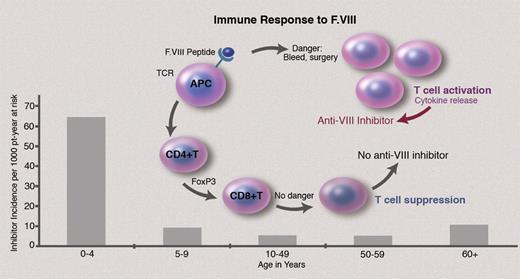
In this issue of Blood, Hay and colleagues report the previously unrecognized bimodal distribution of hemophilia factor VIII inhibitor antibodies, peaking both in early childhood and in older age,1 raising critical questions regarding our understanding of inhibitor formation.
Inhibitor formation is a major complication of hemophilia treatment in which alloantibody directed against infused factor VIII (FVIII) disrupts hemostasis and leads to poorly controlled bleeding, significant morbidity, and expense. Risk factors are well recognized, including young age, high-intensity factor exposure, African American race, and family history, but despite the recognition of inhibitor formation as a T cell–dependent immune response, based on inhibitor disappearance in HIV-positive patients with CD4+ T cell < 200/μL,2 the pathogenesis is not well understood.
FVIII-specific immune response involves FVIII peptide binding to human leukocyte antigen (HLA) class II molecules, presentation to CD4+ T cells, and recognition by the T-cell receptor (TCR), with CD4+ T-cell differentiation and interaction with regulatory cytokines (see figure).3 In the hemophilia exon 16 knockout mouse model, which predictably develops anti-FVIII inhibitor antibodies after 2 or 3 FVIII exposures, splenic T-cell proliferative responses to human FVIII occur even before an inhibitor develops,4 suggesting T-cell activation underlies inhibitor formation. Yet, why inhibitors develop in only 25% of such patients rather than in all patients with severe hemophilia A, who lack circulating normal FVIII, is poorly understood.
Inhibitor formation in hemophilia, a T cell–dependent immune response to infused FVIII, involves binding to HLA class II molecules, presentation to CD4+ T cells, and recognition by the T-cell receptor. In the 25% of severe hemophilia A patients who develop inhibitors, it is hypothesized that if the initial interaction of FVIII and CD4+ T cells occurs in the presence of “danger” signals, that is, severe bleeding or surgery, the innate immune response is activated, with up-regulation of immune response to FVIII. Whether this model also applies to inhibitor formation in the aging adult with hemophilia A is not established.
Inhibitor formation in hemophilia, a T cell–dependent immune response to infused FVIII, involves binding to HLA class II molecules, presentation to CD4+ T cells, and recognition by the T-cell receptor. In the 25% of severe hemophilia A patients who develop inhibitors, it is hypothesized that if the initial interaction of FVIII and CD4+ T cells occurs in the presence of “danger” signals, that is, severe bleeding or surgery, the innate immune response is activated, with up-regulation of immune response to FVIII. Whether this model also applies to inhibitor formation in the aging adult with hemophilia A is not established.
There is increasing evidence that inhibitor risk is related to the intensity and context of first FVIII treatment. If the initial FVIII is administered at the time of a major bleed or surgery, so-called danger signals, the latter activate the innate immune system and, thereby, up-regulate antibody response to FVIII.5 Supporting this hypothesis, several studies have demonstrated that when the first FVIII dose in infants is given before danger, that is, before the first bleed, inhibitor risk is up to 8-fold lower than when FVIII is given after danger, that is, after an existing bleed.6 A randomized, controlled trial is planned to address this question (The Hemophilia INHIBIT trial).
How does this liaison dangereuse, that is, the first meeting between FVIII and the CD4+ T cell, in the context of danger, the purported mechanism of inhibitor formation, explain the increasing inhibitor prevalence in those 60 years of age and older, and why is this liaison less likely in those with HIV infection, most of whom receive immune-reconstituting HAART (highly active antiretroviral therapy)?
The answers to these questions will require careful future study, but there are several possibilities. An increase in inhibitor formation in 60+ year-olds, which includes HIV+ patients, the bulk of whom are in their 50s and 60s, while much less common than in children, may relate to ascertainment bias. First, with antiviral agents rendering HIV and HCV (hepatitis C virus) treatable chronic diseases, the lifespan of adult hemophilia patients, including those with HIV infection, is now approaching that of the general population. Thus, more adult hemophilic men are surviving into their 60s and are potentially at risk for inhibitor formation. Second, better assays that improve detection of low-level inhibitors, for example, the Nijmegen assay, are increasingly available to bolster inhibitor detection by the standard Bethesda assay. Third, data collection with the organizational strength and comprehensive design of the United Kingdom National Hemophilia Database (NHD) by Hay and colleagues is to be applauded, as it underscores the critical importance of close monitoring, careful follow-up, and attention to detail in recognition of late age inhibitor formation.
Other possibilities may be pertinent. A previously undetected inhibitor, for example, from childhood, during long periods with intermittent or less frequent FVIII treatment (exposure), could be first recognized when a 60+ year-old requires high-intensity FVIII treatment for surgery or severe bleeding, resulting in an anemnestic response to FVIII, thereby uncovering a new inhibitor antibody. This would seem unlikely as most severe hemophilia patients would be unlikely to go untreated, although adult patients often have fewer bleeds and reduce factor use as they age, potentially allowing for relapse of an old inhibitor after resumption of heavy treatment, such as for a bleed or surgery. In that regard, factor treatment records, with dates and intensity of FVIII exposure, will be indispensable in determining if late, high-intensity exposure during danger is an important risk in late age inhibitor detection.
It is also possible that inhibitor formation in the elderly could accompany a switch to a new generation FVIII product that the immune system might recognize as foreign, triggering immune activation. After a lifetime of multiple product exposures and negative inhibitor tests, and in whom inhibitor formation would be unanticipated, this seems unlikely. Yet, it is not known whether a transient inhibitor could accompany a switch to a new product, or, under danger and immune stimulation, might persist. Unless regular inhibitor monitoring is conducted from birth, with uniform assessment no less than 48 hours off treatment to avoid inhibitor neutralization, and is performed each time a new product is introduced, it remains difficult to assess the contribution of immune response to new products in the aging adult with hemophilia to new inhibitor formation. We look forward to future analyses to illuminate the intriguing findings of Hay et al.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal