Abstract
Thrombin is a positive mediator of thrombus formation through the proteolytic activation of protease-activated receptors (PARs), fibrinogen, factor XI (fXI), and other substrates, and a negative regulator through activation of protein C, a natural anticoagulant with anti-inflammatory/cytoprotective properties. Protease-engineering studies have established that 2 active-site substitutions, W215A and E217A (fIIWE), result in dramatically reduced catalytic efficiency with procoagulant substrates while largely preserving thrombomodulin (TM)–dependent protein C activation. To explore the hypothesis that a prothrombin variant favoring antithrombotic pathways would be compatible with development but limit inflammatory processes in vivo, we generated mice carrying the fIIWE mutations within the endogenous prothrombin gene. Unlike fII-null embryos, fIIWE/WE mice uniformly developed to term. Nevertheless, these mice ultimately succumbed to spontaneous bleeding events shortly after birth. Heterozygous fIIWT/WE mice were viable and fertile despite a shift toward an antithrombotic phenotype exemplified by prolonged tail-bleeding times and times-to-occlusion after FeCl3 vessel injury. More interestingly, prothrombinWE expression significantly ameliorated the development of inflammatory joint disease in mice challenged with collagen-induced arthritis (CIA). The administration of active recombinant thrombinWE also suppressed the development of CIA in wild-type mice. These studies provide a proof-of-principle that pro/thrombin variants engineered with altered substrate specificity may offer therapeutic opportunities for limiting inflammatory disease processes.
Introduction
The conversion of prothrombin to the active serine protease thrombin is a central event in hemostasis/thrombosis and in the regulation of embryonic development, vascular barrier function, tissue repair, tumor progression, and inflammatory processes.1 In the context of hemostasis, thrombin directly promotes the formation and stabilization of thrombi through the proteolytic activation of protease-activated receptors (eg, PAR-1 and PAR-4), the proteolytic conversion of fibrinogen to fibrin, and the activation of factor XI (fXI), fVIII, fV, fXIII, and other substrates. However, in direct opposition to these procoagulant pathways, when bound to the endothelial cell receptor thrombomodulin (TM), the proteolytic specificity of thrombin is dramatically altered such that its ability to activate procoagulant factors is suppressed in favor of enhanced activation of the natural anticoagulant protein C.2 Thrombin-mediated cleavage of PARs, protein C, complement proteins, cytokines, and growth factors is proposed to be important in the control of cellular proliferation, migration, apoptosis, adhesion, process outgrowth, and other fundamental elements of cell biology.3-9 This extraordinary capacity to engage a wide array of biologically important substrates and to dramatically switch proteolytic specificity in vivo has made thrombin a centerpiece of detailed structural and biological analyses.
Structure-function studies have identified 2 active-site variants, W215A and E217A, which strongly diminish procoagulant activity, but largely retain TM-dependent anticoagulant function as defined by the capacity to activate protein C.10,11 Analyses of the compound mutant, thrombinW215A/E217A (thrombinWE) established that this variant exhibited even greater selectivity for protein C over procoagulant substrates. The catalytic efficiency of human thrombinWE with fibrinogen and PAR-1 expressed in terms of kcat/Km was found to be diminished approximately 19000-fold and 1200-fold, respectively, with a far more modest reduction in activity toward protein C (ie, ∼7-fold change in kcat/Km).12 Therefore, the net effect of these active-site alterations was a molecule with little procoagulant function but preserved anticoagulant potential. Another striking feature of the thrombinWE variant was a pronounced resistance to inactivation by the physiologic inhibitor antithrombin III, the kon of which was reduced 3000-fold relative to wild-type thrombin.12 Studies of human thrombinWE infused into experimental animals have established that this engineered protease can act as a potent and long-lasting anticoagulant molecule in vivo.13,14 A few notable differences exist between human and murine thrombin. The murine enzyme is constitutively stabilized in the high-activity state similar to the Na+-bound form of human thrombin.15 Nevertheless, imposition of the W215A/E217A mutations in murine thrombin was shown to result in a shift in catalytic activity that is qualitatively comparable to that observed with human enzyme, albeit quantitatively less profound. Specifically, the kcat/Km of murine thrombinWE with murine fibrinogen and PAR-1 is diminished approximately 1200-fold and 60-fold, respectively, while the kcat/Km for protein C is diminished a more modest 6-fold.15 These data would suggest that, in balance, murine thrombinWE, like the human variant, would tend to significantly favor anticoagulant over procoagulant substrates.
Multiple lines of evidence suggest a role for thrombin-mediated proteolysis, and procoagulant function in particular, in the pathogenesis of arthritis and other inflammatory diseases.16-18 The specific thrombin inhibitor hirudin was shown to be effective in limiting both collagen-induced arthritis (CIA) and antigen-induced arthritis (AIA) in mice.19,20 Complementary studies of fibrinogen gene–targeted mice established that fibrin is at least one thrombin substrate that drives CIA disease progression and severity.21 Additional studies have shown that mice deficient in PAR-1 were partially resistant to AIA.22 These observations, and provocative recent findings establishing that the thrombin product activated protein C (APC) has potent anti-inflammatory/barrier-stabilization/cytoprotective activities in vitro and in vivo,23-27 lend support to the concept that a pro/thrombin variant with limited activity for fibrinogen and PARs but retained activity for protein C would attenuate the progression of inflammatory joint disease. To explore the general hypothesis that prothrombin active-site mutations favoring an anticoagulant phenotype would be compatible with developmental success but limit inflammatory processes in vivo, gene-targeted mice were generated and characterized carrying the fIIWE mutations within the endogenous prothrombin gene.
Methods
Generation of fIIWE gene-targeted mice
The fIIWE-targeting vector was generated by PCR-based mutagenesis in which 4 nucleotide substitutions were introduced into exon 14, which converted amino acids tryptophan215 and glutamic acid217 (residues numbered according to the chymotrypsin numbering system) into alanine residues. The complete targeting vector included a mutagenized 710-bp short arm, an approximately 4.7-kb long arm, a 3.6-kb HPRT minigene, and a 2-kb HSV-tk minigene. E14Tg2a embryonic stem cell clones that incorporated the targeting vector by homologous recombination were identified by PCR analysis using primers complementary to HPRT (5′-AAATGCTCCAGACTGCCTTG-3′) and the prothrombin gene (5′- GTACAAGAGAAGGCTGTGACTTGGCCAGAG-3′) that generated an 838-bp product. The presence of the mutation was confirmed by PCR using primers upstream (5′-AGGTATGCTTCTTTAAAGGCCAGGGGTTGG-3′) and downstream (5′-TCACTTTTATTGAGAACAGAAACAAACCAC-3′) of the mutated sequence in conjunction with a diagnostic PvuII restriction enzyme digest. Homologous recombination of the targeting vector was also confirmed by Southern blot analysis. Mice carrying the mutant fIIWE allele were backcrossed 6 generations to C57Bl/6J (The Jackson Laboratory) for studies of developmental success and in vivo hemostatic parameters. Mice carrying the fIIflox allele were described previously.28 The fIIWE allele was similarly placed onto the DBA/1J genetic background for studies of collagen-induced arthritis. All mouse experiments were approved by the Cincinnati Children's Hospital Research Foundation Animal Care and Use Committee and complied with National Institutes of Health guidelines.
Analysis of fIIWE expression and hematologic profiles
Comparative analyses of hepatic mRNA were done by RT-PCR using total adult liver RNA. First-stand cDNA synthesis was performed with 1 μg of total RNA using the ThermoScript RT-PCR kit (Invitrogen). PCR was subsequently performed with primers complementary to exon 12 (5′-ACAGCCCAGCGTCCTGCAGGTGGTG-3′) and exon 14 (5′-ACACATGCGTGTAGAAGCCGTATTT-3′) that yielded a transcript-specific product of 266 bp. The PCR products were digested with the PvuII restriction enzyme, which produced fragments of 215 and 51 bp for the fIIWE transcript, but not the wild-type transcript. Western blot immunodetection of circulating prothrombin was performed using citrate plasma prepared either from adult mice or from P1 neonates exhibiting no overt hemorrhage using a rabbit anti–human prothrombin antiserum (Nordic Immunologic Laboratories) cross-reactive with mouse prothrombin. Complete blood cell counts and plasma coagulation tests were performed on blood drawn from the inferior vena cava into a one-tenth volume of 0.1M citrate. Prothrombin times (PTs) and activated partial thromboplastin times (aPTTs) were determined by the use of a STA-Hemostasis Analyzer using the STA-Neoplastine Cl and STAPTT reagents (Diagnostica Stago). Comparative thrombin generation assays (TGAs) were done using a Technothrombin TGA kit (Technoclone) as described by the manufacturer for mouse citrate plasma. Thrombin generation was initiated in vitro by addition of calcium and lipids containing recombinant tissue factor, and thrombin activity was measured by cleavage of a fluorogenic thrombin substrate using a FLx800 fluorescence plate reader (Biotek). Tail-bleeding times were established in ketamine/xylazine–anesthetized mice by excising 3 mm of tail tip and submerging it in Tris-buffered saline (pH 7.5) containing 2mM CaCl2 at 37°C.
Comparative real-time analyses of thrombus formation by intravital microscopy
Intravital microscopic analysis of thrombus formation within injured mesenteric arterioles was done in tribromoethanol-anesthetized (0.15 mL of 2.5% per 10 g of body weight) 3- to 4-week-old animals (14-18g) infused with fluorescent platelets (2.5 × 109 platelets/kg) through the retro-orbital plexus, as described previously.29 Vessel centerline velocities were measured using an optical Doppler velocity meter (Microcirculation Research Institute) and the shear rate was calculated as described previously.29 An endothelial injury was induced by a 5-minute topical application of Whatman paper saturated with 10% FeCl3. Vessels were monitored by fluorescence microscopy for 40 minutes after injury or until occlusion. Key experimental end points were the time required for the formation of a thrombus larger than 30 μm and the occlusion time (ie, the time required for blood to stop flowing for 30 seconds).
Survival and plasma APC levels after endotoxin challenge
Kaplan-Meier analyses were done using cohorts of 8- to 12-week-old control and fIIWE-expressing mice challenged with 15 mg/kg of IP endotoxin (lipopolysaccharide [LPS] O127:B8; Sigma Chemicals). APC levels were measured immunologically using a murine-specific APC antibody, as described previously.30 Mice were infused with a large bolus (150 μg) of human APC 3 minutes prior to blood collection to displace any endothelial cell protein C receptor–bound murine APC into the circulation.
CIA
Cohorts of 6- to 8-week-old male mice backcrossed to the CIA-susceptible DBA/1J genetic background were twice immunized with 100 μg of bovine collagen type II (CII; Elastin Products) in complete Freund adjuvant (CFA) on days 1 and 21. Mice were evaluated for arthritis using an arthritic index macroscopic scoring system ranging from 0 to 4 (0 = no arthritis, 1 = swelling and/or redness of paw or one digit, 2 = 2 arthritic joints, 3 = 3 arthritic joints, and 4 = severe arthritis of the entire paw and digits).21 CII-specific cell proliferation assays with cultured T cells from popliteal lymph node cells and CII-specific plasma antibody titers were determined as described previously.31 In experiments using recombinant thrombinWE, inbred DBA/1 mice (The Jackson Laboratory) were immunized as described and given daily retroorbital injections of the purified recombinant protein beginning at day 21 of the CIA protocol at a dose of 0.1 mg/kg until harvested at day 42.
Histologic analysis
Tissues were fixed for 48 hours in 10% neutral-buffered formalin (Sigma); knee joints were also decalcified in TBD-2 (ThermoShandon) for 10 days. Four-micron sections of paraffin-embedded tissue were processed for hematoxylin/eosin and Masson trichrome staining or immunodetection of fibrin using a rabbit anti–mouse fibrin(ogen) polyclonal serum.21 Images were captured using an Axioplan 2 microscope with Axiovision Image analysis software Version 4.8.1 (Carl Zeiss Microimaging). A semiquantitative histopathology analysis was performed for each knee joint based on the following scoring criteria for CIA: inflammation (0-3), synovial hyperplasia (0-3), edema (0-3), pannus (0-1), and bone/cartilage loss (0-3), for a total histopathology index (0-26) for knees of a given animal.
Statistical analysis
The distribution of embryonic and neonatal genotypes was determined by χ2 analysis. Mouse survival data were analyzed using the Kaplan-Meier log-rank test. Differences in bleeding times, vessel occlusion times, thrombin generation, plasma APC levels, and arthritis parameters were analyzed using the Mann-Whitney U test or the paired t test, as indicated.
Results
Mice carrying the prothrombin W215A/E217A allele
To determine the physiologic and pathologic consequences of constitutive expression of thrombin zymogen with a specificity favoring protein C over procoagulant substrates, we generated founder mice carrying substitutions within the prothrombin gene that simultaneously resulted in (1) the conversion of tryptophan215 and glutamic acid217 to alanines, and (2) the introduction of a novel PvuII restriction enzyme site to assist in genotyping (Figure 1A-C). The mutant allele was transmitted through the germline to yield heterozygous mice carrying one wild-type allele and one W215A/E217A allele (hereafter referred to as fIIWT/WE mice). Expression of the mutant allele was readily detected by RT-PCR analysis of hepatic mRNA isolated from adult mice. The 266 bp RT-PCR products (complementary to exons 12-14) derived from the mutant and wild-type alleles were easily distinguished because PvuII digestion of PCR products derived from the mutant template yielded diagnostic 215- and 51-bp fragments (Figure 1D.) Plasma prothrombin protein levels were immunologically indistinguishable in adult fIIWT/WE and wild-type mice and were comparable in blood collected from fIIWE/WE, fIIWT/WE, and fIIWT/WT embryos harvested at embryonic day 18.5 (E18.5) based on Western blot analysis (Figure 1E). FIIWT/WE mice identified at weaning consistently survived well into adulthood (the oldest fIIWT/WE mice in our colony have survived well over a year of age) without developing spontaneous bleeding events or other pathologies.
Generation and characterization of prothrombin W215A/E217A gene–targeted mice. (A) Structure of the prothrombin W215A/E217A (fIIWE) gene–targeting vector, the wild-type prothrombin gene, and the targeted fIIWE allele. The positions of the nucleotide substitutions that result in the introduction of the W215A/E217A mutations and a unique PvuII endonuclease site are indicated. Brackets indicate the position of SacI fragments diagnostic by Southern blot analysis for wild-type and fIIWE alleles. The region of the prothrombin gene used as a hybridization probe is highlighted with a thick line labeled “SB probe.” Arrowheads indicate the position of PCR primers used to detect and distinguish the mutant and wild-type alleles. Partial nucleotide sequence of exon 14 of the wild-type and fIIWE alleles are indicated. Asterisks highlight the nucleotides that were mutated within the fIIWE allele and the mutated amino acids are indicated in bold. (B) Southern blot analysis of genomic DNA from wild-type, heterozygous fIIWT/WE, and homozygous fIIWE/WE mice digested with SacI. DNA fragments of 6.2 and 9.9 kb were detected for the wild-type and fIIWE alleles, respectively. (C) Representative PCR analyses of genomic DNA from each of 2 individual wild-type, fIIWT/WE, and fIIWE/WE mice. Primers G1 and G3 produced a 567-bp product specific to the wild-type allele, and primers G1 and HPRT1 produced a 621-bp product specific to the fIIWE targeted allele (top panel). Primers G1 and G2 coupled with a PvuII digest produced a 375-bp product specific to the wild-type allele and 202- and 173-bp products specific to the targeted fIIWE allele (bottom panel). (D) RT-PCR analysis of total hepatic RNA isolated from each of 4 individual wild-type and fIIWT/WE mice. Primers specific to sequences within exons 12 and 14 were used to generate a 266-bp PCR product (top panel). The product generated from a wild-type transcript was insensitive to cleavage by PvuII, whereas the 266-bp product generated from the mutant fIIWE transcript was cleaved into fragments that were 215 and 51 bp in length. Primers specific to the cDNA of β-actin were used in control reaction mixtures for each RT product (bottom panel). (E) Western blot analysis of plasma from wild-type, fIIWT/WE, and fIIWE/WE mice using a polyclonal anti-prothrombin antibody. Comparable levels of prothrombin were observed in 0.5-μL aliquots of citrate plasma collected from 3 individual adult wild-type and 2 individual adult fIIWT/WE mice (top panel). Comparable levels of prothrombin were observed in plasma samples (30 μg of total plasma protein per lane) collected from wild-type, fIIWT/WE, and fIIWE/WE day 1 neonates that were viable and free from overt hemorrhage (bottom panel).
Generation and characterization of prothrombin W215A/E217A gene–targeted mice. (A) Structure of the prothrombin W215A/E217A (fIIWE) gene–targeting vector, the wild-type prothrombin gene, and the targeted fIIWE allele. The positions of the nucleotide substitutions that result in the introduction of the W215A/E217A mutations and a unique PvuII endonuclease site are indicated. Brackets indicate the position of SacI fragments diagnostic by Southern blot analysis for wild-type and fIIWE alleles. The region of the prothrombin gene used as a hybridization probe is highlighted with a thick line labeled “SB probe.” Arrowheads indicate the position of PCR primers used to detect and distinguish the mutant and wild-type alleles. Partial nucleotide sequence of exon 14 of the wild-type and fIIWE alleles are indicated. Asterisks highlight the nucleotides that were mutated within the fIIWE allele and the mutated amino acids are indicated in bold. (B) Southern blot analysis of genomic DNA from wild-type, heterozygous fIIWT/WE, and homozygous fIIWE/WE mice digested with SacI. DNA fragments of 6.2 and 9.9 kb were detected for the wild-type and fIIWE alleles, respectively. (C) Representative PCR analyses of genomic DNA from each of 2 individual wild-type, fIIWT/WE, and fIIWE/WE mice. Primers G1 and G3 produced a 567-bp product specific to the wild-type allele, and primers G1 and HPRT1 produced a 621-bp product specific to the fIIWE targeted allele (top panel). Primers G1 and G2 coupled with a PvuII digest produced a 375-bp product specific to the wild-type allele and 202- and 173-bp products specific to the targeted fIIWE allele (bottom panel). (D) RT-PCR analysis of total hepatic RNA isolated from each of 4 individual wild-type and fIIWT/WE mice. Primers specific to sequences within exons 12 and 14 were used to generate a 266-bp PCR product (top panel). The product generated from a wild-type transcript was insensitive to cleavage by PvuII, whereas the 266-bp product generated from the mutant fIIWE transcript was cleaved into fragments that were 215 and 51 bp in length. Primers specific to the cDNA of β-actin were used in control reaction mixtures for each RT product (bottom panel). (E) Western blot analysis of plasma from wild-type, fIIWT/WE, and fIIWE/WE mice using a polyclonal anti-prothrombin antibody. Comparable levels of prothrombin were observed in 0.5-μL aliquots of citrate plasma collected from 3 individual adult wild-type and 2 individual adult fIIWT/WE mice (top panel). Comparable levels of prothrombin were observed in plasma samples (30 μg of total plasma protein per lane) collected from wild-type, fIIWT/WE, and fIIWE/WE day 1 neonates that were viable and free from overt hemorrhage (bottom panel).
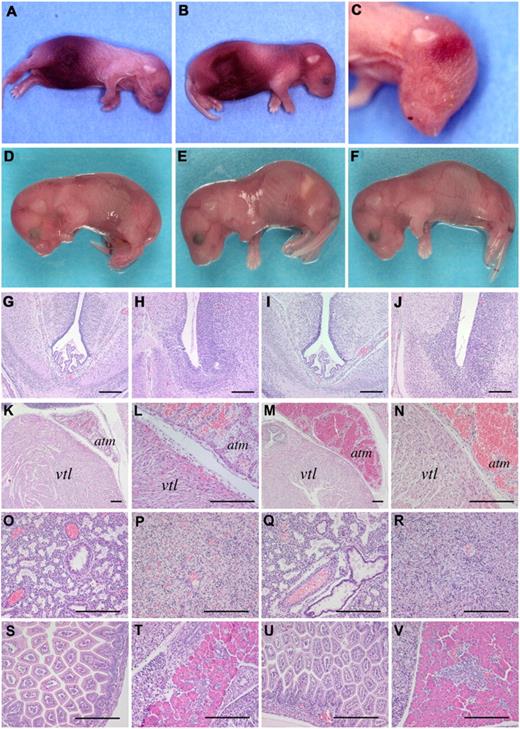
Homozygosity for fIIWE is compatible with development to term but results in perinatal lethality
The phenotypic consequence of homozygous prothrombin W215A/E217A expression was first examined by intercrossing heterozygous mice to evaluate development and postnatal life. The number of fIIWE/WE offspring noted on postnatal day 1 was significantly less than expected (Table 1). Among 141 postpartum animals, the anticipated 1:2 ratio of fIIWT/WT (46) and fIIWT/WE (78) offspring was found; however, less than half the expected number of fIIWE/WE mice were observed (P = .001 by χ2 analysis). Furthermore, 16 of the 17 fIIWE/WE offspring identified died within 1-2 days of birth. The fIIWE/WE neonates typically presented with overt abdominal hemorrhage (Figure 2A-B) and subcutaneous bleeding within the head and neck region (Figure 2B-C). Interestingly, one fIIWE/WE animal lived for more than 2 weeks, but died with signs of hemorrhage shortly after an ear biopsy was taken for genotype analysis. In contrast, 74 of 78 (∼ 95%) fIIWT/WE neonates identified survived to weaning, and all of these ultimately survived to adulthood with no signs of hemorrhage or other spontaneous abnormalities (Table 2). The general long-term success of fIIWT/WE mice was further affirmed based on separate tracking studies. Of 301 weaned offspring derived from crosses of fIIWT/WT and fIIWT/WE mice, 162 (54%) were fIIWT/WT and 139 (46%) were fIIWT/WE mice, proportions that were not significantly different from what was expected (P = .18 by χ2 analysis). Both fIIWT/WE males and females were fertile, with females capable of carrying multiple litters successfully to term.
Summary of neonates collected from heterozygous (fIIWT/WE) breeding pairs
| . | fIIWT/WT (wild-type) . | fIIWT/WE (hemizygous) . | fIIWE/WE (homozygous) . |
|---|---|---|---|
| Number observed* | 46 | 78† | 17‡ |
| Percentage expected | 25% | 50% | 25% |
| Percentage observed | 32.6% | 55% | 12% |
| Number (percentage) of expected based on wild-type | 46 (100%) | 92 (85%) | 46 (37%) |
| . | fIIWT/WT (wild-type) . | fIIWT/WE (hemizygous) . | fIIWE/WE (homozygous) . |
|---|---|---|---|
| Number observed* | 46 | 78† | 17‡ |
| Percentage expected | 25% | 50% | 25% |
| Percentage observed | 32.6% | 55% | 12% |
| Number (percentage) of expected based on wild-type | 46 (100%) | 92 (85%) | 46 (37%) |
P < 0.002 by χ2 analysis.
Four died within 1 day of birth: 3 with abdominal hemorrhage and 1 with head and neck bleed.
All 17 nulls died prior to weaning: 1 mouse lived 14 days, 1 died after 2 days, and the others died after 1 day or at birth.
Mice homozygous for fIIWE complete embryonic development but uniformly experience fatal postnatal bleeding events. (A-C) Representative examples of fIIWE/WE homozygous neonates. Note that most of the fIIWE/WE postnatal animals displayed appreciable abdominal hemorrhage. In addition, subcutaneous bleeding events in the head and neck region were also routinely observed. (D-F) Representative examples of fIIWT/WT, fIIWT/WE, and fIIWE/WE embryos, respectively, collected at E18.5. Each animal was alive at the time of harvest, macroscopically normal, and free of overt hemorrhage. (G-V) Microscopic analysis revealing a normal pattern of development with no signs of hemorrhage in any organ system of fIIWE/WE offspring at E18.5. Note that hematoxylin/eosin–stained sections prepared from embryonic brain (G-J), heart (K-N), lung (O,Q), liver (P, R), small intestine (S,U), and pancreas (T,V) were virtually indistinguishable in wild-type offspring (panels G, H, K, L, O, P, S, T) and homozygous fIIWE/WE offspring (panels I, J, M,N, Q, R, U, V). The heart ventricle and atrium are annotated by vtl and atm, respectively. The scale bar indicates 100 μm.
Mice homozygous for fIIWE complete embryonic development but uniformly experience fatal postnatal bleeding events. (A-C) Representative examples of fIIWE/WE homozygous neonates. Note that most of the fIIWE/WE postnatal animals displayed appreciable abdominal hemorrhage. In addition, subcutaneous bleeding events in the head and neck region were also routinely observed. (D-F) Representative examples of fIIWT/WT, fIIWT/WE, and fIIWE/WE embryos, respectively, collected at E18.5. Each animal was alive at the time of harvest, macroscopically normal, and free of overt hemorrhage. (G-V) Microscopic analysis revealing a normal pattern of development with no signs of hemorrhage in any organ system of fIIWE/WE offspring at E18.5. Note that hematoxylin/eosin–stained sections prepared from embryonic brain (G-J), heart (K-N), lung (O,Q), liver (P, R), small intestine (S,U), and pancreas (T,V) were virtually indistinguishable in wild-type offspring (panels G, H, K, L, O, P, S, T) and homozygous fIIWE/WE offspring (panels I, J, M,N, Q, R, U, V). The heart ventricle and atrium are annotated by vtl and atm, respectively. The scale bar indicates 100 μm.
Summary of E18.5 embryos collected from heterozygous (fIIWT/WE) breeding pairs
| . | fIIWT/WT (wild-type) . | fIIWT/WE (hemizygous) . | fIIWE/WE (homozygous) . |
|---|---|---|---|
| Number observed* | 34 | 83 | 39 |
| Percentage expected | 25% | 50% | 25% |
| Percentage observed | 21.8% | 53.2% | 25.0% |
| Number (percentage) of expected based on wild-type | 34 (100%) | 68 (122%) | 34 (114%) |
| . | fIIWT/WT (wild-type) . | fIIWT/WE (hemizygous) . | fIIWE/WE (homozygous) . |
|---|---|---|---|
| Number observed* | 34 | 83 | 39 |
| Percentage expected | 25% | 50% | 25% |
| Percentage observed | 21.8% | 53.2% | 25.0% |
| Number (percentage) of expected based on wild-type | 34 (100%) | 68 (122%) | 34 (114%) |
P = N.S. by χ2 analysis.
A more formal analysis of the developmental success of homozygous fIIWE/WE mice was performed by evaluation of term embryos surgically delivered from fIIWT/WE females on E18.5. Analysis of 156 embryos harvested from heterozygous fIIWT/WE breeding pairs revealed that the genotypes of the term embryos closely followed a Mendelian pattern (34 fIIWT/WT, 83 fIIWT/WE, and 39 fIIWE/WE; P = .62 by χ2 analysis; Table 2). More interestingly, all 39 homozygous fIIWE/WE embryos were fully developed, and 35 (90%) were viable and overtly indistinguishable from fIIWT/WT and fIIWT/WE littermates (see Figure 2D-F for representative examples). The few nonviable fIIWE/WE embryos appeared to have failed proximal to the time of collection, and these embryos generally exhibited signs of hemorrhage (including one abdominal hemorrhage and one subcutaneous bleed), possibly induced by investigator manipulation during surgical collection. Virtually all of the heterozygous fIIWT/WE littermate embryos were found to be viable at E18.5 and none showed signs of hemorrhage.
To further explore the potential developmental consequences of constitutive prothrombinWE expression, a histologic analysis was performed with tissue sections prepared from 5 fIIWT/WT and 5 fIIWE/WE randomly selected E18.5 embryos. Similar to the gross macroscopic appearance of the embryos, the microscopic features of fIIWT/WT and fIIWE/WE offspring were indistinguishable. FIIWE/WE term embryos were microscopically unremarkable and displayed no signs of hemorrhage or other pathologies in any organ system, including the brain, heart, lungs, liver, gut, and pancreas (see Figure 2G-V for representative comparative sections), as well as the thymus, spleen, and kidneys (data not shown).
Heterozygous fIIWE mice exhibit a prolongation in bleeding time
The fact that fIIWT/WE mice routinely survived to adulthood permitted a more comprehensive evaluation of the physiologic and pathologic consequences, if any, of the constitutive expression of 50% wild-type prothrombin and 50% prothrombinWE. Analyses of complete blood counts in control and fIIWT/WE mice established that all blood cell parameters and differentials were comparable, including platelet counts (Table 3 and data not shown). Despite the presence of a mutant form of prothrombin with little procoagulant potential, there was no genotype-dependent difference in standard plasma coagulation tests. Unlike prior findings showing that the infusion of exogenous human thrombinWE resulted in extended plasma PTs and aPTTs,13,14 no significant differences were observed between control and fIIWT/WE mice in either the mean PT (10.4 ± 0.1 vs 11.2 ± 0.1 seconds, respectively) or the mean aPTT (26.3 ± 0.6 vs 24.9 ± 1.9 seconds, respectively). The dual findings that PT and aPTT values were similar in control and fIIWT/WE mice and prolonged in thrombinWE-infused mice relative to control mice might seem initially counterintuitive. However, each of these observations is compatible with other data. First, mice carrying half-normal levels of wild-type prothrombin have PT and aPTT values similar to wild-type mice (Table 3). Second, infusion of active thrombinWE results in a prompt, TM-dependent increase in circulating APC, leading to a predictable secondary extension in PT and aPTT values, whereas unchallenged fIIWT/WE mice carrying a combination of the wild-type zymogen and the prothrombinWE zymogen have very low and similar levels of plasma APC (see Figure 5). Finally, thrombinWE-mediated activation of protein C, like wild-type thrombin, is dependent on endothelial cell–associated TM, but TM would be distinctly absent in simple plasma PT and aPTT assays.
Hematologic profile of heterozygous (fIIWT/WE) mice
| . | fIIWT/WT . | fIIWT/WE . | fIIWT/Null . |
|---|---|---|---|
| WBCs, ×109/L | 3.2 ± 0.5 | 3.5 ± 1.2 | 7.4 ± 2.6 |
| RBCs, ×1012/L | 8.1 ± 1.6 | 7.9 ± 2.4 | 7.7 ± 1.5 |
| Hemoglobin, g/dL | 13.9 ± 0.4 | 13.8 ± 0.8 | 11.4 ± 0.3 |
| Hematocrit, % | 42.4 ± 8.4 | 40.4 ± 12.2 | 39.4 ± 7.5 |
| Platelets, ×109/L | 620 ± 129 | 580 ± 128 | 755 ± 114 |
| PT, s | 10.4 ± 0.1 | 11.2 ± 0.1 | 11.3 ± 0.1 |
| aPTT, s | 26.3 ± 0.6 | 24.9 ± 1.9 | 26.5 ± 0.9 |
| Tail bleeding time, s | 119 ± 12 | 164 ± 7* | 119 ± 7 |
| . | fIIWT/WT . | fIIWT/WE . | fIIWT/Null . |
|---|---|---|---|
| WBCs, ×109/L | 3.2 ± 0.5 | 3.5 ± 1.2 | 7.4 ± 2.6 |
| RBCs, ×1012/L | 8.1 ± 1.6 | 7.9 ± 2.4 | 7.7 ± 1.5 |
| Hemoglobin, g/dL | 13.9 ± 0.4 | 13.8 ± 0.8 | 11.4 ± 0.3 |
| Hematocrit, % | 42.4 ± 8.4 | 40.4 ± 12.2 | 39.4 ± 7.5 |
| Platelets, ×109/L | 620 ± 129 | 580 ± 128 | 755 ± 114 |
| PT, s | 10.4 ± 0.1 | 11.2 ± 0.1 | 11.3 ± 0.1 |
| aPTT, s | 26.3 ± 0.6 | 24.9 ± 1.9 | 26.5 ± 0.9 |
| Tail bleeding time, s | 119 ± 12 | 164 ± 7* | 119 ± 7 |
WBC indicates white blood cells.
P < .02, Student t test comparing fIIWT/WT or fIIWT/Null with fIIWT/WE (n = 6 per genotype except for tail bleeding time, which is n = 7).
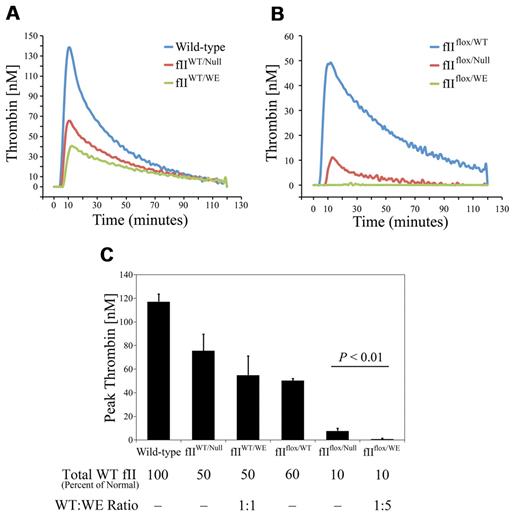
One potential mechanism by which prothrombinWE limits overall thrombin activity in heterozygous fIIWT/WE mice is by competitively inhibiting the conversion of the wild-type prothrombin to thrombin by the prothrombinase complex. Plasma TGAs were performed to evaluate this possibility. As shown in representative tracings in Figure 3, plasma isolated from wild-type mice exhibited robust thrombin generation, with a peak thrombin concentration of 120nM and a lag phase of ∼ 7 minutes. Consistent with the expectation that rates of prothrombinase activity would be slowed by diminished plasma prothrombin concentration, plasma from fIIWT/Null mice carrying 50% of the normal level of prothrombin exhibited a peak thrombin concentration that was approximately half of that observed in wild-type mice (mean value of 75nM). Interestingly, the peak thrombin concentration achieved with plasma from fIIWT/WE animals was 55nM, a value that was modestly lower than that observed for fIIWT/Null mice, but this did not achieve statistical significance. Nevertheless, the phenotypic significance, if any, of the local competitive inhibition of wild-type prothrombin activation by prothrombinWE in fIIWT/WE mice remains unknown in vivo. The potential of prothrombinWE to act as a competitive inhibitor of prothrombinase may be documented in comparative studies of thrombin generation under conditions in which plasma prothrombinWE appreciably exceeded wild-type prothrombin. We took advantage of mice described previously, which carry a floxed prothrombin allele that drives 10% of the normal level of wild-type prothrombin expression, to establish cohorts of mice that either carry no mutant protein (fIIflox/Null mice) or carry a 5:1 ratio of prothrombinWE over wild-type prothrombin (fIIflox/WE mice). As illustrated by the representative plasma thrombin generation curves in Figure 3B and as summarized in Figure 3C, thrombin-generation rates were reduced as a function of diminished wild-type prothrombin in plasma. However, thrombin generation was significantly diminished in plasma from fIIflox/WE mice relative to fIIflox/Null mice. This result is consistent with the concept that excess prothrombinWE can competitively limit wild-type thrombin generation by the prothrombinase complex.
ProthrombinWE limits wild-type thrombin generation in plasma. (A) Representative TGA tracings using plasma isolated from individual wild-type, fIIWT/Null, and fIIWT/WE mice. As expected based on rate-limiting prothrombin concentration, fIIWT/Null plasma supported the generation of approximately half of the thrombin activity observed using wild-type plasma. Thrombin generation tended to be incrementally less in TGA studies using plasma collected from fIIWT/WE mice relative to samples collected from fIIWT/Null mice, but this was not statistically different over multiple specimens (see panel C). (B) Representative TGA tracings using plasma from fIIflox/WT mice (carrying wild-type prothrombin at a concentration ∼ 60% of normal), fIIflox/Null mice (carrying wild-type prothrombin at a concentration ∼ 10% of normal), and fIIflox/WE mice (carrying wild-type prothrombin at a concentration ∼ 10% of normal and an ∼ 5-fold molar excess of prothrombinWE over wild-type prothrombin). Note that thrombin generation with plasma from fIIflox/Null mice was limited (consistent with wild-type prothrombin being present at approximately 10% of normal levels), but was barely detectable with plasma from fIIflox/WE, with a similar concentration of wild-type prothrombin and appreciably more prothrombinWE. (C) Comparison of peak thrombin concentration. The TGA assay was performed in duplicate on plasma collected from 3 mice per genotype. Note that the mean peak thrombin concentration in fIIflox/WE plasma, in which the ratio of fIIWE thrombin to wild-type thrombin was approximately 5:1, was significantly lower than that observed for fIIflox/null plasma. Data are presented as the means ± SD and were analyzed using the Student t test.
ProthrombinWE limits wild-type thrombin generation in plasma. (A) Representative TGA tracings using plasma isolated from individual wild-type, fIIWT/Null, and fIIWT/WE mice. As expected based on rate-limiting prothrombin concentration, fIIWT/Null plasma supported the generation of approximately half of the thrombin activity observed using wild-type plasma. Thrombin generation tended to be incrementally less in TGA studies using plasma collected from fIIWT/WE mice relative to samples collected from fIIWT/Null mice, but this was not statistically different over multiple specimens (see panel C). (B) Representative TGA tracings using plasma from fIIflox/WT mice (carrying wild-type prothrombin at a concentration ∼ 60% of normal), fIIflox/Null mice (carrying wild-type prothrombin at a concentration ∼ 10% of normal), and fIIflox/WE mice (carrying wild-type prothrombin at a concentration ∼ 10% of normal and an ∼ 5-fold molar excess of prothrombinWE over wild-type prothrombin). Note that thrombin generation with plasma from fIIflox/Null mice was limited (consistent with wild-type prothrombin being present at approximately 10% of normal levels), but was barely detectable with plasma from fIIflox/WE, with a similar concentration of wild-type prothrombin and appreciably more prothrombinWE. (C) Comparison of peak thrombin concentration. The TGA assay was performed in duplicate on plasma collected from 3 mice per genotype. Note that the mean peak thrombin concentration in fIIflox/WE plasma, in which the ratio of fIIWE thrombin to wild-type thrombin was approximately 5:1, was significantly lower than that observed for fIIflox/null plasma. Data are presented as the means ± SD and were analyzed using the Student t test.
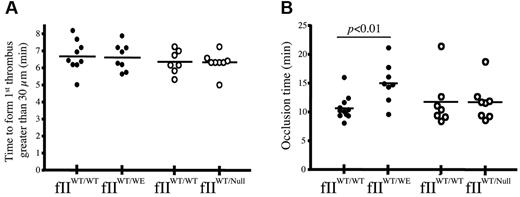
Tail-bleeding time analyses were performed to determine whether a general shift in hemostatic function could be seen in vivo in fIIWT/WE mice. As shown in Table 3, fIIWT/WE mice displayed a significant prolongation in bleeding times relative to wild-type animals. The cessation of bleeding was achieved in 119 ± 12 seconds in wild-type mice, whereas 164 ± 8 seconds were required to control blood loss in fIIWT/WE mice, a 40% increase in bleeding time (P < .02). The prolongation in tail-bleeding time was not simply a function of the fIIWT/WE mice carrying half-normal wild-type prothrombin, because fIIWT/Null mice displayed tail-bleeding times of 119 ± 7 seconds, which is virtually identical to that observed for wild-type mice (Table 3). As a more direct analysis of the formation of thrombi in fIIWT/WE and control mice, vessel occlusion was compared by real-time intravital microscopy within ∼ 100-μm–diameter mesenteric arterioles after FeCl3 injury (Figure 4). No differences were observed in the time to initial thrombus formation after injury (Figure 4A); however, consistent with the appreciably extended bleeding times observed in fIIWT/WE mice, the time to complete vessel occlusion within injured mesenteric arterioles was significantly prolonged in fIIWT/WE mice relative to wild-type control animals (an average of 15.0 ± 1.2 minutes compared with 10.6 ± 0.6 minutes, respectively, P < .01; Figure 3B). The longer average occlusion time observed in fIIWT/WE mice was not a function of any genotype-dependent differences in animal weight, vessel diameter, or vessel shear rate, because these parameters were all comparable between genotypes (Table 4). The extended vessel occlusion times for the fIIWT/WE mice also did not appear to be merely a function of the half-normal level of wild-type prothrombin in these animals. Unlike the extended times to complete vessel occlusion observed in fIIWT/WE mice, fIIWT/Null mice were indistinguishable from wild-type mice in this parameter (an average of 11.7 ± 1.0 minutes for fIIWT/Null compared with 11.7 ± 1.1 minutes for wild-type animals; Figure 4D). Experimental parameters of animal weight, vessel diameter, and shear rates were also similar between fIIWT/Null and control mice (Table 4).
Mice expressing fIIWE exhibit an extended time-to-occlusion in mesenteric arterioles after FeCl3 injury. Thrombus formation was evaluated and quantified using intravital videomicroscopy. (A) The time to initial formation of a thrombus greater than 30 μm was similar between cohorts of wild-type (fIIWT/WT) and fIIWT/WE mice (●), with average times of 6.7 ± 0.3 minutes and 6.6 ± 0.3 minutes, respectively. Similar results were obtained for this parameter in cohorts of fIIWT/WT animals and heterozygous mice carrying one fII null allele and one wild-type fII allele (fIIWT/Null) and known to carry 50% of the normal level of entirely wild-type prothrombin; the average values in cohorts of fIIWT/Null and littermate wild-type mice were 6.3 ± 0.2 minutes and 6.4 ± 0.2 minutes, respectively (○). (B) The time to complete vessel occlusion and cessation of blood flow after injury in fIIWT/WE mice was significantly extended relative to wild-type mice, 15.0 ± 1.2 minutes compared with 10.6 ± 0.6 minutes, respectively. In contrast, the time-to-occlusion after injury in fIIWT/Null mice carrying 50% of normal wild-type prothrombin (average time of 11.7 ± 1.0 minutes) was distinctly shorter than fIIWT/WE mice and virtually identical to that of fIIWT/WT control mice (average time of 11.7 ± 1.1 minutes). Horizontal bars represent the mean of each group, and data were analyzed using the Student t test.
Mice expressing fIIWE exhibit an extended time-to-occlusion in mesenteric arterioles after FeCl3 injury. Thrombus formation was evaluated and quantified using intravital videomicroscopy. (A) The time to initial formation of a thrombus greater than 30 μm was similar between cohorts of wild-type (fIIWT/WT) and fIIWT/WE mice (●), with average times of 6.7 ± 0.3 minutes and 6.6 ± 0.3 minutes, respectively. Similar results were obtained for this parameter in cohorts of fIIWT/WT animals and heterozygous mice carrying one fII null allele and one wild-type fII allele (fIIWT/Null) and known to carry 50% of the normal level of entirely wild-type prothrombin; the average values in cohorts of fIIWT/Null and littermate wild-type mice were 6.3 ± 0.2 minutes and 6.4 ± 0.2 minutes, respectively (○). (B) The time to complete vessel occlusion and cessation of blood flow after injury in fIIWT/WE mice was significantly extended relative to wild-type mice, 15.0 ± 1.2 minutes compared with 10.6 ± 0.6 minutes, respectively. In contrast, the time-to-occlusion after injury in fIIWT/Null mice carrying 50% of normal wild-type prothrombin (average time of 11.7 ± 1.0 minutes) was distinctly shorter than fIIWT/WE mice and virtually identical to that of fIIWT/WT control mice (average time of 11.7 ± 1.1 minutes). Horizontal bars represent the mean of each group, and data were analyzed using the Student t test.
Characteristics of mice analyzed by FeCl3 injury of mesenteric arterioles
| Genotype . | Weight of mice, g . | Diameter of vessel, μm . | Shear rate, s−1 . |
|---|---|---|---|
| fIIWT/WT (n = 11) | 8.6 ± 0.3 | 104.5 ± 4.2 | 1780 ± 110 |
| fIIWT/WE (n = 8) | 9.1 ± 0.3 | 112.5 ± 5.8 | 1760 ± 90 |
| fIIWT/WT (n = 7) | 9.5 ± 0.2 | 108.9 ± 1.8 | 1730 ± 50 |
| fIIWT/Null (n = 8) | 10.0 ± 0.3 | 106.3 ± 2.4 | 1770 ± 90 |
| Genotype . | Weight of mice, g . | Diameter of vessel, μm . | Shear rate, s−1 . |
|---|---|---|---|
| fIIWT/WT (n = 11) | 8.6 ± 0.3 | 104.5 ± 4.2 | 1780 ± 110 |
| fIIWT/WE (n = 8) | 9.1 ± 0.3 | 112.5 ± 5.8 | 1760 ± 90 |
| fIIWT/WT (n = 7) | 9.5 ± 0.2 | 108.9 ± 1.8 | 1730 ± 50 |
| fIIWT/Null (n = 8) | 10.0 ± 0.3 | 106.3 ± 2.4 | 1770 ± 90 |
Heterozygous fIIWE mice exhibit no survival advantage after acute endotoxemia challenge and display modestly reduced APC generation
Exogenous APC can improve survival in the contexts of both bacterial sepsis and acute endotoxemia. Given that the substrate specificity of both human and murine thrombinWE favors protein C activation, we hypothesized that mice carrying prothrombinWE would exhibit a survival advantage associated with elevated plasma levels of APC relative to wild-type animals after challenge with lethal doses of endotoxin. Contrary to these expectations, Kaplan-Meier analyses of cohorts of fIIWT/WE and wild-type mice challenged with high-dose LPS (15 mg/kg) revealed a similar survival profile (Figure 5A). Furthermore, even at lower doses of LPS (ie, ranging from the LD50 over a 2-week period to a LD100), no survival advantage was appreciable in fIIWT/WE mice relative to control mice (data not shown). Complementary studies were done to compare plasma APC levels directly in control and mutant mice after LPS challenge using an established assay for murine APC. Unchallenged wild-type and fIIWT/WE mice exhibited similar levels of plasma APC at baseline (2.8 vs 3.5 ng/mL, respectively) and, as expected, plasma APC levels rose dramatically in mice of each genotype within hours after LPS challenge (Figure 5B). However, circulating APC levels were modestly but significantly lower in fIIWT/WE mice relative to wild-type mice at both 2 hours (11.8 vs 6.6 ng/mL; P < .003) and 10 hours (13.2 vs 9.3 ng/mL; P < .02) after LPS challenge. Although they were contrary to our initial expectations, the distinctly lower APC levels observed in fIIWT/WE mice are compatible with overall diminished wild-type thrombin generation (ie, mutant and wild-type thrombin competing for TM-binding sites and the known modest reduction in fIIWE catalytic efficiency with protein C). Consistent with this notion, thrombin-antithrombin complexes were higher in the plasma of wild-type mice compared with fIIWT/WE mice after LPS challenge (Figure 5C). Whether rates of local APC generation are elevated in fIIWE mice in other contexts, particularly in the context of a local rather than a systemic challenge, is an unresolved question that will require additional studies.
Heterozygous fIIWE mice exhibit no survival advantage after challenge with acute endotoxemia and modestly lower circulating APC relative to wild-type mice. (A) Kaplan-Meier survival analysis of wild-type (n = 10) and fIIWT/WE (n = 11) mice after IP injection of LPS (15 mg/kg). (B) Determination of plasma APC concentration in wild-type and fIIWT/WE mice. Plasma was collected from unchallenged (n = 3 per genotype) mice or from mice challenged with 15 mg/kg of LPS by IP injection for 2 hours (n = 6 per genotype) and 10 hours (n = 6 per genotype). Note that 2 hours after LPS challenge, wild-type mice had approximately 2-fold more APC than fIIWT/WE mice, whereas at 10 hours the differences were more modest. (C) Thrombin-antithrombin (TAT) levels in plasma of LPS-challenged (15 mg/kg) wild-type and fIIWT/WE mice (n = 6 per genotype). Note that TAT levels were higher in the wild-type plasma at both time points. *P < .05; **P < .02; ***P < .003 by Student t test.
Heterozygous fIIWE mice exhibit no survival advantage after challenge with acute endotoxemia and modestly lower circulating APC relative to wild-type mice. (A) Kaplan-Meier survival analysis of wild-type (n = 10) and fIIWT/WE (n = 11) mice after IP injection of LPS (15 mg/kg). (B) Determination of plasma APC concentration in wild-type and fIIWT/WE mice. Plasma was collected from unchallenged (n = 3 per genotype) mice or from mice challenged with 15 mg/kg of LPS by IP injection for 2 hours (n = 6 per genotype) and 10 hours (n = 6 per genotype). Note that 2 hours after LPS challenge, wild-type mice had approximately 2-fold more APC than fIIWT/WE mice, whereas at 10 hours the differences were more modest. (C) Thrombin-antithrombin (TAT) levels in plasma of LPS-challenged (15 mg/kg) wild-type and fIIWT/WE mice (n = 6 per genotype). Note that TAT levels were higher in the wild-type plasma at both time points. *P < .05; **P < .02; ***P < .003 by Student t test.
fIIWE attenuates the development of inflammatory joint disease in mice challenged with CIA
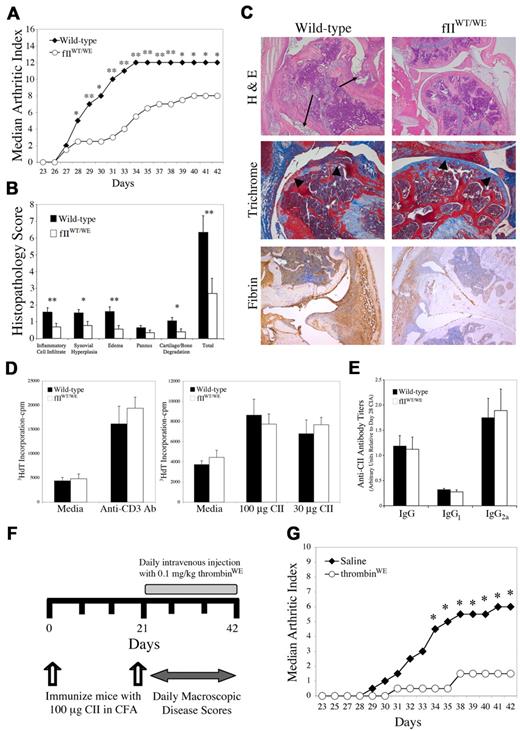
Based on the identification of thrombin and multiple thrombin substrates as critical determinants of inflammatory processes in vivo, we hypothesized that the fIIWE mutation would result in at least one phenotypic benefit: the amelioration of inflammatory disease processes such as the development of CIA. To evaluate this hypothesis, we backcrossed mice carrying the fIIWE allele to the CIA-susceptible strain DBA/1J and challenged cohorts of fIIWT/WE and control mice with CII/CFA immunization to induce CIA. Macroscopic disease was evaluated by enumerating the total number of affected digits in the fore and hind paws of the mice. CIA-challenged fIIWT/WE mice developed dramatically diminished macroscopic inflammatory joint disease relative to wild-type mice, as reflected by significantly lower median arthritic index scores (Figure 6A). Genotype-dependent differences were appreciable just days after the secondary CII immunization on day 21 and persisted to the end of the evaluation period. This analysis was performed 3 times with similar results. Similar to studies of bleeding time and vessel occlusion, the differences in CIA observed in fIIWT/WE mice did not appear to be merely a function of half-normal levels of wild-type prothrombin, because CIA severity was found to be generally similar in DBA/1 fIIWT/Null and wild-type mice (data not shown).
Attenuation of collagen-induced arthritis by pro/thrombinWE. (A) Representative experiment of wild-type (n = 9) and fIIWE (n = 8) mice in which animals were immunized with CII and the development of arthritis within distal joints was scored by investigators blinded to genotype and expressed as a median arthritic index. Note that within a week of secondary CII immunization and to the end of the evaluation period, fIIWE mice exhibited significantly less macroscopic arthritis relative to wild-type animals. Daily scores were analyzed by Mann-Whitney U test and found to be consistently significant beyond day 28. *P < .05; **P < .02. Three independent experiments yielded similar results. (B) Knee joint sections prepared from cohorts of wild-type (n = 16) and fIIWE (n = 12) animals processed for histologic evaluation on day 42 of the CIA protocol. The data for each parameter and the total histopathology score are expressed as the means ± SEM and were analyzed by Mann-Whitney U test. *P < .05; **P < .03. (C) Representative examples of knee histopathology from wild-type and fIIWT/WE CIA-challenged animals. Hematoxylin/eosin–stained sections revealed a profound loss of joint architecture in CIA-challenged wild-type animals, with significant inflammatory cell infiltrates and synovial hyperplasia (arrows). In contrast, knee joints from CIA-challenged fIIWT/WE mice displayed only mild inflammatory cell infiltrates and little synovial hyperplasia. Mason trichrome staining of CIA-challenged knee joint sections revealed a loss of articular cartilage in wild-type mice (arrowheads), whereas cartilage surfaces were smooth and well preserved in fIIWT/WE mice. Fibrin appeared qualitatively more widespread and pronounced in the knee joints of CIA-challenged wild-type mice relative to the knee joints of CIA-challenged fIIWT/WE analyzed in parallel. (D) The adaptive immune response at the level of T cells was similar in CII-immunized control and mutant mice. Popliteal lymph node cells were harvested from wild-type (n = 4) and fIIWT/WE (n = 4) mice 10 days after footpad injection with CII/CFA, and cultured T cells were stimulated with either 100 or 30 μg/mL CII. Anti-CD3–activating T cell–receptor antibody was used as a positive control. Results are expressed as the mean value of 3H-dT incorporated in counts per minute (cpm) ± SEM (E) Determination of anti–CII-specific IgG antibody titers by ELISA using plasma harvested at day 42 of the CIA protocol from wild-type (n = 9) and fIIWT/WE (n = 9) mice. Titers are expressed as arbitrary units relative to titers found in wild-type DBA/1 mice at day 28 of CIA. Results are expressed as the means ± SEM (F) Summary of experimental manipulations in cohorts of CIA-challenged mice treated with recombinant murine thrombinWE. Beginning at day 21 of the CIA protocol, mice were given daily administrations of either 0.1 mg/kg murine thrombinWE or normal saline as a vehicle control. (G) Analysis of macroscopic CIA in the paws of wild-type mice treated with either thrombinWE or saline (n = 6 per group). Note that thrombinWE treatment significantly limited the development of inflammatory joint disease relative to saline-treated control mice over a wide observation period. * P < .05 by Mann-Whitney U test.
Attenuation of collagen-induced arthritis by pro/thrombinWE. (A) Representative experiment of wild-type (n = 9) and fIIWE (n = 8) mice in which animals were immunized with CII and the development of arthritis within distal joints was scored by investigators blinded to genotype and expressed as a median arthritic index. Note that within a week of secondary CII immunization and to the end of the evaluation period, fIIWE mice exhibited significantly less macroscopic arthritis relative to wild-type animals. Daily scores were analyzed by Mann-Whitney U test and found to be consistently significant beyond day 28. *P < .05; **P < .02. Three independent experiments yielded similar results. (B) Knee joint sections prepared from cohorts of wild-type (n = 16) and fIIWE (n = 12) animals processed for histologic evaluation on day 42 of the CIA protocol. The data for each parameter and the total histopathology score are expressed as the means ± SEM and were analyzed by Mann-Whitney U test. *P < .05; **P < .03. (C) Representative examples of knee histopathology from wild-type and fIIWT/WE CIA-challenged animals. Hematoxylin/eosin–stained sections revealed a profound loss of joint architecture in CIA-challenged wild-type animals, with significant inflammatory cell infiltrates and synovial hyperplasia (arrows). In contrast, knee joints from CIA-challenged fIIWT/WE mice displayed only mild inflammatory cell infiltrates and little synovial hyperplasia. Mason trichrome staining of CIA-challenged knee joint sections revealed a loss of articular cartilage in wild-type mice (arrowheads), whereas cartilage surfaces were smooth and well preserved in fIIWT/WE mice. Fibrin appeared qualitatively more widespread and pronounced in the knee joints of CIA-challenged wild-type mice relative to the knee joints of CIA-challenged fIIWT/WE analyzed in parallel. (D) The adaptive immune response at the level of T cells was similar in CII-immunized control and mutant mice. Popliteal lymph node cells were harvested from wild-type (n = 4) and fIIWT/WE (n = 4) mice 10 days after footpad injection with CII/CFA, and cultured T cells were stimulated with either 100 or 30 μg/mL CII. Anti-CD3–activating T cell–receptor antibody was used as a positive control. Results are expressed as the mean value of 3H-dT incorporated in counts per minute (cpm) ± SEM (E) Determination of anti–CII-specific IgG antibody titers by ELISA using plasma harvested at day 42 of the CIA protocol from wild-type (n = 9) and fIIWT/WE (n = 9) mice. Titers are expressed as arbitrary units relative to titers found in wild-type DBA/1 mice at day 28 of CIA. Results are expressed as the means ± SEM (F) Summary of experimental manipulations in cohorts of CIA-challenged mice treated with recombinant murine thrombinWE. Beginning at day 21 of the CIA protocol, mice were given daily administrations of either 0.1 mg/kg murine thrombinWE or normal saline as a vehicle control. (G) Analysis of macroscopic CIA in the paws of wild-type mice treated with either thrombinWE or saline (n = 6 per group). Note that thrombinWE treatment significantly limited the development of inflammatory joint disease relative to saline-treated control mice over a wide observation period. * P < .05 by Mann-Whitney U test.
At day 42 of the CIA protocol, knee joints from both fIIWT/WE and wild-type mice were harvested for microscopic analysis. Midline sagittal sections of knee joints were graded by investigators blinded to animal genotype for histologic features of inflammatory joint disease, including inflammatory cell infiltrate, synovial hyperplasia, edema, pannus formation, and cartilage/bone degradation, using an established scoring system (see “Histologic analysis” for details). Individual parameter scores were summed for each mouse to establish a total histopathology score. As expected, unchallenged control and fIIWE mice were indistinguishable with regard to overall joint architecture and microscopic appearance (data not shown). However, the knee joints from CIA-challenged fIIWT/WE mice exhibited significantly less evidence of joint pathology than wild-type mice with regard to each of the microscopic parameters evaluated (Figure 6B). Furthermore, the total histopathology score affirmed a significant diminution in joint disease in mice expressing fIIWE relative to wild-type animals challenged in parallel. Representative sections of knee joints from control and mutant mice (Figure 6C) highlight the substantial qualitative benefit of the presence of fIIWE in limiting local inflammatory joint disease. The knee joints of CIA-challenged wild-type animals were typically characterized by significant articular cartilage erosion with frequent obliteration of articular surfaces, invading granulation tissue, and even loss of bone (Figure 6C), whereas the articular surfaces in challenged fIIWE mice were often smooth, with largely unperturbed cartilage and intact bone. Finally, qualitatively less local fibrin deposition was apparent within knee joint tissues of CIA-challenged fIIWE mice relative to challenged wild-type animals (Figure 6C). Notably, the diminution in arthritis severity observed in fIIWT/WE mice was not due to a reduced adaptive immune response to the CII/CFA immunization. Cultured T cells isolated from draining lymph nodes from mice of each genotype responded similarly when challenged with CII antigen or when directly stimulated with a T cell–receptor antibody (Figure 6D). Similarly, anti-CII antibody titers of total IgG and the IgG1 and IgG2A subclasses were found to be similar between wild-type and fIIWT/WE mice (Figure 6E).
To determine whether enzymatically active exogenous thrombinWE could also limit the development of arthritis, complementary studies were done in CIA-challenged wild-type mice treated with recombinant murine thrombinWE. Wild-type mice were immunized with CII in CFA and, beginning at the time of the second immunization, cohorts were administered either recombinant murine thrombinWE or saline vehicle control (Figure 6F). Similar to results in mice carrying a prothrombinWE allele, wild-type mice treated with active thrombinWE developed significantly diminished macroscopic CIA relative to saline-treated control mice (Figure 6G). It was not possible to use wild-type murine thrombin as a control for these experiments, because dosing strategies for wild-type thrombin identical to those used for the mutant thrombinWE resulted in significant mortality after just a single administration. These results demonstrate that pro/thrombinWE can significantly limit the development of inflammatory joint disease when introduced either genetically as a zymogen or pharmacologically in the activated form.
Discussion
Thrombin-mediated proteolysis is central to the control of hemostasis and thrombosis, but it is increasingly understood that prothrombin and multiple thrombin substrates play a pivotal role in embryonic development, tissue repair, malignancy, and inflammation. In the present study, we examined for the first time the in vivo consequences of constitutive expression of a prothrombin active site mutant, fIIWE, with a radically altered protease substrate specificity limiting procoagulant function. Homozygous fIIWE/WE mice were found to be fundamentally distinct from fII-deficient mice and from mice with mutations influencing thrombin generation (eg, TF, fV, or fX deficiencies) or thrombin substrates (eg, PAR-1 deficiency), in that fIIWE/WE embryos consistently developed to term. However, like mice with combined deficits in coagulation and platelet function, fIIWE/WE homozygous mice uniformly developed fatal hemorrhagic events immediately after birth.32,33 These findings reinforce the notion that there is a critical requirement for thrombin prothrombotic function to maintain hemostasis beyond the perinatal period, but that embryonic developmental success can still be supported by appreciably more limited thrombin-mediated proteolysis. Heterozygous expression of fIIWE was compatible with survival to adulthood; evidence of perinatal hemorrhage was very rare in these individuals (< 4%) and event-free survival to advanced age was effectively a uniform finding in postnatal fIIWT/WE offspring. However, fIIWT/WE mice exhibited significantly prolonged time-to-occlusion after vascular injury in direct analyses of thrombus formation, as well as prolonged bleeding times. Consistent with the notion that thrombin serves in a regulatory nexus between the hemostatic and inflammatory pathways, there is at least one major phenotypic benefit of carrying circulating fIIWE: attenuation of an inflammatory disease process. When challenged with CIA, fIIWT/WE mice developed dramatically diminished macroscopic and microscopic disease in both distal and proximal joints relative to wild-type animals. Arthritis development was also profoundly limited in wild-type mice treated with active recombinant murine thrombinWE, indicating that the redirection of protease activity was one potential determinant of phenotype. These findings underscore the idea that thrombin-mediated proteolysis is a potent regulator of local inflammatory events in vivo.
Multiple independent studies have established that the constitutive elimination of prothrombin commonly results in midgestation developmental failure (eg, 60%-80% embryonic lethality at E9.5).34,35 However, the failure of prothrombin-deficient embryos does not appear to be related to hemostasis, but rather appears to be a deleterious consequence of lost thrombin-mediated PAR-1 signaling.36 The finding that homozygous fIIWE/WE mice, unlike either fII- or PAR-1–deficient mice, uniformly develop to term suggests (1) that the thrombinWE active-site mutant retains biologically meaningful enzymatic activity in vivo, and (2) that thrombinWE either directly or indirectly generates a sufficient level of PAR-1 activation to consistently support development to term. The residual activity of murine thrombinWE for murine PAR-1 is very low (< 2% of wild-type murine thrombin) but may be adequate for developmental success.15 Alternatively, PAR-1 activation in fIIWE/WE embryos may be indirectly achieved via retained TM-dependent activation of protein C- and APC-mediated PAR-1 signaling. Complementary studies of development in fIIWE/WE embryos with and without a secondary deficit in protein C activation (eg, TMPro/Pro mice37 ) would be instructive in testing this latter concept.
Heterozygous fIIWT/WE mice developed normally, exhibited normal reproductive success, and survived long into adulthood. However, consistent with an alteration in the prothrombin active site limiting procoagulant function and favoring anticoagulant function, fIIWT/WE mice exhibited prolongations in bleeding/occlusion times that were phenotypically distinct from mice heterozygous for a fII-null allele (fIIWT/Null animals). A combination of mechanisms may contribute to the overall phenotype of fIIWT/WE mice. The most intriguing of these is the local generation of thrombin with little procoagulant function but appreciable residual capacity to activate protein C. However, thrombinWE may also influence phenotypic outcomes through the unique engagement of binding partners, including the platelet VWF receptor component GPIbα.38,39 ThrombinWE binding to GPIbα has been shown to antagonize platelet interactions with VWF under shear conditions, which could competitively limit both platelet engagement of VWF and platelet-associated prothrombotic activity.38 ThrombinWE may also contribute to the overall phenotype by competitively acting to limit wild-type thrombin interactions with a variety of downstream substrates, including fXI, fVIII, fV, fXIII, and PARs. Even the zymogen form of prothrombinWE may contribute to overall phenotype in fIIWT/WE mice. The dominant-negative competition between wild-type prothrombin and prothrombinWE for activation by prothrombinase complexes would be expected to limit the rate of wild-type thrombin generation at sites of injury, leading to both a proximal reduction in platelet activation and fibrin conversion, as well as a distal reduction in TM-regulated APC generation. Results of plasma-based thrombin-generation assays have suggested that the fIIWE zymogen can competitively limit wild-type prothrombin activation by prothrombinase under some conditions. Together with the modest reduction in catalytic efficiency of thrombinWE for protein C, this is likely to account for the modest, but initially unanticipated, lower levels of plasma APC observed in fIIWT/WE mice after LPS challenge. The impact of fIIWE is likely multifaceted, and the premiere effect mechanisms may differ based on the underlying challenge (eg, vascular injury, infection, or immunologic challenge), location, and time.
Consistent with the hypothesis that thrombin-mediated proteolysis can mediate inflammatory processes, the results of the present study revealed that at least one striking benefit of circulating prothrombinWE is the amelioration of inflammatory joint disease. Because thrombin engages multiple substrates and receptors that participate in the control of inflammation, this approach may offer some advantages over interventions at the level of individual thrombin targets. Fibrin deposition within the joint space is a conspicuous and consistent feature of both human rheumatoid arthritis and murine CIA.16,18,21,40 We recently demonstrated that the genetic elimination of fibrin(ogen) dramatically reduced CIA disease severity.21 The finding herein that fibrin deposition is qualitatively reduced within the joints of CIA-challenged fIIWT/WE mice points to one simple mechanism that would account for the reduced disease severity. However, other mechanisms may contribute to the outcome in CIA-challenged fIIWT/WE mice. First, thrombinWE is known to be an inefficient activator of PARs, and both PAR-1 and PAR-4 were reported to contribute to the severity of inflammatory joint disease.22,41 Second, thrombinWE-mediated antagonism of GPIbα interactions with VWF and/or wild-type thrombin may limit the pro-arthritic activity of platelets.38 Platelets and platelet-derived microparticles were recently shown to be potent drivers of CIA based on platelet depletion and platelet receptor modification studies.42 Finally, thrombinWE could potentially limit the progression of inflammatory joint disease via local generation of the anticoagulant APC, an enzyme also known to have potent anti-inflammatory, cytoprotective, antiapoptotic, and barrier-stabilization activities. Exogenous thrombinWE administration was reported previously to result in advantageous endogenous APC generation.43 Based on the well-documented beneficial in vivo effects of APC in sepsis,23 ischemic stroke,44-47 amyotrophic lateral sclerosis,48 lung inflammation,49 and neuroinflammatory disease,50 and the documented role of thrombin and thrombin targets in inflammatory joint disease,19-22 APC and APC derivatives with anti-inflammatory/barrier protective properties are likely to limit arthritic disease. Whatever the precise benefits of fIIWE in CIA, they were not tied to the capacity of mutant mice to develop an autoimmune response, but rather were coupled to changes in proteolysis within the joint space to alter subsequent disease progression. Consistent with this view, a significant extension of the genetic analyses was the demonstration that intravenous administration of active murine thrombinWE to CIA-challenged mice also significantly dampened the development of inflammatory joint disease. These results provide a proof-of-principle that pharmacologic interventions imposing pro/thrombin specificity variants or small-molecule agents that impose alterations in thrombin specificity may offer therapeutic opportunities for the treatment of inflammatory diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sara Welch, Joni Prasad, Elizabeth Blevins, and Harini Raghu for their technical assistance.
This work was supported by grants from the National Institutes of Health (R01 AR056990 to M.J.F.; R01 HL085357 and R01 HL096126 to J.L.D.; R01 HL041002 to D.D.W.; and R01 HL049413, R01 HL058141, R01 HL073813, and R44 HL095315 to E.D.C.), by the Howard Hughes Medical Institute (to C.T.E.), and by the Cincinnati Rheumatic Diseases Center (P30 AR047363).
National Institutes of Health
Authorship
Contribution: M.J.F., A.K.C., K.E.T., E.S.M., J.S.P., W.M., M.F., K.W.K., and X.Z. performed research; M.J.F., A.K.C., S.T., N.L.E., C.T.E., D.D.W., A.B., L.A.P, E.D.C., and J.L.D. designed experiments, analyzed data, and interpreted results; and M.J.F. and J.L.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jay L. Degen, Division of Experimental Hematology, Cincinnati Children's Hospital Research Foundation and the University of Cincinnati College of Medicine, 3333 Burnet Ave, Cincinnati, OH 45255; e-mail: jay.degen@cchmc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal