Abstract
The age-adjusted incidence of new factor VIII inhibitors was analyzed in all United Kingdom patients with severe hemophilia A between 1990 and 2009. Three hundred fifteen new inhibitors were reported to the National Hemophilia Database in 2528 patients with severe hemophilia who were followed up for a median (interquartile range) of 12 (4-19) years. One hundred sixty (51%) of these arose in patients ≥ 5 years of age after a median (interquartile range) of 6 (4-11) years' follow-up. The incidence of new inhibitors was 64.29 per 1000 treatment-years in patients < 5 years of age and 5.31 per 1000 treatment-years at age 10-49 years, rising significantly (P = .01) to 10.49 per 1000 treatment-years in patients more than 60 years of age. Factor VIII inhibitors arise in patients with hemophilia A throughout life with a bimodal risk, being greatest in early childhood and in old age. HIV was associated with significantly fewer new inhibitors. The inhibitor incidence rate ratio in HIV-seropositive patients was 0.32 times that observed in HIV-seronegative patients (P < .001). Further study is required to explore the natural history of later-onset factor VIII inhibitors and to investigate other potential risk factors for inhibitor development in previously treated patients.
Introduction
The development of factor VIII inhibitor antibodies is the most important complication of the treatment of severe hemophilia A. Risk factors for early inhibitor development include a family history of inhibitors, nonwhite ethnicity, factor VIII mutation, and intense factor VIII replacement therapy.1-3 It is usually assumed that most factor VIII inhibitors arise after relatively few factor VIII exposure days, early in the patient's life, and that the risk of inhibitor development is subsequently very low.4-6 Reports to the United Kingdom National Hemophilia Database (NHD) suggest that new inhibitors may present throughout life in patients with severe hemophilia and that this risk increases in older patients. We have analyzed prospective reports to the United Kingdom NHD of all new inhibitors reported between 1990 and 2009.
Methods
The study population comprises all patients of all ages with severe hemophilia A (factor VIII ≤ 1 IU/dL) who were alive and treated in the United Kingdom between 1990 and 2009 and who were at risk of developing a new inhibitor, ie, who lacked a history of an inhibitor before 1990. All patients with severe hemophilia are registered with the NHD at the time of diagnosis and followed up prospectively until death. All new factor VIII inhibitors that arise in the United Kingdom have been reported prospectively to the NHD since 1990. We analyzed all reports made between January 1, 1990, and December 31, 2009. The frequency and method of inhibitor testing were at the discretion of the individual center.
We describe a complete and consecutive cohort of a large number of patients. Neither case subjects nor control subjects are affected by reporting bias, because all United Kingdom patients with severe hemophilia A are reported to the NHD and are included in the analysis. The size of the study population was calculated as the number of severe hemophilia A patients lacking a history of an inhibitor before 1990 who were registered with the NHD at any point between 1990 and 2009. The incidence of high-titer (≥ 5 Bethesda units [BU]/mL) and low-titer newly reported inhibitors per 1000 treatment-years at risk was calculated and compared across age groups. Years at risk for each patient were calculated by subtracting 1990 (or the year of registration, if later) from either (1) year of inhibitor onset, (2) year of death, or (3) the end of 2009, whichever was earliest. This figure was then divided for each patient according to years at risk within each age group. The total years at risk within the study period were then calculated for each age group to provide the denominators. The effect of age on inhibitor development was assessed with Poisson regression to calculate incident rate ratios (IRRs). The same methodology was used to analyze the effect of HIV on the risk of developing a new inhibitor.
Results and discussion
The number of patients with severe hemophilia A registered within the study period with no prior inhibitor history was 2528. Patients were followed up for a median (interquartile range) of 12 (4-19) years. Three hundred fifteen new inhibitors arose between 1990 and 2009 (169 ≥ 5 BU/mL; 111 < 5 BU/mL) after a median of 2 (1-6) years (Figure 1). The titer was unavailable for 35 subjects.
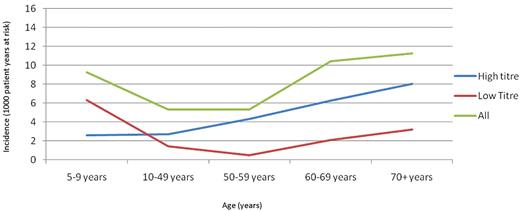
Approximately half of the new inhibitors (49%) presented before the patient was 5 years of age after a median (interquartile range) of 1 (1-2) year (Table 1) of follow-up at a rate of 64.29 new inhibitors per 1000 patient-years at risk. The incidence of inhibitors initially declined with increasing age before rising to a second peak of 10.49 new inhibitors per 1000 patient-years at risk in patients ≥60 years or age (Table 1). Although the incidence of newly reported inhibitors was much lower in patients older than 5 years of age, the cumulative incidence of inhibitors was greater than expected in this group. Half of all reported inhibitors occurred in patients after the age of 5 years after a median follow-up of 6 (4-11) years (Table 1). This is a much higher proportion than is commonly supposed but is compatible with previous reports.4-6
Inhibitor incidence by age, 1990-2009
| . | Years at risk . | n . | Incidence* . | Incident rate ratio . | P . | 95% CI . |
|---|---|---|---|---|---|---|
| Low-titer inhibitors (< 5 BU/mL)† | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 61 | 25.30 | 18.03 | .00 | 11.53-28.20 |
| 5-9 | 2779 | 17 | 6.12 | 4.36 | .00 | 2.39-7.96 |
| 10-49 | 19 953 | 28 | 1.40 | 1.00 | — | — |
| 50-59 | 2094 | 1 | 0.48 | 0.34 | .29 | 0.05-2.50 |
| ≥60 | 1621 | 4 | 2.47 | 1.76 | .29 | 0.62-5.01 |
| Total | 28 858 | 111 | 3.85 | |||
| High-titer inhibitors (≥ 5 BU/mL)† | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 87 | 36.08 | 13.33 | .00 | 9.49-18.72 |
| 5-9 | 2779 | 8 | 2.88 | 1.06 | .87 | 0.51-2.24 |
| 10-49 | 19 953 | 54 | 2.71 | 1.00 | — | — |
| 50-59 | 2094 | 9 | 4.30 | 1.59 | .20 | 0.78-3.22 |
| ≥60 | 1621 | 11 | 6.79 | 2.51 | .01 | 1.31-4.80 |
| Total | 28 858 | 169 | 5.86 | |||
| All inhibitors | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 155 | 64.29 | 12.10 | .00 | 9.45-15.49 |
| 5-9 | 2779 | 26 | 9.36 | 1.76 | .01 | 1.15-2.70 |
| 10-49 | 19 953 | 106 | 5.31 | 1.00 | — | — |
| 50-59 | 2094 | 11 | 5.25 | 0.99 | .97 | 0.53-1.84 |
| ≥60 | 1621 | 17 | 10.49 | 1.97 | .01 | 1.18-3.29 |
| Total | 28 858 | 315 | 10.92 |
| . | Years at risk . | n . | Incidence* . | Incident rate ratio . | P . | 95% CI . |
|---|---|---|---|---|---|---|
| Low-titer inhibitors (< 5 BU/mL)† | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 61 | 25.30 | 18.03 | .00 | 11.53-28.20 |
| 5-9 | 2779 | 17 | 6.12 | 4.36 | .00 | 2.39-7.96 |
| 10-49 | 19 953 | 28 | 1.40 | 1.00 | — | — |
| 50-59 | 2094 | 1 | 0.48 | 0.34 | .29 | 0.05-2.50 |
| ≥60 | 1621 | 4 | 2.47 | 1.76 | .29 | 0.62-5.01 |
| Total | 28 858 | 111 | 3.85 | |||
| High-titer inhibitors (≥ 5 BU/mL)† | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 87 | 36.08 | 13.33 | .00 | 9.49-18.72 |
| 5-9 | 2779 | 8 | 2.88 | 1.06 | .87 | 0.51-2.24 |
| 10-49 | 19 953 | 54 | 2.71 | 1.00 | — | — |
| 50-59 | 2094 | 9 | 4.30 | 1.59 | .20 | 0.78-3.22 |
| ≥60 | 1621 | 11 | 6.79 | 2.51 | .01 | 1.31-4.80 |
| Total | 28 858 | 169 | 5.86 | |||
| All inhibitors | ||||||
| Age, y | ||||||
| 0-4 | 2411 | 155 | 64.29 | 12.10 | .00 | 9.45-15.49 |
| 5-9 | 2779 | 26 | 9.36 | 1.76 | .01 | 1.15-2.70 |
| 10-49 | 19 953 | 106 | 5.31 | 1.00 | — | — |
| 50-59 | 2094 | 11 | 5.25 | 0.99 | .97 | 0.53-1.84 |
| ≥60 | 1621 | 17 | 10.49 | 1.97 | .01 | 1.18-3.29 |
| Total | 28 858 | 315 | 10.92 |
Per 1000 patient-years at risk.
Where titer measurements were known.
The increased incidence of inhibitors in later life was also observed when low- and high-titer inhibitors were analyzed separately. The incidence of high-titer inhibitors increased earlier (from the 50-59-year-old age group) than the incidence for low-titer inhibitors (from 60 years of age).
The Poisson regression analysis used the largest age group (10-49 years of age) as the reference group (Table 1). The rate of inhibitor development relative to the reference group was increased to the greatest degree in patients < 5 years of age (IRR 12.10, 95% confidence interval 9.45-15.49). Subsequent age groups exhibited decreasing relative incidence until the 60 years and above age group, at which point the trend was reversed and a second peak in relative incidence was observed (IRR 1.97, 95% confidence interval 1.18-3.29, P = .01). Similar patterns were exhibited by both low- and high-titer inhibitors, although low-titer inhibitors were relatively more likely to be observed in patients before the age of 5 years (IRR 18.03 and 13.33, respectively). For high-titer inhibitors, the reversal in the trend of declining relative incidence occurred at an earlier age (50-59 years compared with ≥ 60 years) and was greater in magnitude than that for low-titer inhibitors.
The relatively low proportion of low-titer inhibitors reported in older patients probably reflects either ascertainment bias or suppression of or development of tolerance to these inhibitors by ongoing factor VIII replacement therapy. Older patients are generally less intensively monitored for inhibitors than small children, and low-titer inhibitors may therefore be missed if the patient is not tested regularly. When older patients are tested regularly, during the course of pharmacovigilance or regulatory studies of previously treated patients, for example, undetected low-titer inhibitors are found in up to 8% of patients.7-11 The true incidence of low-titer inhibitors in older patients may therefore be higher than observed in the present study.
Seven hundred fifty-two of 2528 patients were HIV seropositive, of whom 31 (4.1%) developed new inhibitors. HIV infection was associated with significantly fewer new inhibitors. The incidence of inhibitors of all titers in HIV-seropositive patients was 0.32 times that observed in seronegative patients (P < .001). HIV has been reported to be associated with occasional inhibitor loss but has not previously been shown to protect against inhibitor formation. The second peak in inhibitor incidence that began at the age of 60 years remained after adjustment for HIV status (all inhibitors: IRR 1.83, P = .02; high-titer inhibitors: IRR 2.10, P = .03). The number of subjects was too small to analyze the effect of the introduction of highly active antiretroviral therapy in the mid-1990s; however, it appears unlikely that highly active antiretroviral therapy will have corrected the effect of HIV on inhibitor risk, because effective antiretroviral therapy was available for 75% of the follow-up period.
The risk factors for inhibitor development in adults with severe hemophilia are poorly understood. An increasing frequency of inhibitors in elderly hemophilic patients, not reflected in earlier reports, may be emerging because patients are living longer.12,13 The mechanism for the increasing incidence of inhibitors in the elderly is unknown but presumably reflects a breakdown of previous tolerance because these patients have had many hundreds of factor VIII exposure days. This breakdown of tolerance may reflect circumstance (danger signals such as surgery and intensive replacement therapy) or a deterioration of immune regulation with advancing age. Anecdotal evidence suggests that these inhibitors frequently arise after intense replacement therapy for surgery or intercurrent bleeding (“peak treatment moments”), a risk factor already established in children with severe hemophilia A and patients with mild hemophilia A.3,14 The United Kingdom Haemophilia Centre Doctors' Organisation is investigating this possibility prospectively.
Patients with a known past history of an inhibitor were excluded from the analysis, and thus, relapse of a previous inhibitor is unlikely. There is currently no conclusive evidence that product switching or recombinant products are associated with an increased risk of inhibitor development.7,8,15 Although the NHD has been collecting inhibitor risk factor data prospectively during the past 2 years, these data are not reported because they are incomplete for this cohort and the denominator is not known.
These previously unreported observations have important implications for patient management, surveillance, and clinical trials for drug licensure. Older patients require more regular monitoring for inhibitors, especially if low-titer inhibitors need to be identified (for example, before surgery). Previously treated patients may be an unsuitable model for the assessment of product immunogenicity in clinical trials because the background inhibitor risk is greater than is commonly supposed and the risk factors for inhibitors are also poorly understood. Our observations in the present study suggest that both the age mix and HIV status may influence the outcome of such studies. Although such trials are traditionally conducted in patients thought to be inhibitor free, 2% to 8% of such patients are found to have an unsuspected, pre-existing, low-titer inhibitor at study entry.7-11 Inhibitors first observed during the course of a clinical trial may have arisen by chance or may have been present but undetected before trial entry and so may be etiologically unrelated to the test product.
As life expectancy increases and the number of individuals with the apparently protective effect of HIV declines, the rising incidence of inhibitor development in older patients with severe hemophilia A will become a more important clinical challenge. Further study is required to explore the natural history of later-onset inhibitors and to investigate other potential risk factors for inhibitor development to inform clinical management.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: The analysis was conceived of by C.R.M.H. with contributions from P.W.C. and the other coauthors; the statistics were conducted by B.P.; and the manuscript was principally written by C.R.M.H., B.P., and P.W.C., with editorial input from E.C., R.L., R.M., S.R., and M.W.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
All authors make an annual declaration of conflicts of interest to the UK Haemophilia Centre Doctors' Organisation.
Correspondence: Charles R.M. Hay, Manchester University Department of Haematology, Manchester Royal Infirmary, Oxford Rd, Manchester M13 9WL, United Kingdom; e-mail: Charles.Hay@cmft.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal