fc or Fc, and retinal vasculature was assessed 24 hours later at P9 by counting the numbers of empty collagen tubes (Figure 3G-H). Quantification revealed a 67% decrease in the number of regressed capillaries in retinas of mice treated with Dll4-Fc (Figure 3I). Thus, Dll4/Notch inhibition can reduce or prevent capillary regression during normal vascular remodeling, resulting in a relative increase in vascular density. These results reveal a new mechanism by which Dll4/Notch signaling regulates normal developmental tissue vascularization: in addition to restraining angiogenic sprouting (tip cell formation) and endothelial cell proliferation, Dll4/Notch signaling also regulates postangiogenic vascular remodeling by promoting capillary regression.
We also evaluated the number of nonperfused blood vessels in retinas of animals treated systemically for 24 hours with Dll4-Fc or Fc control at 25 mg/kg. Blood vessel patency was tested using ConA lectin intracardiac infusion followed by GS lectin staining. Retinas treated for 24 hours with Dll4-Fc displayed reduced number of nonperfused vessels (Figure 3J-L), confirming that Dll4/Notch pathway can also control blood vessel patency in normal remodeling blood vessels.
Dll4-Fc and VEGF differentially regulate the expression of vasoactive genes
To further explore the molecular mechanisms underlying the vasoprotective effect of Dll4/Notch inhibition, we conducted a microarray gene expression study of retinas treated with Dll4-Fc or VEGF-A, a potent proangiogenic and vasodilatory factor. It has previously been shown that, during periods of active angiogenesis, Dll4 is induced by VEGF in the endothelium of angiogenic vessels and acts as a negative regulator of VEGF-induced vascular sprouting and endothelial cell proliferation.15 Although it has been suggested that inhibition of Dll4/Notch signaling increases VEGF action, at least in part, by increasing the expression of VEGF receptor 2,18 in other studies, the effects of Dll4 blockade on proliferating vessels have been noted before the up-regulation of VEGF receptor 2 expression, indicating that Dll4/Notch signaling can affect vascular development and integrity by mechanisms beyond direct or indirect modulation of the VEGF-A/VEGF receptor 2 pathway.15
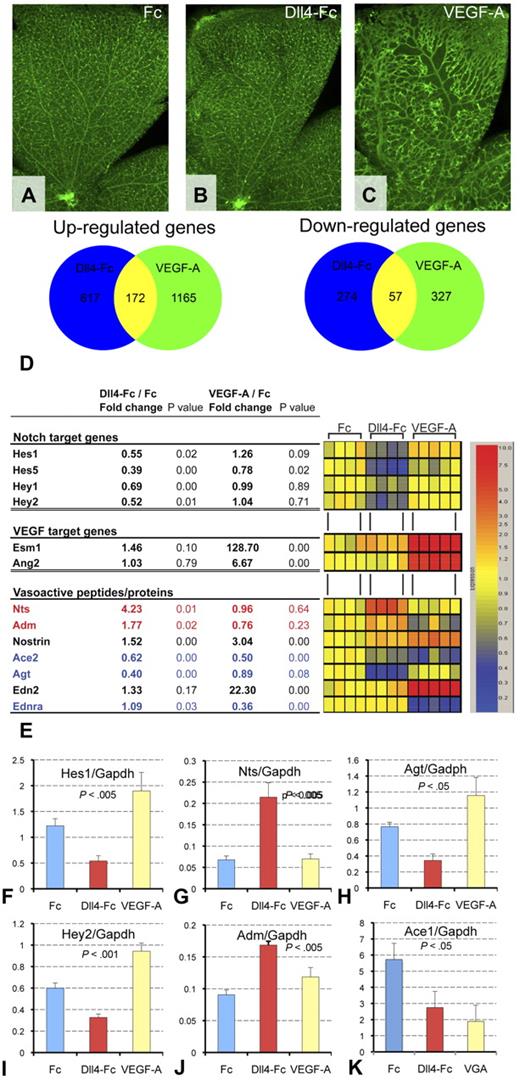
To compare the effects of Dll4-Fc and VEGF-A on normal blood vessels, 8-day-old pups were injected ITV with 4 μg Fc control, 4 μg of Dll4-Fc, or 1 μg VEGF-A165. Within 24 hours of treatment, VEGF-A induced a dramatic increase in the diameter of arteries, veins, and capillaries. In contrast, Dll4-Fc did not induce appreciable changes in blood vessel morphology compared with Fc-treated controls (Figure 4A-C). The distinct effects of administration of Dll4-Fc and VEGF-A on retinal vessel morphology further indicate that Dll4/Notch signaling can affect postangiogenic vascular development and integrity by mechanisms that are largely independent of modulation of VEGF signaling.
Regulation of expression of vasoactive genes by Dll4-Fc and VEGF-A. (A-C) Morphologic changes in the retinal vasculature 24 hours after treatment with 4 μg of Dll4-Fc (B) or 1 μg of VEGF-A (C) compared with 4 μg of Fc (A). (D) Venn diagram showing similarity and difference between Dll4-Fc and VEGF-A significantly regulated genes. (E) Regulation of Dll4/Notch and VEGF-A target genes and vasoactive proteins by Dll4-Fc and VEGF-A. The vasodilators (red) were up-regulated and vasoconstrictors (blue) are down-regulated by Dll4-Fc. Table (left) and corresponding heat map (right) showing changes in the retinal gene expression within 24 hours of treatment. Gene symbols are as follows: Hes indicates hairy and enhancer of split; Hey, hairy/enhancer-of-split related with YRPW motif; Esm1, endothelial cell-specific molecule 1; Ang2, angiopoietin 2; Nts, neurotensin; Adm, adrenomedullin; Nostrin, nostrin; Ace2, angiotensin I-converting enzyme 2; Agt, angiotensinogen; Edn2, endothelin 2; and Ednra, endothelin receptor type A. (F-K) Reverse-transcribed polymerase chain reaction (TaqMan) analysis of Notch target genes (C-D), vasodilators (E-F), and vasoconstrictors (G-H).
Regulation of expression of vasoactive genes by Dll4-Fc and VEGF-A. (A-C) Morphologic changes in the retinal vasculature 24 hours after treatment with 4 μg of Dll4-Fc (B) or 1 μg of VEGF-A (C) compared with 4 μg of Fc (A). (D) Venn diagram showing similarity and difference between Dll4-Fc and VEGF-A significantly regulated genes. (E) Regulation of Dll4/Notch and VEGF-A target genes and vasoactive proteins by Dll4-Fc and VEGF-A. The vasodilators (red) were up-regulated and vasoconstrictors (blue) are down-regulated by Dll4-Fc. Table (left) and corresponding heat map (right) showing changes in the retinal gene expression within 24 hours of treatment. Gene symbols are as follows: Hes indicates hairy and enhancer of split; Hey, hairy/enhancer-of-split related with YRPW motif; Esm1, endothelial cell-specific molecule 1; Ang2, angiopoietin 2; Nts, neurotensin; Adm, adrenomedullin; Nostrin, nostrin; Ace2, angiotensin I-converting enzyme 2; Agt, angiotensinogen; Edn2, endothelin 2; and Ednra, endothelin receptor type A. (F-K) Reverse-transcribed polymerase chain reaction (TaqMan) analysis of Notch target genes (C-D), vasodilators (E-F), and vasoconstrictors (G-H).
To identify changes in gene expression that might confer improved blood vessel perfusion and survival, and to further distinguish the effects of down-regulation of Dll4/Notch signaling from augmentation of VEGF signaling, we compared gene expression profiles in retinas treated from P8 to P9 with 4 μg Fc, 4 μg Dll4-Fc, or 1 μg VEGF-A. A number of genes were commonly regulated by both Dll4-Fc and VEGF-A; however, there were more differentially regulated genes (Figure 4D). Specificity of the effects of Dll4-Fc and VEGF-A administration were further evaluated by comparing the effects of treatment on known Notch target genes16 and VEGF-regulated genes.26 Only Dll4-Fc treatment down-regulated the expression of the Notch target genes Hes1, Hes5 and Hey1, and Hey2. Conversely, only VEGF-A up-regulated expression of Esm1 and Ang2. Notably, microarray gene expression analyses revealed specific and significant changes in the expression of numerous genes encoding vasoactive proteins (Figure 4E-K). For example, genes encoding the potent vasodilators adrenomedullin27 and neurotensin28 were specifically up-regulated by Dll4-Fc (Figure 4D,G,J), whereas the gene encoding angiotensinogen, the precursor of the potent vasoconstrictor angiotensin II, was specifically down-regulated (Figure 4H). In contrast, the expression of endothelin-2 and endothelin receptor A was regulated only by VEGF-A (Figure 4D), and the gene encoding nostrin, a protein involved in nitric oxide trafficking, was more strongly up-regulated by VEGF-A. Very few vasoregulatory genes, such as angiotensin-converting enzyme 2, were similarly regulated by both VEGF-A and Dll4-Fc. A number of other genes encoding structural proteins, regulatory proteins, and their receptors were also discretely regulated by Dll4-Fc or VEGF-A. The microarray gene expression data were confirmed by TaqMan for a subset of differentially regulated genes: Hes1, Hey2, Adm, Nts, Agt, and Ace1 (Figure 4F-K). These data demonstrate that Dll4/Notch and VEGF signaling differentially regulate the expression of multiple genes in the postangogenic retinal vasculature, including genes encoding vasoactive proteins known to be involved in regulation of vasconstriction and vasodilation.
Maintenance of blood flow is a critical requirement for blood vessel survival in normal tissues, whereas flow stasis resulting from vessel occlusion can lead to rapid vessel regression.24,29 Thus, vasoactive proteins can enhance or reduce blood vessel remodeling and regression by controlling blood flow.
The VEGF and Dll4/Notch pathways play distinct roles in postangiogenic vascular remodeling
In the earliest stages of angiogenesis, the VEGF and Dll4/Notch pathways are coregulated and exert diametrically opposing effects on vascular sprouting and endothelial cell proliferation.30 However, little is known about the respective roles and interactions of these 2 critically important regulatory pathways in postangiogenic vascular remodeling and function.
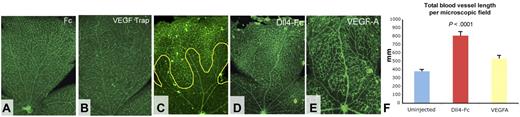
In addition to being an important survival factor for vascular endothelial cells, VEGF is a potent vasodilator.31 VEGF levels are known to be decreased in the central retina by hyperoxia, and ITV injections of VEGF have been reported to prevent capillary regression in OIR.32 Thus, it is widely thought that the degeneration of central retinal capillaries after exposure to hyperoxia is primarily a consequence of reduced VEGF signaling. To test this hypothesis, we evaluated the effect of ITV administration of 5 μg of VEGF Trap at P8 on the postangiogenic retinal vasculature. VEGF Trap is potent inhibitor of VEGF with very high affinity for all isoforms of VEGF-A (< 1 pM). Surprisingly, stringent VEGF inhibition resulted in only a modest increase in capillary pruning around retinal arteries but did not induce pervasive regression of capillaries in the central retina resembling, that is characteristic of OIR, indicating that these vessels are no longer primarily dependent on VEGF-A for survival, or maintenance of blood flow (Figure 5A-B).
Effects of VEGF and Dll4/Notch pathways on blood vessel pruning/regression. (A-B) Eight-day-old pups were injected intravitreally with 4 μg of Fc control (A) or 4 μg of VEGF Trap (B), and retinas were analyzed 24 hours later (original magnifications, ×40). (C-F) Effects of Dll4-Fc and VEGF-A on persistent blood vessel density OIR. Eight-day-old pups were injected ITV with 4 μg of Dll4-Fc or 1 μg of VEGF-A. Animals were placed into high oxygen chamber 24 hours later and retinas harvested on P10. Total blood vessel length was measured per 40× microscopic field (F).
Effects of VEGF and Dll4/Notch pathways on blood vessel pruning/regression. (A-B) Eight-day-old pups were injected intravitreally with 4 μg of Fc control (A) or 4 μg of VEGF Trap (B), and retinas were analyzed 24 hours later (original magnifications, ×40). (C-F) Effects of Dll4-Fc and VEGF-A on persistent blood vessel density OIR. Eight-day-old pups were injected ITV with 4 μg of Dll4-Fc or 1 μg of VEGF-A. Animals were placed into high oxygen chamber 24 hours later and retinas harvested on P10. Total blood vessel length was measured per 40× microscopic field (F).
To compare the effects of ITV injections of Dll4-Fc and VEGF-A on oxygen-induced vaso-obliteration, pups were injected at P8, exposed to high oxygen at P9, and retinal vasculature was assessed at P10. Both Dll4-Fc and VEGF-A administration blocked vaso-obliteration in the central retina (Figure 5C-F). However, in VEGF-A-treated retinas, blood vessels of all classes were grossly enlarged, and the overall number of microvessels, as determined by measurement of total blood vessel length per retina, was significantly reduced compared with Dll4-Fc-treated retinas (Figure 5F).
Thus, although VEGF-A ameliorated oxygen-induced vaso-obliteration, it also produced marked effects on blood vessel morphology and vascular network density in both normal and high oxygen-exposed pups, whereas Dll4-Fc effectively protected blood vessels from oxygen-induced vascular regression without inducing appreciable abnormalities in vascular structure. Taken together, the findings reported herein suggest that the Dll4/Notch pathway plays a critical role in postangiogenic capillary remodeling, both during development and under pathologic conditions, and that the role of VEGF in these processes appears to be rather modest.
To directly test the effect of vasoconstriction on blood vessel survival, angiotensin II was injected ITV in 8-day-old mouse pups, and the effect on retinal vessels was assessed 24 hours later using combined GS lectin and collagen IV staining. Angiotensin II induced dramatic vasoconstriction, collapse, and regression of immature capillaries (Figure 6D-E).
Effects of vasodilation and constriction on blood vessel regression. (A-C) Effect of captopril on oxygen-induced vaso-obliteration. Nine-day-old pups were injected periocularly with 5 μL of vehicle (A) or 300 mg/mL captopril (B); 4 hours later, animals were placed into high oxygen chamber and retinas were analyzed after another 24 hours. (C) Quantification of avascular areas. Fellow untreated eyes were used as a control. Original magnifications, ×40. (D-E) Effect of ITV mock injection (D) or injection of 0.75 μL of 0.2M of angiotensin II (Ang II) (E) on retinal blood vessels. Collagen IV staining (red) and GS lectin staining (green). Original magnifications, ×200. (F-I) Effect of ITV mock injection (F) or injection of 0.25 μL of 20 mg/mL of adrenomedullin (G) on retinal blood vessels exposed to high oxygen for 6 hours (F-G) or 24 hours (H-I). GS lectin staining (green) and ConA perfusion (red). Original magnifications, ×40.
Effects of vasodilation and constriction on blood vessel regression. (A-C) Effect of captopril on oxygen-induced vaso-obliteration. Nine-day-old pups were injected periocularly with 5 μL of vehicle (A) or 300 mg/mL captopril (B); 4 hours later, animals were placed into high oxygen chamber and retinas were analyzed after another 24 hours. (C) Quantification of avascular areas. Fellow untreated eyes were used as a control. Original magnifications, ×40. (D-E) Effect of ITV mock injection (D) or injection of 0.75 μL of 0.2M of angiotensin II (Ang II) (E) on retinal blood vessels. Collagen IV staining (red) and GS lectin staining (green). Original magnifications, ×200. (F-I) Effect of ITV mock injection (F) or injection of 0.25 μL of 20 mg/mL of adrenomedullin (G) on retinal blood vessels exposed to high oxygen for 6 hours (F-G) or 24 hours (H-I). GS lectin staining (green) and ConA perfusion (red). Original magnifications, ×40.
Moreover, treatment of eyes with captopril, an angiotensin-converting enzyme 1 inhibitor (Figure 6A-C), and losartan, an Ang II receptor antagonist (supplemental Figure 5), significantly reduced acute blood vessel loss in OIR, albeit not to the extent as Dll4-Fc.
Importantly, in Dll4-Fc pretreated retinas, the effect of AngII was significantly attenuated (supplemental Figure 6), indicating that Dll4-Fc can attenuate AngII-induced blood vessel regression. ITV administration of vasodilator adrenomedullin also reduced retinal capillary nonperfusion at 6 hours (Figure 6F-G) and regression at 24 hours (Figure 6H-I) in OIR. These data support the hypothesis that angiotensin and adrenomedullin play an important role in regulation of Dll4/Notch-mediated blood vessel regression.
Discussion
Our data indicate that Dll4/Notch pathway regulates blood vessel remodeling and regression by rapidly fine-tuning the expression levels of a distinct subset of vasoactive genes. We also demonstrate, for the first time, a nonangiogenic role of Dll4/Notch in the remodeling of maturing vasculature, which in contrast to its role in sprouting angiogenesis appears to be largely independent of VEGF signaling. Thus, Dll4/Notch inhibition acts not only to modulate angiogenic sprouting but also maintains capillary perfusion and prevents pathologic blood vessel regression induced by exposure to hyperoxia. A number of diseases, such as cerebral, cardiac, and retinal ischemias, are characterized by vascular insufficiency resulting in reduced tissue perfusion.33 In these settings, attenuating vessel loss, improving flow in existing vessels and/or promoting angiogenesis might have beneficial effects. Taken together with previous observations that Dll4/Notch inhibition can promote productive angiogenesis and vessel regrowth in the ischemic retina,15 the present data suggest that Dll4/Notch inhibitors might be useful for ameliorating other ischemic conditions characterized by progressive loss of blood vessels and/or insufficient compensatory angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank colleagues Gavin Thurston, John Rudge, Nick Gale, Lori Morton, WeiCheng Su, and Yang Liu for their many helpful suggestions on these studies and the manuscript.
Authorship
Contribution: I.B.L. designed and performed research, analyzed data, and wrote the paper; E.C. and R.W. performed research and analyzed data; J.C., G.H., Y.W., H.C.L., and G.D.Y. analyzed data; A.E. and N.P. contributed vital new reagents; and S.J.W. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: All authors are employees of Regeneron Pharmaceuticals Inc.
Correspondence: Ivan B. Lobov, Ophthalmology, Regeneron Pharmaceuticals Inc, 777 Old Saw Mill River Rd, Tarrytown, NY 10591-6707; e-mail: ivan.lobov@regeneron.com; and Stanley J. Wiegand, Ophthalmology, Regeneron Pharmaceuticals, Inc, 777 Old Saw Mill River Rd, Tarrytown, NY 10591-6707; e-mail: stanley.wiegand@regeneron.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal