Have you ever wondered what is next for genetically modified mouse research after knockout, floxed-out, and Cre-nased mice? If the study by Flick and colleagues in this issue of Blood is representative of what is yet to come, then we are in for a thrill.1
By introducing an engineered anticoagulant-selective prothrombin mutant within the endogenous prothrombin gene (F2), Flick and colleagues generated a multifaceted phenotype that opens doors for new dimensions in genetically modified mouse research. Endogenous expression of engineered WE-thrombin (W215A/E217A), which conveys only the anticoagulant activity profile of thrombin but not its procoagulant activity profile, permits investigators to “peel the layers of the onion” of thrombin's multisubstrate specificity while learning important lessons about thrombin's diverse activities in normal physiology and pathophysiology. This innovative approach presents numerous unique opportunities, but also identifies new challenges and highlights the need to refurbish our current view on structure-function of coagulation proteases.
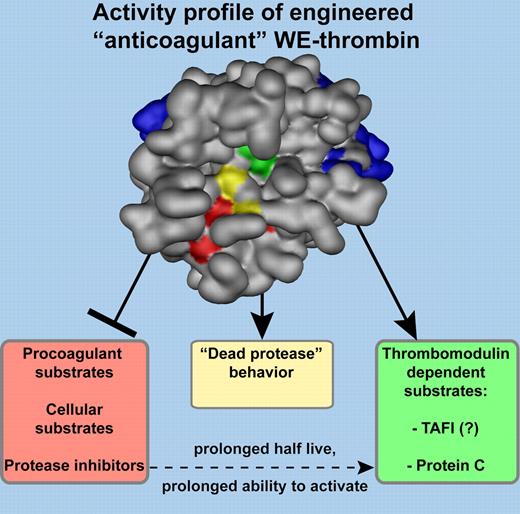
Engineered WE-thrombin and its “anticoagulant” activity profile. Schematic representation of WE-thrombin with active site triad residues indicated in green. The active site cleft is running from left to right with W215A/E217A mutations near the entrance to the active site cleft shown in yellow. The collapse of the 215-217 polypeptide strand blocks access of substrates to the active site resulting in loss of enzymatic activity for all substrates, including procoagulant substrates, cellular substrates, and protease inhibitors. Only in the presence of both thrombomodulin and protein C, and possibly TAFI, is enzymatic activity of WE-thrombin restored, resulting in the generation of activated protein C with anticoagulant and cytoprotective activities, and of activated TAFI with antifibrinolytic and anti-inflammatory activities. “Dead protease” behavior of WE-thrombin, such as competitive inhibition of prothrombinase or blocking the interaction of GPIbα with von Willebrand factor, may contribute to the anticoagulant phenotype of WE-prothrombin/thrombin in vivo.1 Exosite I (right) and II (left) are shown in blue and residues important for Na+ allostery are indicated in red.
Engineered WE-thrombin and its “anticoagulant” activity profile. Schematic representation of WE-thrombin with active site triad residues indicated in green. The active site cleft is running from left to right with W215A/E217A mutations near the entrance to the active site cleft shown in yellow. The collapse of the 215-217 polypeptide strand blocks access of substrates to the active site resulting in loss of enzymatic activity for all substrates, including procoagulant substrates, cellular substrates, and protease inhibitors. Only in the presence of both thrombomodulin and protein C, and possibly TAFI, is enzymatic activity of WE-thrombin restored, resulting in the generation of activated protein C with anticoagulant and cytoprotective activities, and of activated TAFI with antifibrinolytic and anti-inflammatory activities. “Dead protease” behavior of WE-thrombin, such as competitive inhibition of prothrombinase or blocking the interaction of GPIbα with von Willebrand factor, may contribute to the anticoagulant phenotype of WE-prothrombin/thrombin in vivo.1 Exosite I (right) and II (left) are shown in blue and residues important for Na+ allostery are indicated in red.
Thrombin is the archetype multifunctional coagulation protease. Its eminent role in hemostasis, thrombosis, vascular biology, inflammation, angiogenesis, tumor biology, etc, undoubtedly relates to its multiple substrate specificity involving more than 20 different physiologic substrates. However, thrombin does not just proteolyse whatever it encounters; instead, it relies on a series of intricate mechanisms to regulate its substrate specificity depending on location and (micro)environmental factors. Learning when, how, and why thrombin employs these mechanisms in complex biologic systems, such as in vivo, provides a wealth of biologic insights that ultimately will facilitate discoveries of novel therapeutic approaches.
However, this is not just about thrombin. This is also about other coagulation proteases with well-documented additional activities beside their function in coagulation, such as activities on vascular cells.2,3 This is about a growing awareness that coagulation proteases are not just part of a linear network of coagulation reactions but are in fact networking hubs collecting, integrating, and executing signals from multiple sources and through multiple activities. This is about refurbishing our views on coagulation proteases according to this network model, about novel conceptual advances that reinforce our views with in vivo proof of principal studies, and about better insights into the complex biology of protease specificity that may one day lead to the identification of new, improved, or safer therapeutic entities.
To understand how the WE mutations (W215A/E217A) affect thrombin's activities, it is important to realize which features comprise the basis of thrombin's substrate specificity.4,5 These include: (1) active site interactions; (2) exosite interactions; (3) the use of cofactors, such as thrombomodulin, which shields thrombin's procoagulant exosite I and mediates a substrate specificity switch; and (4) the phenomenon of thrombin's Na+-induced “allostery,” characterized by a Na+-containing “procoagulant conformation” and an “anticoagulant conformation” devoid of Na+. The WE mutations located near the entrance to the active site cleft result in a collapse of the 215-217 polypeptide strand thereby blocking access of substrates to the active site.4 The 215-217 blockade of the active site is stabilized in human WE-thrombin by adoption of the Na+-devoid “anticoagulant conformation,” causing an additional 20-fold drop in activity. Lack of 215-217 blockade stabilization in mouse WE-thrombin is the basis for the ∼ 14-fold difference in relative anticoagulant potency between human and mouse WE-thrombin.6 Nevertheless, all proteolytic activity of WE-thrombin is severely diminished and exosite binding of substrates to thrombin cannot overcome the blockade of the active site.7 Thus far, only when both thrombomodulin and protein C are present is enzymatic activity of WE-thrombin restored. Although protein C activation is compromised (∼ 6-fold), a prolonged half-live, because of reduced reactivity with serpin plasma protease inhibitors, increases its ability to generate activated protein C and makes WE-thrombin a potent anticoagulant in vivo.6-8
The discovery that WE-thrombin reduced collagen-induced arthritis (CIA) attests to the value of protease engineering and the application thereof in genetically modified mice. As demonstrated by Flick and colleagues, this beneficial effect of thrombin is overshadowed in wild-type thrombin by its procoagulant activity, but unmasked in WE-thrombin.1 Through application of neat tricks, the authors probe the question of what property of endogenous WE-prothrombin is responsible for these observed beneficial effects in arthritis. In addition to possibilities listed by the authors, one must also consider that active Thrombin Activatable Fibrinolysis Inhibitor (TAFI) may be an important element because this carboxypeptidase B-like enzyme has antifibrinolytic and anti-inflammatory activities and has demonstrated beneficial effects in arthritis models.9 TAFI activation, similar to protein C activation, requires thrombin-thrombomodulin. Although WE-thrombin's ability to activate TAFI is not yet characterized, relatively unaffected TAFI activation by its half brothers E217A-thrombin in vitro and E217K-thrombin in vivo suggests that thrombomodulin-mediated activation of TAFI by WE-thrombin is conceivable.9,10
Is a new chapter for genetically modified mouse research in the making? Possibly, but the product of new studies can only be as good as its ingredients. First, species specificity of biochemical reactions between man and mouse is an inherent complexity of using the mouse as a model system for human disease, which we have learned to overcome or accept. Second, extrapolation of engineered human mutants to the mouse works in principle, although with a general loss of specificity as seen for WE-thrombin and for engineered activated protein C mutants with altered activity profiles.2,6 Historically, human proteins are used for structure-function studies, but application of engineered proteases in transgenic models or mouse models in general, begs the question of whether a more detailed understanding of mouse protein structure-function is needed. Third, successful knockin studies will rely on normal endogenous expression and processing of engineered zymogen mutants, requiring intact physiologic activation pathways, and this demand puts additional pressure on specific engineering strategies for any given protease zymogen. No doubt, innovative approaches and concerted efforts in protease engineering will overcome these hurdles in the future. These new studies using engineered zymogen knockins will provide basic knowledge from which physiologic and pathophysiologic insights will emerge that may one day lead to the design and development of novel protease biologics with improved activity profiles.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal