Abstract
Thrombin and activated protein C (APC) signaling can mediate opposite biologic responses in endothelial cells. Given that thrombin induces procoagulant tissue factor (TF), we examined how TF activity is affected by APC. Exogenous or endogenously generated APC led to increased TF-dependent factor Xa activity. Induction required APC's proteolytic activity and binding to endothelial cell protein C receptor but not protease activated receptors. APC did not affect total TF antigen expression or the availability of anionic phospholipids on the apical cell membrane. Western blotting and cell surface immunoassays demonstrated that APC sheds the Kunitz 1 domain from tissue factor pathway inhibitor (TFPI). A TFPI Lys86Ala mutation between the Kunitz 1 and 2 domains eliminated both cleavage and the enhanced TF activity in response to APC in overexpression studies, indicating that APC up-regulates TF activity by endothelial cell protein C receptor-dependent shedding of the Kunitz 1 domain from membrane-associated TFPI. Our results demonstrate an unexpected procoagulant role of the protein C pathway that may have important implications for the regulation of TF- and TFPI-dependent biologic responses and for fine tuning of the hemostatic balance in the vascular system.
Introduction
Blood coagulation is initiated when tissue factor (TF)–bound factor VIIa activates zymogen factors X and IX to the active serine proteases factor Xa and IXa.1 Under normal physiologic conditions, procoagulant TF is constitutively expressed only by extravascular cells but TF expression can also be induced on vascular cells such as monocytes and endothelial cells by inflammatory stimuli. Current evidence indicates that cells can regulate the procoagulant activity of TF through mechanisms other than simply changing the expression level, and the TF with reduced activity has been referred to as encrypted.2 The active coagulation factors produced by TF/VIIa are recruited to the surface of activated platelets and support, together with cofactors Va and VIIIa, the rapid thrombin generation that is required for fibrin formation and hemostasis. Procoagulant pathways are tightly controlled by inhibitors to prevent excessive intravascular thrombosis. Tissue factor pathway inhibitor (TFPI) is a serine protease inhibitor with 3 Kunitz-type inhibitory domains.3,4 The first and second Kunitz domain bind and block factors VIIa and Xa, respectively, thus locking in a quaternary TF-VIIa-Xa-TFPI complex on the cell surface and shutting down TF's procoagulant activity. The anticoagulant protein C pathway is initiated when thrombin bound to thrombomodulin on the endothelial cell surface produces activated protein C (APC). APC degrades cofactors Va and VIIIa and thus down-regulates further thrombin formation in a negative feedback loop.5,6 Endothelial cell protein C receptor (EPCR) binds both protein C and APC and enhances protein C activation by the thrombomodulin-thrombin complex.7
The serine proteases of the coagulation system can affect cellular responses through protease-activated receptor (PAR) signaling.8 In recent years it has been established that the prototypical thrombin receptor PAR1 can mediate not only proinflammatory signaling by thrombin but also cytoprotective and barrier-protective APC signaling in cultured endothelial cells.9-11 PAR1 signaling by APC is linked to protein C activation by the thrombomodulin-thrombin complex and requires EPCR binding.12 Studies in animal models indicate that EPCR-dependent APC-PAR1 signaling contributes to APC's beneficial effects in systemic inflammation.13-15
It has long been known that thrombin can induce the expression of procoagulant TF in endothelial cells.16,17 Given that thrombin and APC can induce opposite biologic responses by signaling through the same receptor, PAR1, we investigated how APC affects procoagulant TF on endothelial cells. Here, we show that APC up-regulates TF-dependent Xa generation in an EPCR-dependent but PAR1-independent manner by inducing the shedding of the Kunitz 1 domain from TFPI on the endothelial cell surface.
Methods
Reagents, antibodies, and assays
Human thrombin was as described previously.9,18 Human plasma–derived APC, protein C, and factor Xa were from Hematologic Technologies. Human APC blocked with dansyl-glutamyl-glycyl-arginyl-chloromethylketone was from Hematologic Technologies. Recombinant human TFPI was from R&D Systems. All experiments involving stimulation with APC included hirudin (Calbiochem) unless cells were costimulated with thrombin. Control experiments demonstrated that hirudin alone had no effect in any of our assays. The PI3K inhibitor wortmannin was from Calbiochem.
Monoclonal rat anti-EPCR RCR-252 (blocks protein C/APC binding to EPCR) and RCR-92 (nonblocking) antibodies were kindly provided by Dr Kenji Fukudome and were used at 25 μg/mL.19 PAR1 cleavage-blocking monoclonal antibodies ATAP2 and WEDE15 have been characterized previously and were used at 10 and 25 μg/mL, respectively.9,20 The activity-blocking C1 anti–protein C monoclonal antibody was a kind gift from Dr John Griffin.21 The monoclonal anti–human TF (10H10) was as described previously.18 Murine monoclonal antibodies directed against human TFPI were obtained from American Diagnostica. Antibody 4903 is specific for the Kunitz 1 domain (amino acids 22-87) and 4904 is specific for the Kunitz 2 domain (amino acids 88-160). Affinity-purified polyclonal goat anti–human TFPI was from R&D Systems. Rabbit neutralizing polyclonal antibody against TFPI was a kind gift from Drs Samuel Rapaport and Vijay Rao.
APC activity was measured using the chromogenic substrate Spectrozyme PCa (no. 336; American Diagnostica) as described previously.12 Negatively charged phospholipid-rich cell membrane domains were quantified using biotinylated annexin V (Calbiochem) and HRP-coupled streptavidin (Invitrogen) as described previously.22 Prothrombin activation on the cell surface was analyzed as another measure for negatively charged phospholipids as described by Pendurthi et al23 In brief, agonist-treated cells were washed, followed by a 10-minute incubation with factor Va (12nM), factor Xa (0.5nM), and substrate prothrombin (500nM). Thrombin activity in an aliquot was measured by chromogenic assay using the substrate S-2238 (DiaPharma).
Cell culture, transfection, and site-directed mutagenesis
The transformed human endothelial cell line EAhy926 was provided by Dr Cora Edgell (University of North Carolina at Chapel Hill).24 Cells were grown to confluence in a humidified atmosphere at 37°C in DMEM (Invitrogen) supplemented with 10% FBS (HyClone). EAhy926 cells were passaged for up to 40 generations. We found a slight decrease in thrombin and increase in APC effects on Xa generation with higher passage number in these cells. Primary HUVECs (Clonetics) were maintained in EGM-2 medium (basal endothelial cell medium supplemented with hydrocortisone, bovine brain extract, epidermal growth factor, and 10% FBS). They were passaged for no more than 7 generations. In experiments involving gene silencing, cells were plated together with complexes of small interfering RNA (siRNA; 30pM final concentration) and Lipofectamine 2000 (Invitrogen) as described previously.22 Cells were used for experiments 48 hours after transfection. Chemically synthesized, double-stranded siRNA with 19-nucleotide duplex RNA and 2-nucleotide 3′ dTdT overhangs was obtained from Ambion. The siRNA sequences were GUACAAGAGAUAAUGCAAAtt (targeting TFPI) and GGAUCAAACUCUGCUUCCUtt (targeting PAR4 and used as a control).
Human embryonic kidney (HEK293t) cells were grown in DMEM supplemented with 10% FBS. The mammalian expression vector pcDNA3.1/Hygro+ with the coding sequence for human EPCR was as described previously.22 The complete coding sequence for human TFPIα was PCR-amplified from EAhy926 cell cDNA, cloned into pcDNA3.1/Hygro+, and confirmed by sequencing. A construct containing the coding sequence for human TFPIβ in pcDNA3.1D/V5-His-TOPO was kindly provided by Dr George Broze (Washington University, St Louis, MO).25 Full-length human TF in pcDNA3.1/Zeo+ was kindly provided by Dr Wolfram Ruf (The Scripps Research Institute, La Jolla, CA). The QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene) was used to create the expression construct for TFPIβ-K86A. HEK293t cells were transiently transfected using Lipofectamine 2000 and the different expression constructs. Unless indicated otherwise, 0.2 μg DNA for TFPIα or TFPIβ, 0.05 μg DNA for EPCR, and 0.02 μg DNA for TF were used per well on a 96-well plate. Transfected cells were used for experiments 18 hours after transfection.
Xa generation assay
TF-dependent Xa activity was analyzed as a measure of procoagulant TF activity. Cells were grown to confluence in 96-well plates and incubated for 5 hours in serum-free DMEM with 0.4% BSA containing 5nM tumor necrosis factor-α (TNFα) to induce TF expression, unless indicated otherwise. Preliminary experiments established that procoagulant TF activity reaches a plateau at 5 hours of TNFα induction, and no apoptosis was detected at this time point. Other agonists such as APC were added for the final 3 hours of TNFα stimulation unless indicated differently. The medium was replaced with 100 μL DMEM/BSA containing 20nM factor VIIa and 100nM zymogen factor X. After 20 minutes at 37°C, a 50 μL sample was removed and mixed with 150 μL HEPES-buffered saline containing 100mM EDTA and 50nM hirudin. The chromogenic factor Xa substrate Spectrozyme Xa (American Diagnostica) was added (50 μL of a 0.25mM solution) followed by determination of the initial rate of substrate hydrolysis at 405 nm using a SpectraMax 190 spectrophotometer (Molecular Devices). A standard curve with known factor Xa concentrations was used to calculate the Xa activity in the samples. In a typical experiment, approximately 2nM factor Xa was generated on TNFα-induced cells in the absence of agonists, and results are shown relative to this level.
Cell surface immunoassays and Western blotting
For cell surface ELISAs, the cells were fixed with 2% paraformaldehyde, blocked with 1% BSA, and probed with anti-TF or anti-TFPI antibodies at 2 μg/mL for 30 minutes. An HRP-coupled goat anti–mouse antibody and tetramethylbenzidine were used for spectrophotometric quantification of anti-TF or anti-TFPI binding. Residual unspecific staining was found to be unaffected by agonists and was subtracted to correct for background. In all experiments non–agonist-treated cells were included, and surface staining in these cells was defined as 100%. For TFPI detection by Western blotting, cells were lysed in nonreducing sample buffer followed by SDS-PAGE (10% gel) and transfer to Immobilon P membranes (Millipore Corporation). Blots were blocked with dry milk and stained using anti-TFPI and HRP-coupled secondary antibodies. Bound antibodies were visualized using the Femto detection system (Pierce). TF was detected in reduced samples by staining with monoclonal anti-TF 10H10. Staining with anti–β-actin (Sigma-Aldrich) of the stripped membranes served as a loading control.
Statistical analysis
A 2-sample, 2-tailed homoscedastic t test was used to calculate the indicated P values.
Results
Effect of APC on TF-dependent factor Xa generation on endothelial cells
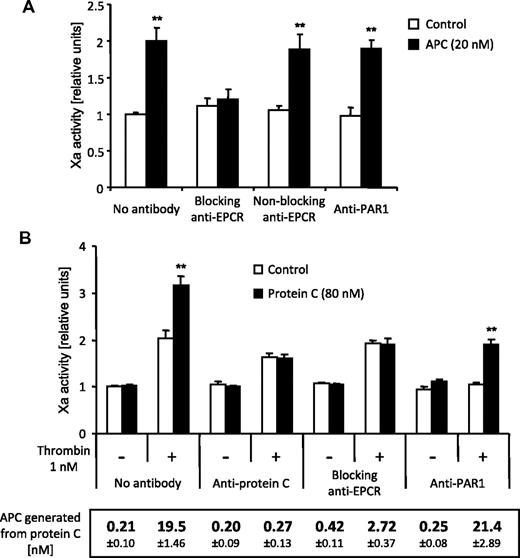
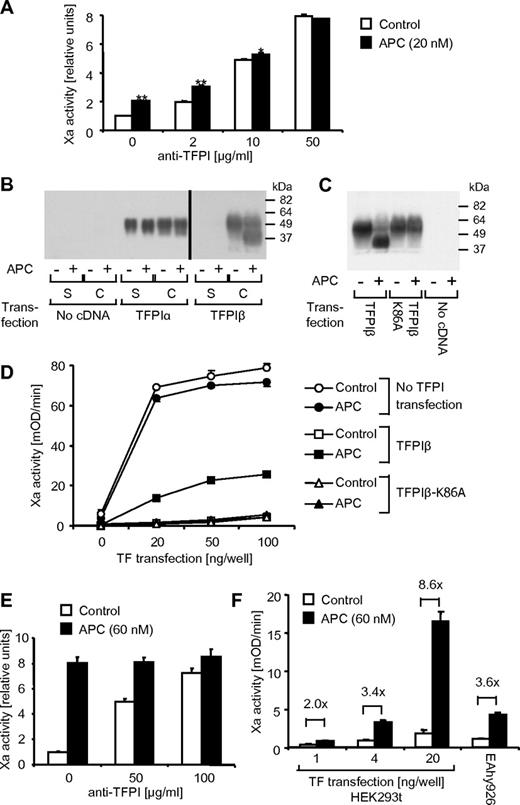
Conversion of zymogen factor X to factor Xa in the presence of factor VIIa was analyzed on the surface of endothelial EAhy926 cells as a measure of TF procoagulant activity. Control experiments demonstrated that very little factor Xa was generated unless the cells were perturbed with the proinflammatory cytokine TNFα, and factor Xa generation reached a plateau after a 5-hour incubation with 5nM TNFα. No factor Xa was detected in the absence of cells, factor X, or factor VIIa, demonstrating that indeed procoagulant TF is measured. If TNFα-induced cells were treated for 3 hours with various concentrations of APC (2-40nM), a dose-dependent increase in Xa activity was found, reaching significance at 5nM APC (Figure 1A). APC's proteolytic activity was required for this response because active site–blocked APC had no effect. Time course studies (5 minutes-5 hours) showed that the increase in factor Xa activity in response to 20nM APC was significant after a 90-minute or longer incubation with APC (Figure 1B). Thrombin is required to generate APC, and it is known to induce TF in endothelial cells. We therefore tested whether APC could still affect procoagulant TF activity in the presence of increasing concentrations of thrombin. As shown in Figure 1C, thrombin alone had a moderate effect on Xa generation, and APC was highly effective even in the presence of 10nM thrombin. Active site–blocked APC was ineffective even in the presence of high-dose thrombin, and the agonists had no effect in cells that were not perturbed with TNFα. We have previously shown that APC can induce relevant PAR1 cleavage in the presence of only up to approximately 1nM thrombin,26 and therefore our current results suggest that the APC effect is probably not PAR1 mediated. The results are consistent with APC being more effective in cells with higher TF expression levels. Inhibition of PI3K with compounds such as Wortmannin can increase TF expression27 and Wortmannin-treated cells indeed strongly up-regulated Xa generation in response to APC (Figure 1D).
APC enhances TF procoagulant activity on endothelial cells. EAhy926 cells were treated in all experiments with TNFα (5nM) for 5 hours to induce TF expression, unless otherwise noted. (A) Different concentrations of APC or active site–blocked APC (APC blocked with dansyl-glutamyl-glycyl-arginyl-chloromethylketone [DEGR-APC]) were added for the final 3 hours of TNFα incubation. Procoagulant TF activity was analyzed as described in “Xa generation assay.” Activity of generated factor Xa relative to only TNFα-stimulated controls is shown. (B) APC (20nM) was added for the indicated period at the end of the TNFα incubation. (C) Cells were incubated for 5 hours in the presence or absence of TNFα, and different concentrations of thrombin and APC (10nM) were added for the final 3 hours as indicated. (D) APC (20nM) and/or the PI3K inhibitor wortmannin (100nM) was added for the final 3 hours of TNFα incubation. Means ± SEM are shown; n = 5 (A, B), 6 (C), or 3 (D). *P < .05; **P < .005, compared with corresponding sample without APC.
APC enhances TF procoagulant activity on endothelial cells. EAhy926 cells were treated in all experiments with TNFα (5nM) for 5 hours to induce TF expression, unless otherwise noted. (A) Different concentrations of APC or active site–blocked APC (APC blocked with dansyl-glutamyl-glycyl-arginyl-chloromethylketone [DEGR-APC]) were added for the final 3 hours of TNFα incubation. Procoagulant TF activity was analyzed as described in “Xa generation assay.” Activity of generated factor Xa relative to only TNFα-stimulated controls is shown. (B) APC (20nM) was added for the indicated period at the end of the TNFα incubation. (C) Cells were incubated for 5 hours in the presence or absence of TNFα, and different concentrations of thrombin and APC (10nM) were added for the final 3 hours as indicated. (D) APC (20nM) and/or the PI3K inhibitor wortmannin (100nM) was added for the final 3 hours of TNFα incubation. Means ± SEM are shown; n = 5 (A, B), 6 (C), or 3 (D). *P < .05; **P < .005, compared with corresponding sample without APC.
Role of EPCR and PAR1 in the up-regulation of procoagulant TF by the protein C pathway
Most cellular effects of APC require PAR1 activation by EPCR-bound APC, and we directly tested the role of these receptors in the up-regulation of TF activity using blocking antibodies. An anti-EPCR that precludes APC binding to EPCR abolished the APC effect, whereas cleavage-blocking anti-PAR1 had no effect (Figure 2A). Thus, the up-regulation of procoagulant TF activity requires APC binding to EPCR but not PAR1 cleavage. EPCR binding of zymogen protein C enhances APC generation by the thrombin-thrombomodulin complex on the endothelial cell surface, and we have previously shown that APC-PAR1 signaling is mechanistically linked to protein C activation.12 We therefore analyzed the effect of endogenously generated APC on TF activity. Xa generation was increased when cells were incubated with thrombin in the presence of protein C (Figure 2B). This effect was abolished in the presence of an antibody that blocks the proteolytic activity of APC, demonstrating that the effect is mediated by endogenously generated APC. Anti-EPCR prevented enhanced Xa generation in the presence of protein C, whereas anti-PAR1 blocked the thrombin-dependent but not the protein C-dependent effect on Xa generation. APC generation was strongly reduced in the presence of anti-EPCR but not anti-PAR1 (Figure 2B, box). Taken together, these results indicate that the effects of thrombin versus exogenous and endogenously generated APC on procoagulant TF activity are mediated by different mechanisms. Thrombin requires PAR1, whereas the protein C pathway relies on EPCR but is PAR1 independent. PAR2 or PAR3 may mediate APC signaling under certain conditions.9,28 In additional experiments using siRNA-mediated down-regulation, we could not establish a role of these receptors in the procoagulant APC effect (not shown). Likewise, antagonism of the apolipoprotein E receptor 229 did not block the APC effect (not shown).
Increased TF procoagulant activity in response to exogenous and endogenously generated APC requires EPCR binding but not PAR1 cleavage. TNFα-induced (5 hours) cells were treated for the final 3 hours with the indicated agonists. Blocking anti-EPCR (RCR-252, 25 μg/mL), nonblocking anti-EPCR (RCR-92, 25 μg/mL), cleavage blocking anti-PAR1 (ATAP2 and WEDE15, 10 and 25 μg/mL, respectively), and anti–protein C (C1, 25 μg/mL) were added 30 minutes before the agonists. Factor Xa generation is shown in panels A and B. For the experiments shown in panel B, APC activity in the cell medium at the end of the incubation time was also analyzed. No amidolytic activity was detected in the absence of protein C and only the results in the presence of protein C are shown. Means ± SEM; n = 5 (A) and 9 (B). **P < .005 compared with the corresponding sample without APC or protein C.
Increased TF procoagulant activity in response to exogenous and endogenously generated APC requires EPCR binding but not PAR1 cleavage. TNFα-induced (5 hours) cells were treated for the final 3 hours with the indicated agonists. Blocking anti-EPCR (RCR-252, 25 μg/mL), nonblocking anti-EPCR (RCR-92, 25 μg/mL), cleavage blocking anti-PAR1 (ATAP2 and WEDE15, 10 and 25 μg/mL, respectively), and anti–protein C (C1, 25 μg/mL) were added 30 minutes before the agonists. Factor Xa generation is shown in panels A and B. For the experiments shown in panel B, APC activity in the cell medium at the end of the incubation time was also analyzed. No amidolytic activity was detected in the absence of protein C and only the results in the presence of protein C are shown. Means ± SEM; n = 5 (A) and 9 (B). **P < .005 compared with the corresponding sample without APC or protein C.
TF expression and availability of negatively charged phospholipids in APC-treated cells
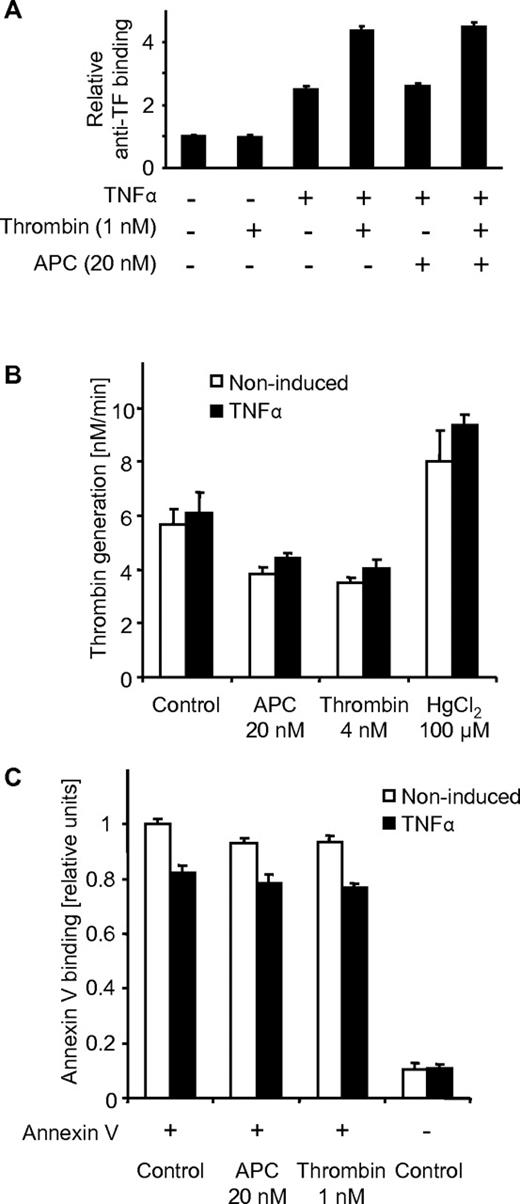
To determine whether APC regulates total TF on the endothelial cell surface, cells were stimulated with APC in the absence and presence of thrombin followed by analysis of TF antigen on the cell surface. APC had no effect on surface TF in the absence or presence of thrombin (Figure 3A), indicating that the induction of TF procoagulant activity by APC is not mediated by up-regulation of TF protein on the cell surface. Even if the expression of TF protein on the cell surface is constant, TF procoagulant activity can increase as a result of changes in the cell membrane microenvironment, that is, from an increased expression of negatively charged phospholipids at the outer leaflet of the cell surface membrane.2 We analyzed whether APC up-regulates prothrombinase activity (Figure 3B) and annexin V binding (Figure 3C) as measures for the expression of anionic phospholipids. Prothrombinase activity was induced by the oxidizing agent HgCl2, as reported previously,23 but was actually slightly down-regulated by APC. Consistent with these results, no increase in annexin V binding was detected in APC-treated cells. Thus, increased availability of acidic phospholipids on the cell surface is not the mechanism by which how APC up-regulates TF procoagulant activity.
APC does not lead to increased surface expression of TF or availability of anionic phospholipids. TNFα- or control-treated cells were incubated for 3 hours with the indicated agonists. (A) Surface expression of TF was determined by immunoassay using monoclonal anti-TF. Prothrombin activation (B) and annexin V binding (C) were analyzed on the cell surface as measures of negatively charged phospholipids as described in “Reagents, antibodies, and assays.” HgCl2 was added only for 30 minutes. Agonists were removed by washing steps before the assays were performed. Means ± SEM with n = 6 are shown.
APC does not lead to increased surface expression of TF or availability of anionic phospholipids. TNFα- or control-treated cells were incubated for 3 hours with the indicated agonists. (A) Surface expression of TF was determined by immunoassay using monoclonal anti-TF. Prothrombin activation (B) and annexin V binding (C) were analyzed on the cell surface as measures of negatively charged phospholipids as described in “Reagents, antibodies, and assays.” HgCl2 was added only for 30 minutes. Agonists were removed by washing steps before the assays were performed. Means ± SEM with n = 6 are shown.
Effect of APC on endothelial TFPI
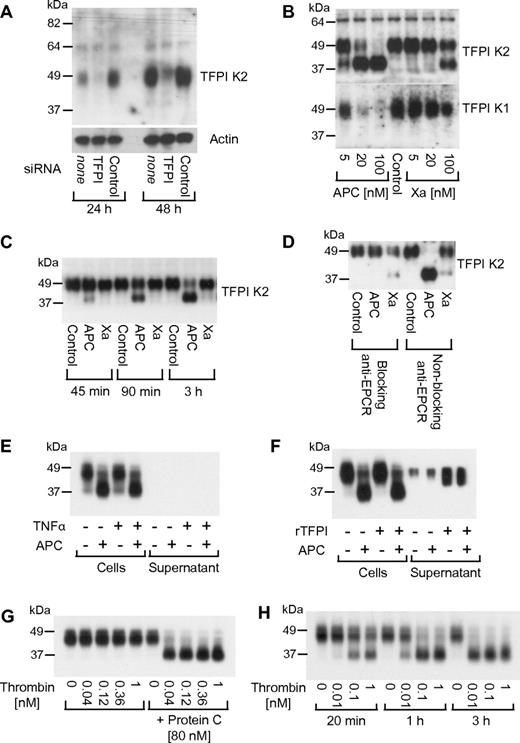
Because endothelial cells express TFPI, we decided to analyze whether up-regulation of TF procoagulant activity may be mediated by decreased TFPI expression. Monoclonal antibodies against the TFPI Kunitz 2 (K2) domain detected a single broad band at approximately 48 kDa on Western blots from whole-cell lysates under nonreducing conditions, approximately the expected size of full-length glycosylated TFPI. Transfection with siRNA targeting the TFPI transcript demonstrated that this band indeed corresponded to TFPI (Figure 4A). An unexpected finding was that when cells were treated for 3 hours with different concentrations of APC (5-100nM), the 48-kDa band disappeared dose dependently and a 38-kDa TFPI species was detected (Figure 4B top panel). The 38-kDa TFPI species was detected already at 5nM APC. A similarly sized 38-kDa band was formed when the cells were incubated with factor Xa, but only at very high concentrations (100nM). When the cell lysates were analyzed with an antibody specific for the TFPI Kunitz 1 (K1) domain, staining of the 48-kDa band decreased in parallel with anti-TFPI K2 domain staining of this band, but no smaller product was detected (Figure 4B bottom panel). These results indicate that APC treatment leads to shedding of the K1 domain from the cell-associated TFPI. The effect of APC on TFPI was time dependent (Figure 4C), mirroring the time course of APC's effect on TF activity in the factor Xa generation assay. TFPI cleavage by APC was dependent on EPCR binding (Figure 4D), whereas blocking anti-PAR1 had no effect (not shown). APC was similarly effective in degrading cellular TFPI in cells that were treated with TNFα using the same conditions as for the experiments analyzing Xa generation (Figure 4E). In these experiments, the growth medium was replaced with serum-free medium for the agonist incubations, and no soluble TFPI was detected in the cell culture medium after the 5-hour incubation. When the growth medium was not replaced, a small amount of soluble TFPI was detected in the cellular conditioned medium. However, neither this endogenous secreted TFPI nor added exogenous recombinant TFPI was susceptible to cleavage by APC (Figure 4F). In the presence of protein C at the plasma concentration (80nM), thrombin supported efficient cleavage of cell-associated TFPI, whereas thrombin alone had no effect (Figure 4G). Dose- and time-response studies established that thrombin, even at extremely low concentrations (10 pM), can support TFPI cleavage in the presence of protein C through the endogenously generated APC (Figure 4H). Experiments in primary HUVECs confirmed that up-regulation of TF-dependent Xa activity and degradation of TFPI are not unique to the EAhy926 cell line (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
APC leads to a loss of the K1 domain from cell-associated TFPI. (A) Cells were treated with siRNA targeting TFPI or control for 24 or 48 hours. TFPI was detected by Western blotting in cell lysates using a K2 domain–specific antibody. (B) Cells were treated for 3 hours with the indicated agonist concentrations followed by detection of TFPI with anti-K2 or anti-K1 domain–specific antibodies. (C) Cells were treated with 20nM APC or factor Xa for the indicated time period followed by detection of TFPI with anti-K2. (D) Cells were preincubated for 15 minutes with 25 μg/mL blocking (RCR-252) or nonblocking (RCR-92) anti-EPCR followed by a 3-hour incubation with 20nM APC or factor Xa and Western blotting using anti-K2. (E) Cells and supernatant were analyzed after incubation with TNFα (5 hours) and 20nM APC (3 hours) as indicated. Growth medium was replaced with TNF-containing serum-free DMEM as in the Xa generation experiments. (F) Cells and supernatant were analyzed without replacing the growth medium for the agonist incubation. Where indicated, 25nM recombinant soluble TFPI (tTFPI) were added before APC. (G) Cells were treated for 3 hours with the indicated concentrations of thrombin in the absence or presence of protein C. (H) Cells were agonist treated as indicated in the presence of 80nM protein C. Polyclonal anti-TFPI was used for detection in panels E through H. Typical results from 2-4 experiments are shown in all panels.
APC leads to a loss of the K1 domain from cell-associated TFPI. (A) Cells were treated with siRNA targeting TFPI or control for 24 or 48 hours. TFPI was detected by Western blotting in cell lysates using a K2 domain–specific antibody. (B) Cells were treated for 3 hours with the indicated agonist concentrations followed by detection of TFPI with anti-K2 or anti-K1 domain–specific antibodies. (C) Cells were treated with 20nM APC or factor Xa for the indicated time period followed by detection of TFPI with anti-K2. (D) Cells were preincubated for 15 minutes with 25 μg/mL blocking (RCR-252) or nonblocking (RCR-92) anti-EPCR followed by a 3-hour incubation with 20nM APC or factor Xa and Western blotting using anti-K2. (E) Cells and supernatant were analyzed after incubation with TNFα (5 hours) and 20nM APC (3 hours) as indicated. Growth medium was replaced with TNF-containing serum-free DMEM as in the Xa generation experiments. (F) Cells and supernatant were analyzed without replacing the growth medium for the agonist incubation. Where indicated, 25nM recombinant soluble TFPI (tTFPI) were added before APC. (G) Cells were treated for 3 hours with the indicated concentrations of thrombin in the absence or presence of protein C. (H) Cells were agonist treated as indicated in the presence of 80nM protein C. Polyclonal anti-TFPI was used for detection in panels E through H. Typical results from 2-4 experiments are shown in all panels.
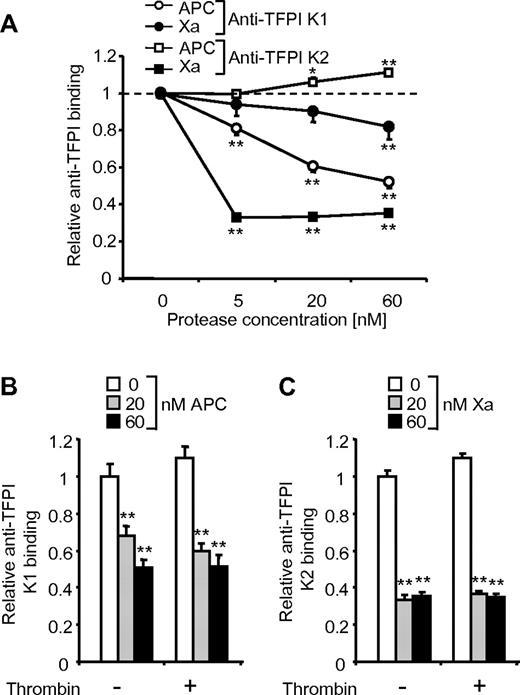
These results from Western blot analysis of denatured samples demonstrate that APC can mediate the generation of a truncated cell-associated TFPI molecule that lacks the K1 domain. We used cell surface immunoassays to test whether the TFPI K1 domain is lost from the endothelial cell surface in response to APC treatment under nondenaturing conditions. Detection of TFPI K1 domain was reduced by APC and by high concentrations of factor Xa with a dose response similar to that for the cleavage results from Western blots (Figure 5A). Thus, APC indeed sheds the TFPI K1 domain from the cell surface. Factor Xa led to a strong decrease in TFPI K2 domain detection, presumably because factor Xa binding to the K2 domain blocks the epitope detected by the antibody. In contrast, APC treatment induced a slight increase in anti-TFPI K2 domain binding, indicating that APC does not interact with the K2 domain in a way similar to factor Xa. The pronounced effect of APC and Xa on the detection of the K1 and K2 domains, respectively, was used to test whether thrombin can affect the interaction of APC and Xa with cell surface TFPI. Thrombin did not influence the observed effects of APC on anti-TFPI K1 binding (Figure 5B) or the effects of factor Xa on anti-TFPI K2 domain binding (Figure 5C).
The TFPI K2 domain is detected by immunoassay on APC-treated but not factor Xa-treated cells. Cells were treated with various concentrations of APC and factor Xa for 3 hours followed by cell surface immunoassay using the indicated antibodies. Thrombin (5nM) was present during the 3-hour incubation in panels B and C where indicated. Means ± SEM with n = 6 are shown, *P < .05; **P < .005, compared with no APC/Xa.
The TFPI K2 domain is detected by immunoassay on APC-treated but not factor Xa-treated cells. Cells were treated with various concentrations of APC and factor Xa for 3 hours followed by cell surface immunoassay using the indicated antibodies. Thrombin (5nM) was present during the 3-hour incubation in panels B and C where indicated. Means ± SEM with n = 6 are shown, *P < .05; **P < .005, compared with no APC/Xa.
Role of TFPI K1 domain shedding in the up-regulation of procoagulant TF by APC
APC did not affect Xa generation in the presence of high concentrations of blocking polyclonal anti-TFPI, consistent with the notion that APC's effect on TFPI mediates the up-regulation of TF activity (Figure 6A). We used an overexpression system to directly test the role of TFPI K1 domain shedding in APC's procoagulant activity. Different mRNA splicing can lead to the formation of full-length TFPI, referred to as TFPIα, or a truncated protein, called TFPIβ, that lacks the third Kunitz domain and has a different C terminus that directs the attachment of a glycosylphosphatidylinositol (GPI) anchor. TFPIα was expressed in transiently transfected HEK293t cells both as a secreted protein in the cell supernatant and as cell associated (Figure 6B). The gel was loaded with 30% of the cell extract but only 5% of the supernatant; thus, the majority of TFPIα was secreted, presumably because the GPI-linked receptor protein that anchors TFPIα to the surface of endothelial cells is missing in HEK293t cells. In contrast, the directly GPI-linked TFPIβ remained cell associated as expected. Consistent with previous results,30 TFPIα and TFPIβ migrated at a similar apparent molecular size on SDS-PAGE. The cell surface TFPIβ but not TFPIα was efficiently cleaved on incubation with APC if EPCR was coexpressed (Figure 6B). APC incubation led to the generation of an approximately 10-kDa smaller TFPI species in TFPIβ-transfected HEK293t cells, similar to the results for endogenous TFPI in the EAhy926 cells, suggesting that endogenous TFPIα and overexpressed TFPIβ get cleaved at the same position. Experiments using the K1 and K2 domain–specific antibodies for Western blotting and surface immunoassay demonstrated that the TFPIβ K1 but not K2 domain is shed on APC incubation (results not shown). We hypothesized that APC may cleave the Lys86-Thr87 peptide bond between the K1 and K2 domains. Results shown in Figure 6C indeed demonstrate that a TFPIβ variant with a substitution of alanine for Lys86 (TFPIβ-K86A) is completely resistant to APC cleavage, suggesting that APC may cleave TFPI at this position. TFPIβ-K86A was as effective as wild-type TFPIβ as an inhibitor of TF procoagulant activity (Figure 6D). However, APC led to increased TF-dependent Xa activity only in cells expressing wild-type but not TFPIβ-K86A, indicating that TFPI K1 domain shedding is necessary for the procoagulant APC effect. In the presence of high concentrations of polyclonal anti-TFPI, APC, even at a high concentration (60nM), had a much reduced effect on Xa generation on TFPIβ-transfected HEK293t cells (Figure 6E), similar to the results in the endothelial cell line (Figure 6A). However, APC was much more efficient in the up-regulation of Xa activity in the overexpression system than in the endothelial cell line compared with polyclonal anti-TFPI. Our results using Eahy926 cells suggest that APC may be more effective in cells that express higher levels of TF (Figure 1D). Consistent with this hypothesis, up-regulation of Xa generation by APC was less efficient and comparable to the results in Eahy926 cells when very low levels of TF were expressed in HEK293t cells (Figure 6F).
TFPI K1 domain shedding is required for the up-regulation of procoagulant TF by APC. (A) TNFα-induced (5 hours) cells were treated for the final 3 hours with APC (20nM). Factor Xa generation was analyzed after a 30-minute pretreatment with different concentrations of blocking polyclonal anti-TFPI. (B) HEK293t cells were transfected with expression constructs for EPCR alone or in the presence of TFPIα or TFPIβ. The following day cells were incubated for 3 hours with control or APC (60nM) and TFPI in the supernatant (S) or cells (C) was analyzed by Western blotting using polyclonal anti-TFPI. (C) HEK293t cells were transfected with EPCR alone or in the presence of wild-type TFPIβ or TFPIβ K86A. The following day cells were incubated for 3 hours with control or APC (60nM) and TFPI expression in the cells was analyzed by Western blotting using polyclonal anti-TFPI. (D) HEK293t cells were triple transfected as indicated with EPCR, TFPI, and different amounts of TF expression construct. Factor Xa generation was analyzed the next day after a 3-hour incubation with control or APC (60nM). (E) HEK293t cells transfected with EPCR, TFPIβ, and TF (20 ng/well) were treated for 3 hours with APC (60nM). Xa generation was analyzed after a 30-minute pretreatment with the indicated concentrations of blocking polyclonal anti-TFPI. (F) HEK293t cells transfected with EPCR, TFPIβ, and different amounts of TF or TNFα-induced EAhy926 cells were treated for 3 hours with APC (60nM) followed by analysis of Xa generation. Means ± SEM with n = 3 are shown in panels A, D, E, and F. *P < .05; **P < .005. Typical results from 2-3 experiments are shown in panels B and C.
TFPI K1 domain shedding is required for the up-regulation of procoagulant TF by APC. (A) TNFα-induced (5 hours) cells were treated for the final 3 hours with APC (20nM). Factor Xa generation was analyzed after a 30-minute pretreatment with different concentrations of blocking polyclonal anti-TFPI. (B) HEK293t cells were transfected with expression constructs for EPCR alone or in the presence of TFPIα or TFPIβ. The following day cells were incubated for 3 hours with control or APC (60nM) and TFPI in the supernatant (S) or cells (C) was analyzed by Western blotting using polyclonal anti-TFPI. (C) HEK293t cells were transfected with EPCR alone or in the presence of wild-type TFPIβ or TFPIβ K86A. The following day cells were incubated for 3 hours with control or APC (60nM) and TFPI expression in the cells was analyzed by Western blotting using polyclonal anti-TFPI. (D) HEK293t cells were triple transfected as indicated with EPCR, TFPI, and different amounts of TF expression construct. Factor Xa generation was analyzed the next day after a 3-hour incubation with control or APC (60nM). (E) HEK293t cells transfected with EPCR, TFPIβ, and TF (20 ng/well) were treated for 3 hours with APC (60nM). Xa generation was analyzed after a 30-minute pretreatment with the indicated concentrations of blocking polyclonal anti-TFPI. (F) HEK293t cells transfected with EPCR, TFPIβ, and different amounts of TF or TNFα-induced EAhy926 cells were treated for 3 hours with APC (60nM) followed by analysis of Xa generation. Means ± SEM with n = 3 are shown in panels A, D, E, and F. *P < .05; **P < .005. Typical results from 2-3 experiments are shown in panels B and C.
Discussion
Here we show that, similar to thrombin, APC can induce increased procoagulant TF activity on endothelial cells. The APC effect is additive with thrombin, is dependent on EPCR binding, does not require activation of PAR1 or other receptors that may mediate APC signaling, and does not involve up-regulation of TF protein expression or increased availability of negatively charged phospholipids on the cell surface. Our results demonstrate that the APC's procoagulant activity is mediated by degradation of TFPI, the major inhibitor of TF-initiated coagulation. TFPI is composed of 3 Kunitz-type protease inhibitor domains. The K2 domain inhibits factor Xa, and the resulting complex binds and blocks TF-bound VIIa via the TFPI K1 domain, forming a tetramolecular complex. APC can up-regulate TF procoagulant activity on endothelial cells dependent on the EPCR-dependent shedding of the TFPI K1 domain. This finding is consistent with studies in purified systems demonstrating that recombinant TFPI variants with a deleted or mutated K1 domain are not able to inhibit factor Xa generation by the TF/VIIa complex.31,32
Our results show that a TFPIβ variant with an alanine for lysine 86 substitution (TFPIβ-K86A) is not cleaved on incubation with APC, and, most importantly, APC was not able to enhance TF-dependent Xa activity in cells expressing the variant TFPI. A previous study has shown that factor Xa can cleave the Lys86-Thr87 peptide bond between the K1 and K2 domains in recombinant TFPI.33 Cleavage after Lys86 leads to the release of an N-terminal peptide with a calculated molecular mass of 10.08 kDa from mature TFPI, closely matching our observed size difference. This finding strongly suggests that the procoagulant effect of APC is mediated by cleavage of the TFPI Lys86-Thr87 peptide bond. However, the possibility that the K86A mutation affects APC-mediated cleavage at another position cannot be ruled out.
Both TFPI34 and EPCR35 are known to localize to caveolae, microdomains that are enriched in proteins anchored to the surface through a GPI anchor. Current evidence indicates that the human EAhy926 endothelial cell line expresses full-length TFPIα, which binds to the cell surface mainly through an association of its Kunitz 3 domain and/or highly basic C-terminal region with an as yet unidentified GPI-anchored receptor protein.36 The overexpressed TFPIβ is GPI-linked and directly anchored to the cell surface. We show that APC can efficiently induce EPCR-dependent shedding of the K1 domain from both of these GPI-anchored TFPI forms. In contrast, TFPI secreted from EAhy926 cells, recombinant soluble TFPI, and overexpressed secreted TFPIα were APC resistant. The overexpressed cell-associated TFPIα is not expected to be bound to a GPI-anchor in the HEK293t cells, and it was also not cleaved by APC. The most straightforward explanation for these findings is that EPCR-bound APC directly cleaves TFPI when it is localized through a GPI-anchor to caveolae. Nevertheless, it is also possible that cell surface proteases that are proteolytically activated by EPCR-bound APC indirectly mediate limited proteolysis of TFPI in response to APC.
TFPI degradation by APC in the endothelial cell line was very efficient as analyzed by Western blotting, whereas the effect on TF-dependent Xa activity was more limited. Our results show that the procoagulant effect of APC is less pronounced in cells that express very low levels of TF, presumably because TFPI degradation needs to be almost complete to prevent rapid inhibition of the limited number of TF molecules. This observation argues that APC will have a stronger procoagulant effect on cells that express relatively large amounts of TF compared with surface-associated TFPI. However, we cannot rule out other explanations for the limited effect of APC on procoagulant TF. It is possible that only a small fraction of the total endothelial surface TFPI has anticoagulant activity, and this fraction may be relatively protected from APC cleavage. Current data indicate that TFPIβ is a more potent TF/VIIa/Xa inhibitor than TFPIα on endothelial cells.30 EAhy926 cells appear to express mainly TFPIα and any TFPIβ present may be less susceptible to cleavage than the TFPIα. As an alternative, cleaved TFPIα on the EAhy926 cells still may have some residual anticoagulant activity by slowing substrate turnover on the TF-VIIa complex.
A previous study using purified proteins has provided evidence that full-length recombinant human TFPI inhibits APC in the presence of heparin. Competition studies with factor Xa suggested that the second Kunitz domain is responsible for APC inhibition.37 Subsequent studies did not corroborate a role of TFPI as an APC inhibitor, and our results demonstrate strong down-regulation of anti-TFPI K2 binding to the cell surface in the presence of factor Xa but not APC, indicating that APC does not interact with the K2 domain of endogenous TFPI in a manner similar to that for factor Xa.
APC has an important and well established role in preventing the intravascular spreading of thrombosis by down-regulating prothrombinase and intrinsic Xase activity on negatively charged phospholipid surfaces such as activated platelets.5 More recent results indicate that APC also has an anti-inflammatory (ie, barrier-protective, antiadhesive, antiapoptotic) role through PAR1-dependent signaling in endothelial cells.9-11 Our current data suggest that yet another role of the protein C pathway is to down-regulate TFPI on endothelial cells in the proximity of an ongoing thrombotic process in which thrombin supports APC generation on the endothelial surface. Thus, in addition to the PAR1-mediated cytoprotective effects, APC lowers the “TFPI threshold” and hence increases the potential procoagulant activity of any TF that may be expressed by these cells, keeping the endothelium adjacent to the thrombotic event on “high alert” to reinitiate coagulation if necessary. This model adds another unexpected level of sophistication to the regulation of thrombosis. APC's negative and positive feedback input into the system targets distinct phases of coagulation. APC is anticoagulant by shutting down the propagation-phase thrombin generation supported by active enzyme complexes on negatively charged lipid surfaces. At the same time, APC is procoagulant by decreasing the threshold of endothelial cells to trigger TF-induced initiation phase coagulation. Another intriguing possibility is that increased factor Xa production and prothrombinase activity on endothelial cells on TFPI degradation by APC could play an anticoagulant role by maintaining further APC generation. Interestingly, the prothrombin activation product meizothrombin binds to thrombomodulin and is an approximately 6 times more efficient protein C activator than thrombin.38
Recombinant APC has been approved to treat patients with severe sepsis, although the use remains controversial.39 It is difficult to predict how degradation of endothelial TFPI by infused APC may affect the outcome in septic patients. TFPI degradation may be detrimental because it may lead to enhanced coagulation activation and consumptive coagulopathy. On the other hand, TFPI degradation may also aid in the host defense against pathogens. Recent results indicate that proteolytic inactivation of TFPI by the neutrophil serine proteases elastase and cathepsin G plays an important role in large vessel thrombosis as well as in microvessel thrombosis as part of the host defense against pathogen invasion into tissues.40 These data suggest that a substantial reduction of TFPI activity is necessary to allow normal hemostasis. APC may similarly aid in the host defense by lowering the TFPI threshold on endothelial cells in proximity to invading microorganisms. Interestingly, the neutrophil proteases inactivate human TFPI by cleavage at T87 and L89,40 positions adjacent to the K86 targeted by APC, suggesting that this sequence stretch has evolved to allow proteolytic removal of the TFPI's K1 domain by proteases with different specificities.
Even though endothelial cells express large quantities of surface-associated TFPI, the precise role of this inhibitor on the endothelial surface is unknown. Whereas it is easy to induce TF in cultured endothelial cells using a variety of perturbing reagents, it is currently not known whether TF expression by the endothelium plays any pathophysiologic role in vivo. It has been difficult to detect endothelial TF expression by immunohistochemical analysis in mouse models of systemic inflammation. Compared with humans, mice are highly resistant to thrombosis in a variety of different models, and the functions of endothelial TF and TFPI in the control of hemostasis and thrombosis may be quite different between the species. For example, human endothelium appears to produce predominantly TFPIα, whereas exclusively TFPIβ is expressed in adult mouse tissues.41 Moreover, TFPI on endothelial and other cells probably has roles beyond the control of TF procoagulant activity,30 such as modulation of innate immunity, angiogenesis, and lipid metabolism.42 It will be interesting to elucidate whether APC affects these functions of TFPI.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Colleen Lau for excellent technical assistance.
This study was supported by grants from the National Institutes of Health (HL73318; to M.R.) and from the Swiss Foundation für medizinisch biologische Stipendien and the Mach-Gaensslen-Stiftung (to R.A.S.).
National Institutes of Health
Authorship
Contribution: R.A.S. and M.R. designed and performed the research, analyzed and interpreted the data, wrote the manuscript, and read and reviewed the manuscript; and K.V. performed the research, analyzed the data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.A.S. is Surgical Intensive Care Medicine, University Hospital Zurich, Zurich, Switzerland.
Correspondence: Matthias Riewald, MD, The Scripps Research Institute, Department of Immunology and Microbial Science, SP30-3040, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: riewald@scripps.edu.

![Figure 1. APC enhances TF procoagulant activity on endothelial cells. EAhy926 cells were treated in all experiments with TNFα (5nM) for 5 hours to induce TF expression, unless otherwise noted. (A) Different concentrations of APC or active site–blocked APC (APC blocked with dansyl-glutamyl-glycyl-arginyl-chloromethylketone [DEGR-APC]) were added for the final 3 hours of TNFα incubation. Procoagulant TF activity was analyzed as described in “Xa generation assay.” Activity of generated factor Xa relative to only TNFα-stimulated controls is shown. (B) APC (20nM) was added for the indicated period at the end of the TNFα incubation. (C) Cells were incubated for 5 hours in the presence or absence of TNFα, and different concentrations of thrombin and APC (10nM) were added for the final 3 hours as indicated. (D) APC (20nM) and/or the PI3K inhibitor wortmannin (100nM) was added for the final 3 hours of TNFα incubation. Means ± SEM are shown; n = 5 (A, B), 6 (C), or 3 (D). *P < .05; **P < .005, compared with corresponding sample without APC.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/23/10.1182_blood-2010-10-316257/4/m_zh89991172220001.jpeg?Expires=1769141090&Signature=hqKHM4jAJJ1E3WwquLBFCSE6QK80eayxQK6lgbwTRJbePaD3qBOwyHNqZHwl1raSlu4s8Db32zH1Zv-6dPCnfYX1NYEMmBpVY0T6QnTzcOQp2q0zxNw-4-nUZ9xC0QqQ5Xx9MECOIR3qY9Bd94Fm4T7uEc~vTHuXyRzxtpm33LAxFMD5sVyCnkfIg6pmkfYH2GgtxEJ5AizuyTtPS-l16V9EG1R4RBLABTdA02M-Nt4Rn7DuSGfFyWxjzkyrKLqgFFmlX8Cq7eY3BEX~T~d6J0fYoGrSalZTS2znHpgDJUUIErdXNGbGAz8GLXvplCgpBC1tU84DPiyblqWgjyPDjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal