Abstract
The contribution of specific cell types to the production of cytokines that regulate hematopoiesis is still not well defined. We have previously identified T cell–dependent regulation of hematopoietic progenitor cell (HPC) numbers and cycling. In this report, we demonstrated that HPC activity is decreased in mice with STAT3-deficient T cells, a phenotype that is not because of decreased expression of IL-17 or RORγt. STAT3 expression in T cells was required for IL-21 production by multiple T helper subsets, and neutralization of IL-21 resulted in decreased HPC activity identical to that in mice with STAT3-deficient T cells. Importantly, injection of IL-21 rescued HPC activity in mice with STAT3-deficient T cells. Thus, STAT3-dependent IL-21 production in T cells is required for HPC homeostasis.
Introduction
T cells are an important source of cytokines that regulate hematopoietic progenitor cell (HPC) homeostasis. Mice deficient in T cells have altered myeloid cell development that is rescued by the transfer of CD4+ T cells.1 Moreover, mice that are deficient in STAT4 or STAT6, transcription factors that respectively promote Th1 and Th2 development, have altered HPC homeostasis due in part to the effects of IL-12–priming Th1 development and the secretion of Oncostatin M.2,3 The ability of other Th subsets to regulate this process has not been examined.
STAT3 promotes the development of Th17 cells,4,5 Tfh cells,6 and Th2 cells,7 by activating genes encoding subset-specific cytokines and transcription factors.7,8 Although the requirement for STAT3 in T cells to regulate HPC homeostasis has not been examined, one of the genes regulated and bound by STAT3 is Il21.9 IL-21 is produced by multiple T-cell subsets and has been reported to promote hematopoiesis.10-13 In this report, we examine the requirement for STAT3-dependent Th subsets in regulating HPC homeostasis.
Methods
Mice
C57BL/6 Stat3fl/fl mice14 with a CD4-Cre (Stat3CD4–/–) transgene and Cre-negative littermates, wild-type mice purchased from Harlan Laboratories, Il17−/− mice provided by Dr Alison Finnegan (Rush University, Chicago, IL) with agreement from Dr Yoichiro Iwakura (University of Tokyo, Tokyo, Japan), and Rorcgfp/gfp mice purchased from The Jackson Laboratory were maintained in specific pathogen-free conditions and experiments were approved by the Indiana University Institutional Animal Care and Use Committee.
In vitro T-cell differentiation and analysis of phospho-STAT proteins
Assay of hematopoietic progenitor numbers and cycling
Results and discussion
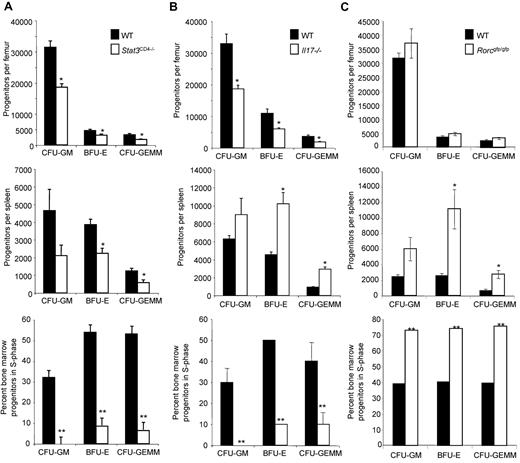
To test the requirement for STAT3-dependent T-cell function in regulating HPC homeostasis, we isolated bone marrow and spleen from mice carrying a floxed Stat3 allele and expressing Cre controlled by a CD4 promoter (noted as Stat3CD4−/−) and from littermates that did not have the Cre-transgene (noted as wild-type). CD4-Cre expression results in conditional deletion of Stat3 in T cells but not HPC. There were no differences in the number of nucleated bone marrow or spleen cells, and no difference in apoptosis of Lineage−Sca-1+c-Kit+ cells that are highly enriched for HPC between mice with wild-type or STAT3-deficient T cells (data not shown). However, there was a significant decrease in absolute numbers of CFU-GM, BFU-E and CFU-GEMM in bone marrow and spleen from mice that lacked STAT3 in T cells, compared with littermate controls (Figure 1A). The proportion of cells that were in S-phase of the cell cycle in the bone marrow, defined using high specific activity tritiated thymidine in parallel cultures, was significantly decreased in mice that lacked STAT3 expression in T cells, compared with littermate controls (Figure 1A). The percentage of cycling cells in the spleen was < 5% and was not different between mice that lacked STAT3 expression in T cells and littermate controls. The percentages of mature CD11b+Gr-1+ granulocytes and single-positive populations were indistinguishable between mice with STAT3-deficient T cells and littermate controls (data not shown). These data suggest that STAT3-dependent T-cell activity is required for HPC homeostasis in vivo.
STAT3 regulates HPC homeostasis in bone marrow and spleen. (A) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom) from wild-type or Stat3CD4−/− mice. Results are the average ± SEM of 6 Stat3CD4−/− and littermate control (WT) mice, all individually assessed from a total of 2 separate experiments. (B-C) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom) from wild-type and Il17−/− mice (B) or wild-type and Rorcgfp/gfp mice (C). Results are the average ± SEM of 3 Il17−/− and Balb/c (WT) mice, all individually assessed and representative of a total of 3 separate experiments (B), or 4 Rorcgfp/gfp and 4 C57BL/6 mice all individually assessed (C). Unless indicated, SEM of the percentage of progenitors in S-phase was < 3%. Asterisks indicate significant difference from wild-type mice: *P < .04; **P < .001. Statistics were performed using the Student t test.
STAT3 regulates HPC homeostasis in bone marrow and spleen. (A) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom) from wild-type or Stat3CD4−/− mice. Results are the average ± SEM of 6 Stat3CD4−/− and littermate control (WT) mice, all individually assessed from a total of 2 separate experiments. (B-C) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom) from wild-type and Il17−/− mice (B) or wild-type and Rorcgfp/gfp mice (C). Results are the average ± SEM of 3 Il17−/− and Balb/c (WT) mice, all individually assessed and representative of a total of 3 separate experiments (B), or 4 Rorcgfp/gfp and 4 C57BL/6 mice all individually assessed (C). Unless indicated, SEM of the percentage of progenitors in S-phase was < 3%. Asterisks indicate significant difference from wild-type mice: *P < .04; **P < .001. Statistics were performed using the Student t test.
Since STAT3 has clearly been shown to promote Th17 development, we next determined if IL-17A, which contributes to regulation of hematopoiesis,18 or the transcription factor RORγt that is required for the development of Lymphoid Tissue Inducer (LTi) and Th17 cells, were involved in HPC homeostasis. Although a decrease in bone marrow HPC numbers and cycling in Il17−/− mice compared with controls was similar to that observed in mice with STAT3-deficient T cells, there was an increase in HPC numbers in the spleen in the absence of IL-17A (Figure 1B). Disruption of Rorc, which results in decreased IL-17–secreting T cells in vivo,19 resulted in no change in bone marrow HPC numbers, though there were increases in spleen HPC numbers and cycling in bone marrow and spleen (Figure 1C and data not shown). The increase in spleen HPC in both the Il17−/− and Rorcgfp/gfp mice corresponded to an increase in the overall cellularity of the spleen but not the bone marrow in these mice, compared with controls. Together, these data suggest that the effects of STAT3-deficiency in T cells on HPC activity are not due only to a deficiency in IL-17A, or Th17, and LTi cells.
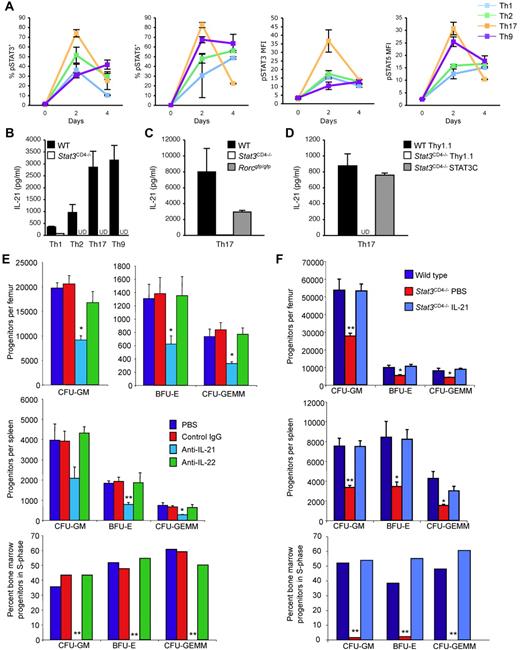
IL-21, secreted by several T helper subsets, is induced by IL-6 in a STAT3-dependent manner, and affects hematopoiesis.9-13 To define STAT3 activation in multiple T helper subsets, we differentiated wild-type and Stat3CD4−/− naive CD4 T cells under Th1, Th2, Th9 and Th17 conditions and tested for phospho-STAT3 (pSTAT3), and pSTAT5 as a control, using intracellular staining. Both pSTAT5 and pSTAT3 were detected in all Th subsets during differentiation as assessed by percent positive cells and MFI (Figure 2A). The highest amounts of IL-21 were produced in Th9 and Th17 cells, with lower amounts produced by Th1 or Th2 cells, minimal amounts produced by Treg cultures and no detectable IL-21 produced by NKT cells (Figure 2B and data not shown). Production of IL-21 was greatly reduced or undetectable in Stat3CD4−/− cultures, but was not as dramatically affected by deficiency of RORγt (Figure 2B-C). Reconstitution of STAT3 in STAT3-deficient Th17 cells recovered IL-21 production (Figure 2D). Thus, STAT3 is required for IL-21 production from multiple Th cell subsets.
STAT3-dependent IL-21 production regulates hematopoiesis. (A) Naive CD4+ T cells from wild-type mice were cultured under conditions that promote Th1, Th2, Th9 or Th17 differentiation. Cells were stained with antibodies for phospho-STAT5 and phospho-STAT3 on culture initiation, and after 2 or 4 days of differentiation. Results are the average ± SEM of 3-5 cultures for percent positive cells, based on quadrants set from staining at day 0, or mean fluorescence intensity (MFI) of the entire population. (B-C) Naive CD4+ T cells from wild-type, Stat3CD4−/− or Rorcgfp/gfp mice were cultured under the conditions indicated and after 5 days of culture, cells were restimulated with anti-CD3 for 24 hours before cell-free supernatants were analyzed for IL-21 concentration using ELISA. UD indicates undetectable. (D) Naive CD4+ T cells from wild-type or Stat3CD4−/− mice were cultured under Th17 differentiation conditions and transduced with control or active STAT3-expressing retroviruses. After 5 days of culture, cells were restimulated with anti-CD3 for 24 hours before cell-free supernatants were analyzed for IL-21 concentration using ELISA. UD indicates undetectable. (E-F) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom). (E) C57BL/6 mice were injected intraperitoneally at 0 and 72 hours with PBS, anti–IL-21, or anti–IL-22 (100 μg/injection; Abs from R&D Systems). Mice were euthanized 24 hours after the second injection for analysis of HPC. Results are the average ± SEM of 3 individually assessed mice for each treatment group. (F) Wild-type and Stat3CD4−/− mice were injected every 12 hours for 48 hours with PBS or Stat3CD4−/− mice were injected with IL-21 (2 μg in 100 μL of PBS twice per day for 2 days; cytokines from R&D Systems or Peprotech), and mice were euthanized for analysis of HPC numbers and cycling 12 hours after the last injection. Results are the average ± SEM of 4 individually assessed mice and are representative of 2 separate experiments. SEM of the percentage of progenitors in S-phase was < 3%. Asterisks indicate significant difference from wild-type or control mice: *P < .05; **P < .005. Statistics were performed using the Student t test.
STAT3-dependent IL-21 production regulates hematopoiesis. (A) Naive CD4+ T cells from wild-type mice were cultured under conditions that promote Th1, Th2, Th9 or Th17 differentiation. Cells were stained with antibodies for phospho-STAT5 and phospho-STAT3 on culture initiation, and after 2 or 4 days of differentiation. Results are the average ± SEM of 3-5 cultures for percent positive cells, based on quadrants set from staining at day 0, or mean fluorescence intensity (MFI) of the entire population. (B-C) Naive CD4+ T cells from wild-type, Stat3CD4−/− or Rorcgfp/gfp mice were cultured under the conditions indicated and after 5 days of culture, cells were restimulated with anti-CD3 for 24 hours before cell-free supernatants were analyzed for IL-21 concentration using ELISA. UD indicates undetectable. (D) Naive CD4+ T cells from wild-type or Stat3CD4−/− mice were cultured under Th17 differentiation conditions and transduced with control or active STAT3-expressing retroviruses. After 5 days of culture, cells were restimulated with anti-CD3 for 24 hours before cell-free supernatants were analyzed for IL-21 concentration using ELISA. UD indicates undetectable. (E-F) Bone marrow (top) or spleen (middle) cells were analyzed for hematopoietic progenitor cell numbers and bone marrow cells for cycling status (bottom). (E) C57BL/6 mice were injected intraperitoneally at 0 and 72 hours with PBS, anti–IL-21, or anti–IL-22 (100 μg/injection; Abs from R&D Systems). Mice were euthanized 24 hours after the second injection for analysis of HPC. Results are the average ± SEM of 3 individually assessed mice for each treatment group. (F) Wild-type and Stat3CD4−/− mice were injected every 12 hours for 48 hours with PBS or Stat3CD4−/− mice were injected with IL-21 (2 μg in 100 μL of PBS twice per day for 2 days; cytokines from R&D Systems or Peprotech), and mice were euthanized for analysis of HPC numbers and cycling 12 hours after the last injection. Results are the average ± SEM of 4 individually assessed mice and are representative of 2 separate experiments. SEM of the percentage of progenitors in S-phase was < 3%. Asterisks indicate significant difference from wild-type or control mice: *P < .05; **P < .005. Statistics were performed using the Student t test.
To determine whether decreased IL-21 results in an HPC phenotype similar to mice that lack STAT3 in T cells, we injected wild-type mice with anti–IL-21, or with control Ig and anti–IL-22 as controls. Although control Ig and anti-IL-22 had no significant effect on HPC numbers or cycling, anti–IL-21 resulted in decreased HPC numbers in bone marrow and spleen, and eliminated HPC cycling in the bone marrow (Figure 2E).
To directly test if IL-21 would rescue the HPC defect observed in mice with STAT3-deficient T cells, Stat3CD4−/− mice were injected with PBS or IL-21. Injection of IL-21 increased HPC numbers in the bone marrow and spleen, and cycling in the bone marrow, in Stat3CD4−/− mice (Figure 2F). Thus, STAT3-dependent T-cell production of IL-21 is required for HPC activity.
Multiple T-cell subsets regulate hematopoiesis through mechanisms that are likely linked to their ability to promote inflammation.20 During immune responses, T-cell cytokines can promote the development of granulocytes that serve to replenish and reinforce cells recruited to sites of inflammation. Through similar mechanisms, T cells also regulate hematopoiesis. CD4+ T cells, and subsets including Th1 cells, maintain HSC and HPC homeostasis.1-3,21 Although an IL-21–expressing transgene had been previously shown to increase hematopoiesis,10 a physiologic role for IL-21 in regulating HPC activity was not previously defined. In this report we demonstrated that STAT3, a transcription factor that is activated during differentiation of multiple Th subsets, is required for IL-21 production, and that in the absence of STAT3 in T cells, decreased IL-21 results in decreased HPC numbers and cycling. Our study reinforces data demonstrating that T cells regulate HPC homeostasis, and defines an additional level of control by T cells on hematopoiesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Public Health Service U19 AI070448 and RO1 AI057459 (to M.H.K.), R21 AI077091 (to R.R.B., J.S.B. and M.H.K.) and RO1 HL56416 and HL67384 (to H.E.B.). G.L.S. and N.Y. were supported by T32 AI060519; J.K., N.L.G. and D.P. were supported by T32 HL07910; J.K. was supported by T32 DK07519; and S.L.R. was supported by T32 CA111198.
National Institutes of Health
Authorship
Contribution: M.H.K. and H.E.B. planned the experiments and wrote the manuscript; N.L.G., G.L.S., N.Y., J.K., S.L.R., R.G., D.P., S.C. and G.H. performed the experiments; and D.E.L., R.R.B., and J.S.B. provided reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Mark H. Kaplan, Departments of Pediatrics, and Microbiology and Immunology, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut St Rm 202, Indianapolis, IN 46202; e-mail: mkaplan2@iupui.edu; or Dr Hal E. Broxmeyer, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 West Walnut St R2 302, Indianapolis, IN 46202; e-mail: hbroxmey@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal