Abstract
Factor XIII (FXIII) generates fibrin-fibrin and fibrin-inhibitor cross-links. Our flow model, which is sensitive to cross-linking, was used to assess the effects of FXIII and the fibrinolytic inhibitor, α2-antiplasmin (α2AP) on fibrinolysis. Plasma model thrombi formed from FXIII or α2AP depleted plasma lysed at strikingly similar rates, 9-fold faster than pooled normal plasma (PNP). In contrast, no change was observed on depletion of PAI-1 or thrombin activatable fibrinolysis inhibitor (TAFI). Inhibition of FXIII did not further enhance lysis of α2AP depleted thrombi. Addition of PNP to FXIII or α2AP depleted plasmas normalized lysis. Lysis rate was strongly inversely correlated with total cross-linked α2AP in plasma thrombi. Reconstitution of FXIII into depleted plasma stabilized plasma thrombi and normalized γ-dimers and α-polymers formation. However, the presence of a neutralizing antibody to α2AP abolished this stabilization. Our data show that the antifibrinolytic function of FXIII is independent of fibrin-fibrin cross-linking and is expressed exclusively through α2AP.
Introduction
Factor XIII (FXIII) is activated by thrombin to form an active transglutaminase, FXIIIa. FXIIIa significantly alters the rheologic properties of fibrin by introducing intramolecular cross-links between fibrin strands.1,2 A deficiency in FXIII results in bleeding, delayed wound healing and spontaneous abortion in humans and mice.3,4 Initially FXIIIa forms a γ-γ dimer between Gln388/389 on one γ-chain of fibrin and Lys406 on another.5,6 High molecular mass polymers of the α-chain follow6 with hybrid γ-α cross-links generated over prolonged periods.7
FXIIIa cross-links inhibitors of fibrinolysis to fibrin, dramatically altering its susceptibility to lysis.8 The most extensively characterized is α2-antiplasmin (α2AP), which cross-links to the Aα chain of fibrin(ogen)9 at Lys303 via Gln2.10 Plasminogen activator inhibitor 2 (PAI-2)11 and thrombin activatable fibrinolysis inhibitor (TAFI)12 are also substrates for FXIIIa. Despite evidence of inhibitor cross-linking, it has been challenging to observe the role of FXIII in modulating fibrinolysis. We recently showed that thrombi formed under flow, even in the absence of cells, allows the impact of FXIII on fibrinolysis to be visualized and quantified.13 The thrombus model has also proved invaluable in determining the role of different inhibitors in regulating fibrinolysis.14 This study examines the contribution of fibrin-fibrin cross-links and fibrin-inhibitor cross-links in conferring resistance to fibrinolysis. We show for the first time that the role of FXIII in protecting fibrin against fibrinolytic degradation is fully explained by its ability to cross-link α2AP into the fibrin network.
Methods
Plasma thrombus formation and lysis
Plasma thrombi were formed in a Chandler loop as described.13 Briefly, FITC-labeled fibrinogen was added to pooled normal plasma (PNP) or plasma depleted of FXIII, α2AP, TAFI or PAI (Affinity Biologicals Inc). Plasma was recalcified with 10.9mM CaCl2 in a total volume of 0.575 mL. A nonreversible transglutaminase inhibitor, 1,3-dimethyl-2-[(2-oxopropyl) thio] imidazolium chloride (1mM)13,15 (TG inhibitor), FXIII (0.1,0.3 or 1 U/mL Fibrogammin P; Aventis) or neutralizing antibody to α2AP14 (150 μg/mL; Technoclone) were added in some experiments before thrombus formation. Thrombi were incubated in 10mM Tris (pH 7.5); 0.01% Tween-20 containing tissue plasminogen activator (tPA; 1 μg/mL) at 37°C. Samples (10 μL) were diluted 1/25 in 10mM phosphate (pH 7.4), 150mM NaCl, and fluorescence measured (excitation 485 nm: emission 530 nm) in a Biotek Instruments Fluorometer.
SDS-PAGE and Western blot
Plasma thrombi, formed as described in the preceding paragraph, were washed 3 times in 0.9% (wt/vol) NaCl before dissolving in 8M urea, 0.2M Tris (pH 8), 40mM dithiothreitol and 4% SDS at 72°C for approximately 1 hour. Samples were diluted in 0.9% NaCl and separated on 7.5% acrylamide gels before transferring to nitrocellulose and immunoblotting for fibrinogen α-chain, γ-chain (Santa Cruz Biotechnology Inc) or α2AP (Affinity Biologicals).
Data analysis
Quantitative data are expressed as mean ± SEM. Data were analyzed in GraphPad Prism 5 (GraphPad Software) and shown as fluorescence units (FU) released or rates of lysis (FU/minutes), as determined by linear regression. Statistical analysis was performed by t test and Western blots were analyzed using Image J software (Version 1.44).
Results and discussion
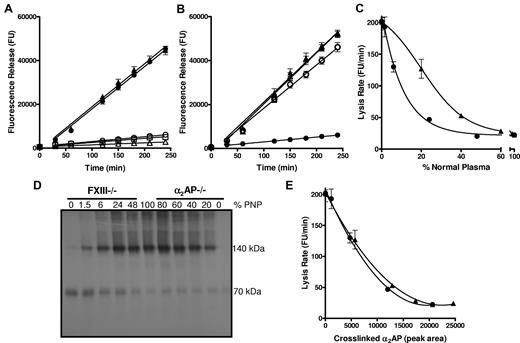
We examined lysis of thrombi prepared from PNP and from plasma immunodepleted of FXIII and the inhibitors, α2AP, TAFI and PAI-1. FXIIIa can cross-link α2AP8 and TAFI12 to fibrin, whereas PAI-1 is not a substrate.11 Depletion of FXIII or α2AP resulted in a 9-fold increase in lysis rate over PNP thrombi (Figure 1A; P < .005). Depletion of TAFI or PAI-1 did not significantly alter thrombus lysis (Figure 1A; P = .133 and P = .285, respectively). These data clearly confirm the major role of cross-linked α2AP in down-regulating fibrinolysis. Consistent with this, addition of a TG inhibitor13 to thrombi formed from α2AP depleted plasma had no effect on lysis (Figure 1B; P = .472), while in PNP a 9-fold (P < .005) increase in lysis was observed, as in Mutch et al.13
Thrombi formed from plasma depleted of FXIII or α2AP show comparable lysis. (A) Plasma thrombi were prepared from pooled normal plasma (PNP; ○; n = 6) or plasma depleted of FXIII (●; n = 6), α2AP (▴; n = 9), TAFI (▵; n = 2) and PAI-1 (□; n = 2) and lysed with 1 μg/mL tissue plasminogen activator (tPA). Lysis was monitored as release of fluorescence and expressed as mean ± SEM. FXIII and α2AP depleted plasmas lysed significantly faster (P < .005) than PNP, TAFI, and PAI-1 depleted plasma, whereas FXIII and α2AP depleted plasma lysed at comparable rates (P = .5). (B) Plasma thrombi were prepared from PNP (●, ○; n = 6) or α2AP depleted plasma (▴, ▵;r n = 3) in the absence (closed symbols) and presence (open symbols) of a TG inhibitor. Thrombi were lysed as described in panel A. Lysis of α2AP depleted plasma thrombi was significantly different from PNP (P < .005) but no difference in lysis was observed on incorporation of TG inhibitor into α2AP depleted plasma before thrombus formation (P = .5). (C-E) Plasma thrombi were prepared from PNP or mixtures of PNP with FXIII (●) or α2AP (▴) depleted plasma, resulting in different percentages of FXIII (0, 1.5, 6, 24, 48, 100%) or α2AP (0, 20, 40, 60, 80, 100%) relative to their plasma concentration. Lysis was recorded as described in panel A and the mean lysis rate (FU/minutes) plotted against % PNP (n = 6; C). Alternatively, thrombi were solubilized in reducing sample buffer and subjected to SDS-PAGE followed by Western blotting for α2AP (D; n = 6). The Western blot (D) was analyzed using Image J densitometry software and the peak area of total cross-linked α2AP was plotted against the mean lysis rate for each sample of PNP and FXIII or α2AP depleted plasma containing different percentages of plasma (E).
Thrombi formed from plasma depleted of FXIII or α2AP show comparable lysis. (A) Plasma thrombi were prepared from pooled normal plasma (PNP; ○; n = 6) or plasma depleted of FXIII (●; n = 6), α2AP (▴; n = 9), TAFI (▵; n = 2) and PAI-1 (□; n = 2) and lysed with 1 μg/mL tissue plasminogen activator (tPA). Lysis was monitored as release of fluorescence and expressed as mean ± SEM. FXIII and α2AP depleted plasmas lysed significantly faster (P < .005) than PNP, TAFI, and PAI-1 depleted plasma, whereas FXIII and α2AP depleted plasma lysed at comparable rates (P = .5). (B) Plasma thrombi were prepared from PNP (●, ○; n = 6) or α2AP depleted plasma (▴, ▵;r n = 3) in the absence (closed symbols) and presence (open symbols) of a TG inhibitor. Thrombi were lysed as described in panel A. Lysis of α2AP depleted plasma thrombi was significantly different from PNP (P < .005) but no difference in lysis was observed on incorporation of TG inhibitor into α2AP depleted plasma before thrombus formation (P = .5). (C-E) Plasma thrombi were prepared from PNP or mixtures of PNP with FXIII (●) or α2AP (▴) depleted plasma, resulting in different percentages of FXIII (0, 1.5, 6, 24, 48, 100%) or α2AP (0, 20, 40, 60, 80, 100%) relative to their plasma concentration. Lysis was recorded as described in panel A and the mean lysis rate (FU/minutes) plotted against % PNP (n = 6; C). Alternatively, thrombi were solubilized in reducing sample buffer and subjected to SDS-PAGE followed by Western blotting for α2AP (D; n = 6). The Western blot (D) was analyzed using Image J densitometry software and the peak area of total cross-linked α2AP was plotted against the mean lysis rate for each sample of PNP and FXIII or α2AP depleted plasma containing different percentages of plasma (E).
The absence of α2AP and FXIII had strikingly similar effects on fibrinolysis. Addition of increasing concentrations of PNP normalized thrombus lysis (Figure 1C), but different concentrations were required to stabilize lysis in FXIII and α2AP depleted plasma. The α2AP data are consistent with previous studies on α2AP deficiency.16,17 Protection against premature fibrinolysis was achieved at approximately 50% plasma FXIII, as we have previously shown.13 We then defined the proportion of α2AP (70 kDa) cross-linked to fibrin by Western blotting of plasma thrombi (Figure 1D). Total cross-linked α2AP (140 kDa band and those of higher mass) was analyzed by densitometry and plotted against the mean lysis rate for each of the mixed plasma thrombi (Figure 1E). The lysis rate inversely correlated with total cross-linked α2AP, whether thrombi were formed from FXIII or α2AP depleted plasma (r2 = 0.998; P = .383).
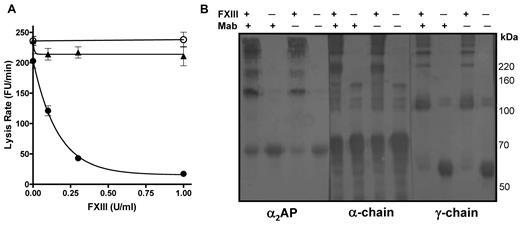
We then assessed the ability of FXIII to stabilize plasma thrombi in the absence of functional α2AP by incorporating a neutralizing antibody to the inhibitor14 before thrombus formation; it should be noted that the antibody inhibits α2AP function but not cross-linking of the inhibitor to fibrin. Reconstitution of FXIII into thrombi prepared from FXIII depleted plasma dramatically decreased the mean lysis rate, with complete stabilization of thrombi at 0.5 U/mL FXIII (Figure 2A closed circles). In contrast, when a neutralizing antibody to α2AP was also added, no change in lysis was observed (Figure 2A open circles). We verified that the addition of FXIII had no effect on lysis of thrombi formed from α2AP depleted plasma (Figure 2A closed triangles). Cross-linking patterns were examined by Western blot at 0 and 1 U/mL FXIII using antibodies to fibrin(ogen) and α2AP. We confirmed that α2AP was still cross-linked to fibrin in the presence of antibody (Figure 2B left panel). Normal fibrin-fibrin cross-links were formed when FXIII was present (Figure 2B). The key finding is that, despite the formation of normal fibrin-fibrin cross-links, the stability of the thrombi to lysis (Figure 2A) depends on FXIIIa cross-linking functional α2AP into the thrombus.
FXIII requires α2AP to down-regulate fibrinolysis. (A) Plasma thrombi were prepared from FXIII depleted plasma in the absence (●) and presence (○) of a neutralizing antibody to α2AP or α2AP depleted plasma (▴). Various concentrations of FXIII (0, 0.1, 0.3, 1 U/mL) were added before thrombus formation and thrombi were lysed with 1 μg/mL tissue plasminogen activator (tPA). Lysis was monitored as release of fluorescence and expressed as mean lysis rate per minute ± SEM (n = 4). (B) Plasma thrombi prepared as described in panel A in the presence of FXIII (1 U/mL) or neutralizing antibody to α2AP (Mab) were solubilized in reducing sample buffer. After separating on 7.5% acrylamide gels samples were immunoblotted with antibodies to α2AP, the α-chain or the γ-chain of fibrinogen.
FXIII requires α2AP to down-regulate fibrinolysis. (A) Plasma thrombi were prepared from FXIII depleted plasma in the absence (●) and presence (○) of a neutralizing antibody to α2AP or α2AP depleted plasma (▴). Various concentrations of FXIII (0, 0.1, 0.3, 1 U/mL) were added before thrombus formation and thrombi were lysed with 1 μg/mL tissue plasminogen activator (tPA). Lysis was monitored as release of fluorescence and expressed as mean lysis rate per minute ± SEM (n = 4). (B) Plasma thrombi prepared as described in panel A in the presence of FXIII (1 U/mL) or neutralizing antibody to α2AP (Mab) were solubilized in reducing sample buffer. After separating on 7.5% acrylamide gels samples were immunoblotted with antibodies to α2AP, the α-chain or the γ-chain of fibrinogen.
Our data show that the ability of FXIII to modulate fibrinolysis is dependent on its action in cross-linking active α2AP to fibrin during thrombus formation. We therefore infer that the introduction of fibrin-fibrin cross-links alone has a negligible impact on fibrinolysis. γ-dimers and α-polymers are reported to play a limited role in stabilizing clots against fibrinolytic degradation; with protection achieved only after prolonged incubation because of γ-multimer formation.7 These studies were performed with purified proteins in the absence of α2AP.7 Our results indicate that α2AP cross-linking determines thrombus stability in a plasma milieu.
Fibrin-fibrin cross-links, particularly α-polymers, γ-multimers and α-γ-hybrids enhance the ability of clots to resist mechanical stresses.18,19 Together with our cross-linking data, these observations indicate that FXIII plays a dual role during clot formation (1) to strengthen individual fibrin fibers against mechanical stress and (2) to incorporate inhibitors into a forming clot to protect against premature degradation by fibrinolytic proteases.
Two forms of α2AP exist in plasma, Met-α2AP and Asn-α2AP. Asn-α2AP is produced on cleavage of Met-α2AP by antiplasmin cleaving enzyme, and is more rapidly cross-linked to fibrin.17 We quantified α2AP by ELISA in plasma and in the serum postthrombus formation. By subtraction, approximately 30%-50% of plasma α2AP was bound into thrombi, consistent with other studies.17,20 Inhibition of lysis occurs with incorporation of increasing amounts of Asn-α2AP, demonstrating the effectiveness of cross-linked α2AP in down-regulating fibrinolysis.17
This study addresses the complex question of whether fibrin-fibrin cross-links or fibrin-inhibitor cross-links regulate fibrinolysis of thrombi. We clearly show that, despite their importance in clot strength,18,19 fibrin-fibrin cross-links do not protect against degradation by fibrinolytic proteases. Instead it is inhibitor cross-linking, specifically cross-linking of α2AP to fibrin, that is the key determinant of fibrinolytic resistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the British Heart Foundation (PG/2001005).
Authorship
Contribution: S.R.F. performed the research and analyzed data; N.A.B. supervised the research and wrote the manuscript; and N.J.M. supervised the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Nicola J. Mutch, School of Medicine & Dentistry, Institute of Medical Sciences, Foresterhill, University of Aberdeen, Aberdeen, AB25 2ZD, UK; e-mail: n.j.mutch@abdn.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal